Anti-Obesity Effects of Ecklonia cava Extract in High-Fat Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. E. cava and Animals
2.2. Extraction and Characterization of E. cava
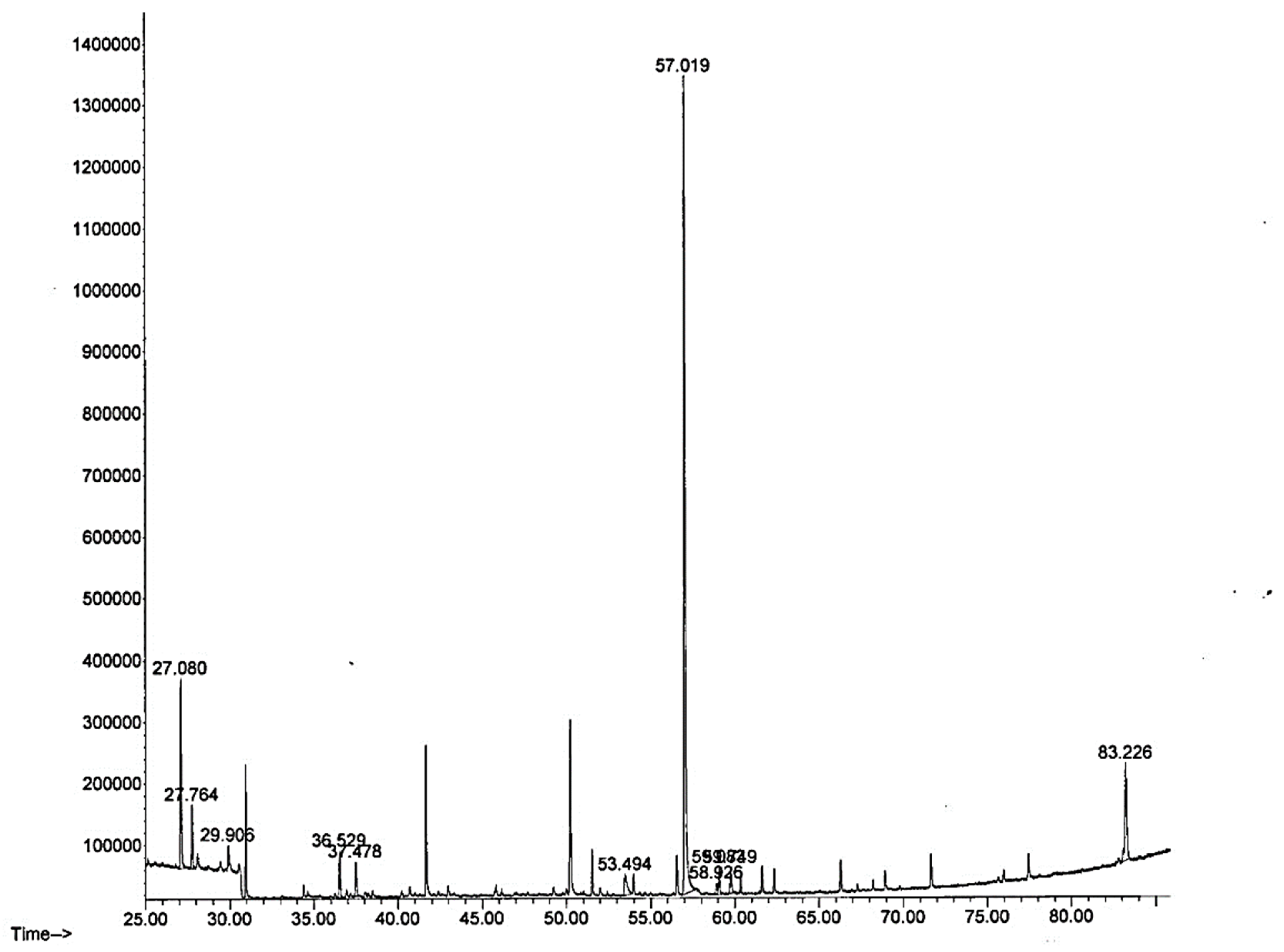
2.3. Gas Chromatography/Mass Spectrometry Analysis (GCMS) of E. cava
2.4. Cell Culture (In Vitro Study)
2.5. Oil Red O Staining
2.6. Cell Viability Determination via MTT Assay
2.7. Antioxidant Activity of E. cava (DPPH and ABTS Assay)
2.8. Experiment Design for In Vivo Study and Animal Housing
2.9. Animal Feed and Administration
2.10. Animal Feeding and FCR
2.11. Blood Glucose Level Determination
2.12. Necropsy and Sample Collection
2.13. Evaluation of Plasma Level of Various Obesity-Related Biomarkers
2.14. Liver and Adipose Histopathology
2.15. Extraction of Total RNA and PCR Analysis
2.16. Receiver Operating Characteristics (ROC) and Area under ROC Curve (AUROC)
2.17. Statistical Analysis
3. Results
3.1. Extraction and Characterization of E. cava
3.2. Gas Chromatography/Mass Spectrometry Analysis (GCMS) of E. cava
3.3. Suppressive and Cytotoxic Effects of E. cava Extract on Adipogenesis in 3T3-L1 Pre-Adipocytes
3.4. Antioxidant Activity of E. cava (DPPH and ABTS Assay)
3.5. Observation of General Body Weight and Feed Conversion Ratio (FCR)
