Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Distillation Procedure
2.2. GC-FID Analysis of the Essential Oil
2.3. Gas Chromatography–Mass Spectroscopy Analysis of the Volatile Oil
2.4. Identification of the Essential Oil Constituents
2.5. Antioxidant Activity of ArJ Essential Oil
2.5.1. Total Antioxidant Capacity (TAC)
2.5.2. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Scavenging Activity (DPPH-SA)
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Metal Chelating Activity Assay (MCA)
2.6. Antimicrobial Activity of ArJ Essential Oil
2.6.1. Preliminary Antimicrobial Activity
2.6.2. Minimum Inhibitory Concentration (MIC) and Minimum Biocidal Concentration (MBC)
2.6.3. Minimum Biofilm Inhibitory Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC)
MBIC Assay
MBEC Assay
2.7. Preparation of Ointment Formulation Loaded ArJ Essential Oil
2.8. In Vivo Wound Healing Animal Experiment
2.9. Skin Burn Induction Model
2.10. Biopsy
2.11. Histological Staining
2.12. Determination of Oxidants and Antioxidants
2.13. Determination of Pro-Inflammatory and Anti-Inflammatory Cytokine Levels: Interleukins, TGF-b, and TNF-α Levels
2.14. Wound Area Measurement
2.15. Statistical Analysis
3. Results and Discussion
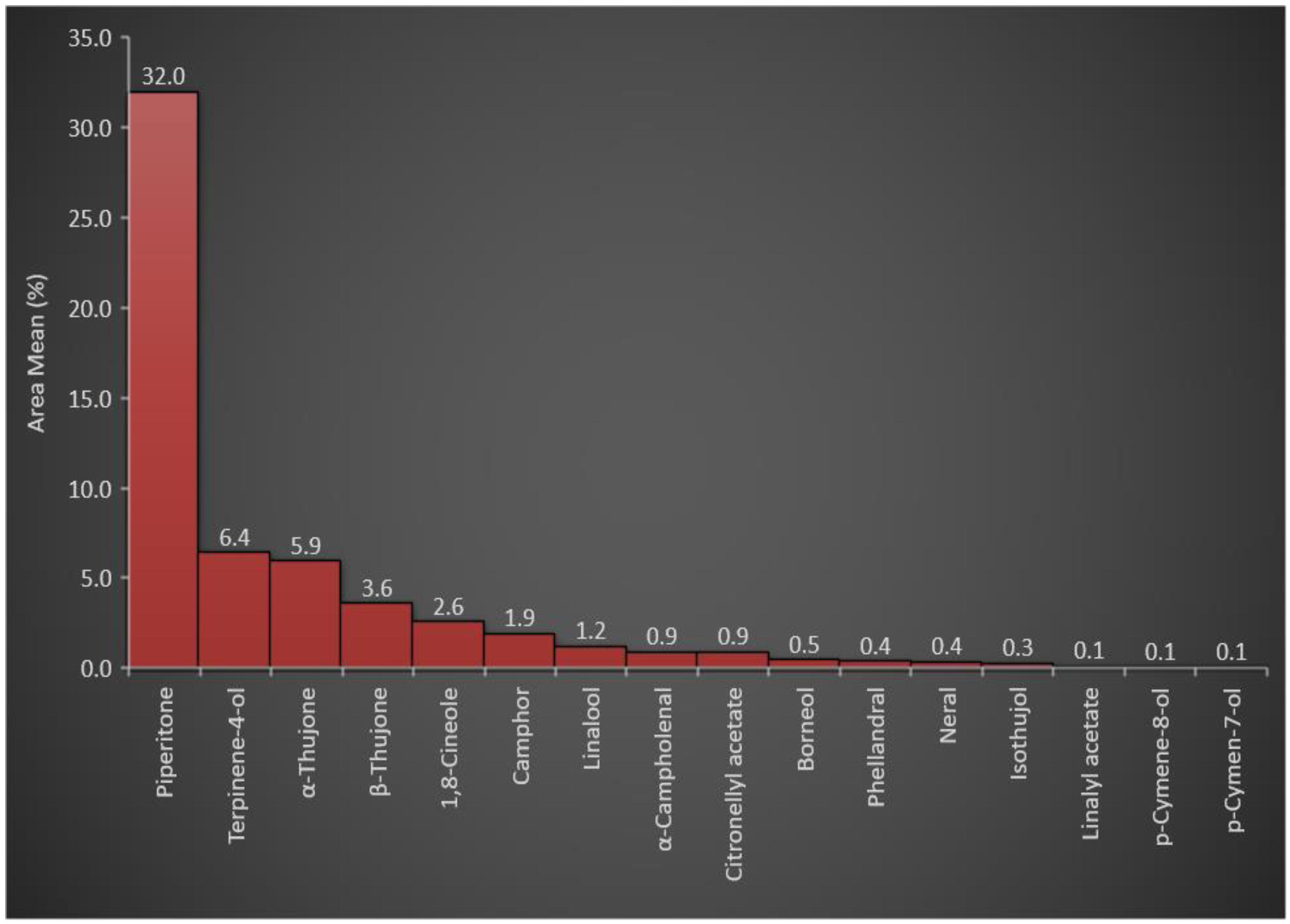
3.1. Essential Oil Constituents of A. judaica