3.6. Effects of E. cava Treatment on Organ Weight
3.7. Effects E. cava Treatment on Visceral Fat
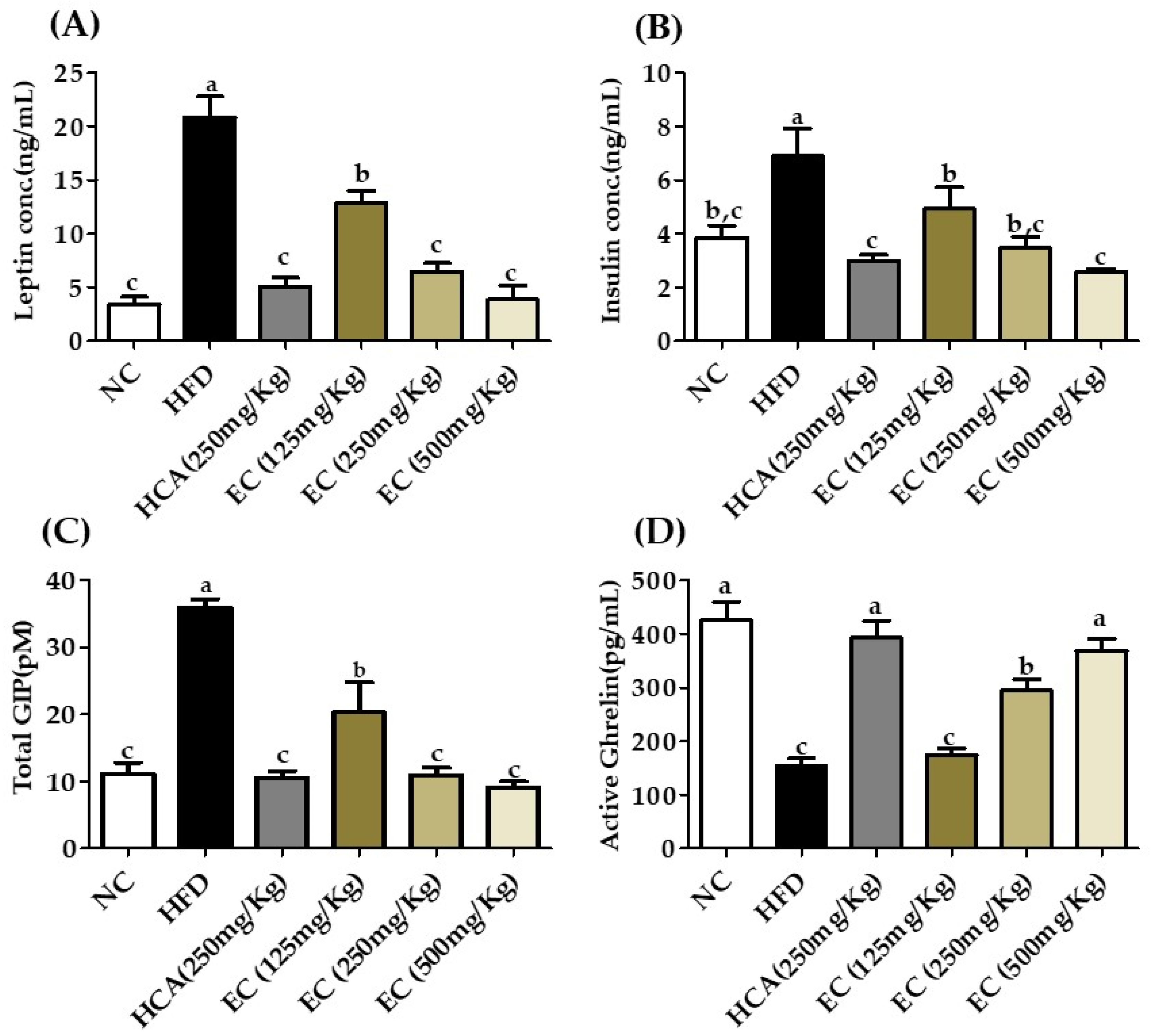
3.8. Effect of E. cava on Adipokines, Incretins, Total GIP, and Insulin
3.9. Effects of E. cava on Blood Glucose Level
3.10. Effect of E. cava on Plasma Level of Pro-Inflammatory Cytokines
3.11. Effects of E. cava on Lipid Profile and Liver Function Biomarkers
3.12. Effects of E. cava on Hepatocytes and Adipocytes
3.13. Effects of E. cava Extract on Adipogenic and Lipogenic Gene Expression
3.14. Receiver Operating Characteristics (ROC) and Area under the ROC Curve
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC/E.cava | Ecklonia cava |
| ECE | Ecklonia cava extract |
| NC | normal control |
| PC | positive control |
| HFD | high-fat diet |
| T1 | low dose of ECE treatment group |
| T2 | intermediate dose of ECE treatment group |
| T3 | high dose of ECE treatment group |
| HCA | hydroxy citric acid |
| BW/b.w | bodyweight |
| FCR | feed conversion rate |
| GIP | gastric inhibitory peptides |
| GGT | gamma-glutamyl transferase |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| TG | triglycerides |
| FFA | free fatty acid |
| AI | atherogenic index |
| RNA | ribonucleic Acid |
| PCR | polymerase chain reaction |
| CDNA | complementary DNA |
| DMEM | Dulbecco’s modified Eagle’s medium |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| LPL | lipo-protein lipase |
| SRBP-1c | sterol regulatory element-binding transcription factor 1 |
| FBS | fetus bovine serum |
| DEX | dexamethasone |
| IBMX | 3-Isobutyl-1-methylxanthine |
| ROC | receiver operating characteristics |
| AUROC | area under receiver operating characteristics curve |
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| GGT | gamma-glutamyl-transferase |
| WHO | World Health Organization |
| COVID | coronavirus disease |
| NAFLD | nonalcoholic fatty liver disease |
| HIV | human immunodeficiency virus |
| SD | Sprague–Dawley |
| DCM | dichloromethane |
| IU | international unit |
| PBS | Phospahte buffer saline |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | dimethyl sulfoxide |
| OD | optical density |
| DPPH | 2,2-diphenyl-1picryl-hydrzyl |
| ABTS | 2,2-azino-bis(3-ethylbenzothiazolin)-6-sulfonic acid |
| RPM | round per minute |
| TA | tannic acid |
| FF | fenofibrate |
| SEM | standard error mean |
| ELISA | enzyme-linked immunoassay |
| TNF-a | tumor necrosis factor-alpha |
| IL-6 | interleukin-6 |
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Obesity and Overweight Fact Sheet; World Health Organizaton: Geneva, Switzerland, 2016.
- Clemmensen, C.; Petersen, M.B.; Sørensen, T.I. Will the COVID-19 pandemic worsen the obesity epidemic? Nat. Rev. Endocrinol. 2020, 16, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Kwon, Y.; Choe, Y.H.; Kim, M.J. COVID-19-related school closing aggravate obesity and glucose intolerance in pediatric patients with obesity. Sci. Rep. 2021, 11, 5494. [Google Scholar] [CrossRef]
- Lee, M. Research trends in obesity & obesogenic environments in Korea. Nutr. Res. Pract. 2019, 13, 461. [Google Scholar]
- Jéquier, E. Pathways to obesity. Int. J. Obes. 2002, 26, S12–S17. [Google Scholar] [CrossRef]
- Obici, S.; Rossetti, L. Minireview: Nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 2003, 144, 5172–5178. [Google Scholar] [CrossRef]
- Gurevich-Panigrahi, T.; Panigrahi, S.; Wiechec, E.; Los, M. Obesity: Pathophysiology and clinical management. Curr. Med. Chem. 2009, 16, 506–521. [Google Scholar] [CrossRef]
- Wang, S.-B.; Cho, Y.-C. Body mass index and subsequent risk of hypertension, hyperglycemia and hypercholesterolemia in health checkup examinees. J. Korea Acad. Ind. Coop. Soc. 2011, 12, 2677–2684. [Google Scholar]
- Park, E.Y.; Kim, E.H.; Kim, M.H.; Seo, Y.W.; Lee, J.I.; Jun, H.S. Polyphenol-rich fraction of brown alga Ecklonia cava collected from Gijang, Korea, reduces obesity and glucose levels in high-fat diet-induced obese mice. Evid. Based Complementary Altern. Med. 2012, 2012, 418912. [Google Scholar] [CrossRef]
- Sergent, T.; Vanderstraeten, J.; Winand, J.; Beguin, P.; Schneider, Y.-J. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012, 135, 68–73. [Google Scholar] [CrossRef]
- Yun, J.-W.; Kim, S.-H.; Kim, Y.-S.; You, J.-R.; Cho, E.-Y.; Yoon, J.-H.; Kwon, E.; Yun, I.-J.; Oh, J.-H.; Jang, J.-J. Enzymatic extract from Ecklonia cava: Acute and subchronic oral toxicity and genotoxicity studies. Regul. Toxicol. Pharmacol. 2018, 92, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jin, Y.B.; Lee, H.; Cha, M.; Sohn, E.-t.; Moon, J.; Park, C.; Chun, S.; Jung, E.-S.; Hong, J.-S. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem. Toxicol. 2010, 48, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Han, J.-S.; Heo, S.-J.; Hwang, J.-Y.; Jeon, Y.-J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. Vitr. 2010, 24, 375–381. [Google Scholar] [CrossRef]
- Lee, S.-H.; Min, K.-H.; Han, J.-S.; Lee, D.-H.; Park, D.-B.; Jung, W.-K.; Park, P.-J.; Jeon, B.-T.; Kim, S.-K.; Jeon, Y.-J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef]
- Yeo, A.-R.; Lee, J.; Tae, I.H.; Park, S.-R.; Cho, Y.H.; Lee, B.H.; Shin, H.C.; Kim, S.H.; Yoo, Y.C. Anti-hyperlipidemic effect of polyphenol extract (Seapolynol™) and dieckol isolated from Ecklonia cava in in vivo and in vitro models. Prev. Nutr. Food Sci. 2012, 17, 1. [Google Scholar] [CrossRef]
- Kang, K.; Hwang, H.J.; Hong, D.H.; Park, Y.; Kim, S.H.; Lee, B.H.; Shin, H.-C. Antioxidant and antiinflammatory activities of ventol, a phlorotannin-rich natural agent derived from Ecklonia cava, and its effect on proteoglycan degradation in cartilage explant culture. Res. Commun. Mol. Pathol. Pharmacol. 2004, 115, 77–95. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies; Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e05003. [Google Scholar]
- Louhimies, S. Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes. Altern. Lab. Anim. 2002, 30, 217–219. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Boby, N.; Abbas, M.A.; Lee, E.-B.; Im, Z.-E.; Hsu, W.H.; Park, S.-C. Protective effect of Pyrus ussuriensis Maxim. extract against ethanol-induced gastritis in rats. Antioxidants 2021, 10, 439. [Google Scholar] [CrossRef]
- Hackbarth, H.; Küppers, N.; Bohnet, W. Euthanasia of rats with carbon dioxide-animal welfare aspects. Lab. Anim. 2000, 34, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, A.; Lima-Leopoldo, A.; Nascimento, A.; Luvizotto, R.; Sugizaki, M.; Campos, D.; Da Silva, D.; Padovani, C.; Cicogna, A. Classification of different degrees of adiposity in sedentary rats. Braz. J. Med. Biol. Res. 2016, 49, e5028. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bays, H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 345. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Obesity and kidney disease: Hidden consequences of the epidemic. Clin. Kidney J. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Gil-Campos, M.; Aguilera, C.; Ramirez-Tortosa, M.; Canete, R.; Gil, A. Fasting and postprandial relationships among plasma leptin, ghrelin, and insulin in prepubertal obese children. Clin. Nutr. 2010, 29, 54–59. [Google Scholar] [CrossRef]
- Zou, C.-C.; Liang, L.; Zhao, Z.-Y. Factors associated with fasting plasma ghrelin levels in children and adolescents. World J. Gastroenterol. WJG 2008, 14, 790. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Yang, Y.; Xiang, X.; Zhu, Y.; Men, J.; He, M. Estimation of the normal range of blood glucose in rats. Wei Sheng Yan Jiu J. Hyg. Res. 2010, 39, 133–137, 142. [Google Scholar]
- Catalán, V.; Gómez-Ambrosi, J.; Ramirez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. Int. Sch. Res. Not. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kinosian, B.; Glick, H.; Garland, G. Cholesterol and coronary heart disease: Predicting risks by levels and ratios. Ann. Intern. Med. 1994, 121, 641–647. [Google Scholar] [CrossRef]
- Bo, M.S.; Cheah, W.L.; Lwin, S.; Moe Nwe, T.; Win, T.T.; Aung, M. Understanding the relationship between atherogenic index of plasma and cardiovascular disease risk factors among staff of an university in Malaysia. J. Nutr. Metab. 2018, 2018, 7027624. [Google Scholar] [CrossRef]
- Burns, C.J.; Boswell, J.M.; Olsen, G.W. Liver enzyme activity and body mass index. J. Occup. Environ. Med. 1996, 38, 1248–1252. [Google Scholar] [CrossRef]
- Choi, J.W. Association between elevated serum hepatic enzyme activity and total body fat in obese humans. Ann. Clin. Lab. Sci. 2003, 33, 257–264. [Google Scholar]
- Adams, L.A.; Knuiman, M.W.; Divitini, M.L.; Olynyk, J.K. Body mass index is a stronger predictor of alanine aminotransaminase levels than alcohol consumption. J. Gastroenterol. Hepatol. 2008, 23, 1089–1093. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Cho, Y.-K.; Kang, M.-S.; Yoo, T.-W.; Park, J.-H.; Kim, H.-J.; Park, D.-I.; Sohn, C.-I.; Jeon, W.-K.; Kim, B.-I. The association between increased alanine aminotransferase activity and metabolic factors in nonalcoholic fatty liver disease. Metabolism 2006, 55, 1604–1609. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Cmaj 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H.; Frierson Jr, H.F. Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J. Histochem. Cytochem. 1996, 44, 1101–1108. [Google Scholar] [CrossRef]
- Alcala, M.; Gutierrez-Vega, S.; Castro, E.; Guzman-Gutiérrez, E.; Ramos-Álvarez, M.P.; Viana, M. Antioxidants and oxidative stress: Focus in obese pregnancies. Front. Physiol. 2018, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.D.; Jakobsdottir, S.; Drent, M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Kim, S.P.; Catalano, K.J.; Hsu, I.R.; Chiu, J.D.; Kabir, M.; Hucking, K.; Ader, M. Why visceral fat is bad: Mechanisms of the metabolic syndrome. Obesity 2006, 14, 16S. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Pozzilli, P. Obesity and glucose metabolism. In Multidisciplinary Approach to Obesity; Springer: Cham, Switzerland, 2015; pp. 107–119. [Google Scholar]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Irwin, N.; Flatt, P. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia 2009, 52, 1724–1731. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Kasarala, G.; Tillmann, H.L. Standard liver tests. Clin. Liver Dis. 2016, 8, 13. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Srinivasan, S.R.; Xu, J.-H.; Chen, W.; Hassig, S.; Rice, J.; Berenson, G.S. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: The Bogalusa Heart Study. Diabetes Care 2011, 34, 2603–2607. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed]
- Ghadir, M.R.; Riahin, A.A.; Havaspour, A.; Nooranipour, M.; Habibinejad, A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010, 10, 285. [Google Scholar] [PubMed]
- Szczygielska, A.; Widomska, S.; Jaraszkiewicz, M.; Knera, P.; Muc, K. Blood lipids profile in obese or overweight patients. Ann. Univ. Mariae Curie-Sklodowska Sectio D Med. 2003, 58, 343–349. [Google Scholar]
- Zhu, X.; Yu, L.; Zhou, H.; Ma, Q.; Zhou, X.; Lei, T.; Hu, J.; Xu, W.; Yi, N.; Lei, S. Atherogenic index of plasma is a novel and better biomarker associated with obesity: A population-based cross-sectional study in China. Lipids Health Dis. 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D. The impact of obesity on liver histology. Clin. Liver Dis. 2014, 18, 33–40. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Adipogenesis and obesity: Rounding out the big picture. Cell 1996, 87, 377–389. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]











| Groups | Daily Intake | |
|---|---|---|
| NC | Normal control | Normal feed |
| HFD | High-fat diet | Feed with 45% high-fat content (HFD) |
| PC | Positive control | 250 mg/kg b.w HCA * + HFD |
| T1 | Dose 1 | 125 mg/kg b.w of ECE ** + HFD |
| T2 | Dose 2 | 250 mg/kg b.w of ECE + HFD |
| T3 | Dose 3 | 500 mg/kg b.w of ECE + HFD |
| Parameters | Weight Gain (gm/rat) | FCR | Glucose (mg/dL) |
|---|---|---|---|
| NC | 388.12 ± 30.3 | 26.01 | 102.3 ± 0.54 *** |
| HFD | 509.13 ± 51.7 | 17.60 | 139.6 ± 1.85 +++ |
| HCA (250 mg/kg) | 431.88 ± 14.6 | 18.88 | 103.9 ± 0.95 *** |
| EC (125 mg/kg) | 448 ± 43.7 | 17.33 | 105.4 ± 0.75 *** |
| EC (250 mg/kg) | 436.5 ± 23.6 | 18.01 | 104.4 ± 0.43 *** |
| EC (500 mg/kg) | 424 ± 14.72 | 20.08 | 102 ± 0.61 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.A.; Boby, N.; Lee, E.-B.; Hong, J.-H.; Park, S.-C. Anti-Obesity Effects of Ecklonia cava Extract in High-Fat Diet-Induced Obese Rats. Antioxidants 2022, 11, 310. https://doi.org/10.3390/antiox11020310
Abbas MA, Boby N, Lee E-B, Hong J-H, Park S-C. Anti-Obesity Effects of Ecklonia cava Extract in High-Fat Diet-Induced Obese Rats. Antioxidants. 2022; 11(2):310. https://doi.org/10.3390/antiox11020310
Chicago/Turabian StyleAbbas, Muhammad Aleem, Naila Boby, Eon-Bee Lee, Joo-Heon Hong, and Seung-Chun Park. 2022. "Anti-Obesity Effects of Ecklonia cava Extract in High-Fat Diet-Induced Obese Rats" Antioxidants 11, no. 2: 310. https://doi.org/10.3390/antiox11020310
APA StyleAbbas, M. A., Boby, N., Lee, E.-B., Hong, J.-H., & Park, S.-C. (2022). Anti-Obesity Effects of Ecklonia cava Extract in High-Fat Diet-Induced Obese Rats. Antioxidants, 11(2), 310. https://doi.org/10.3390/antiox11020310