3.2. In Vitro Antioxidant Activity of the ArJ Essential Oil
3.3. Antimicrobial Profile of ArJ Essential Oil
3.3.1. Preliminary Antimicrobial Activity
3.3.2. Minimum Inhibitory Concentration (MIC), Minimum Biocidal Concentration (MBC), Minimum Biofilm Inhibitory Concentration (MBIC), and Minimum Biofilm Eradication Concentration (MBEC)
3.4. In Vivo Skin Burn Wound Healing
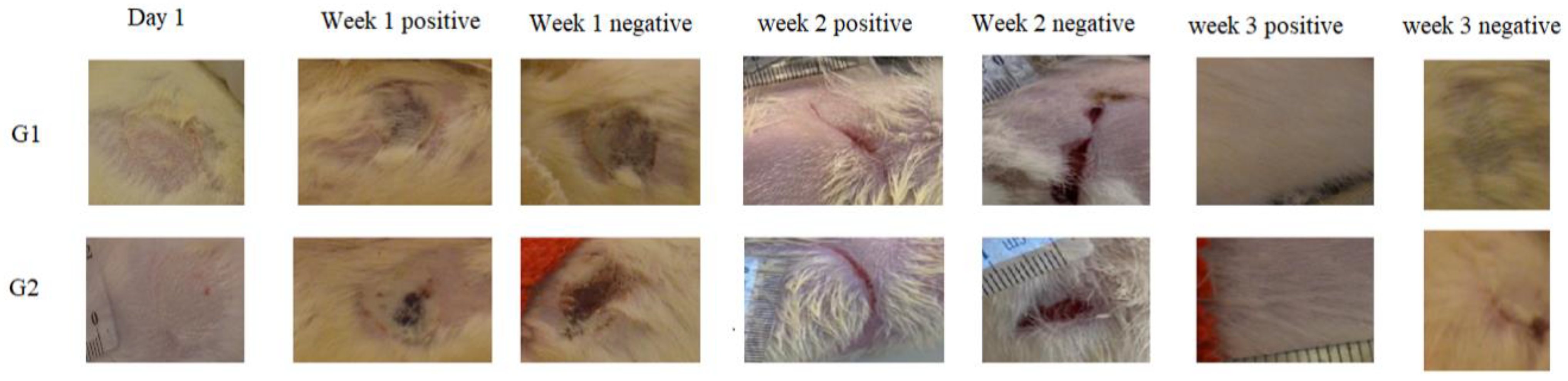
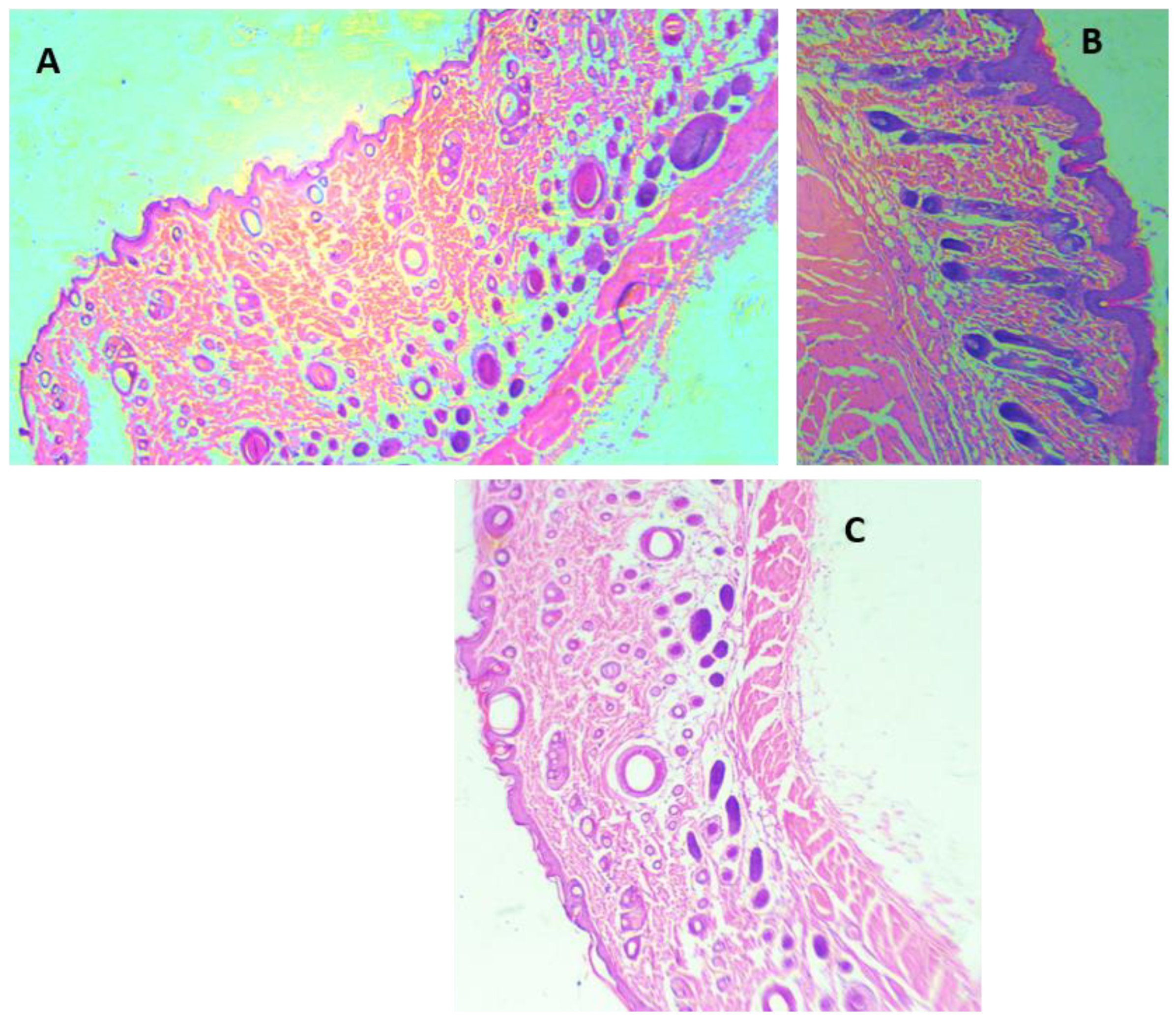
3.4.1. Morphological Appearance and Histological Analysis of the Wounds
3.4.2. Role of Antioxidants and Oxidative Stress Markers in Wound Healing
3.4.3. Role of Pro- and Anti-Inflammatory Markers in Wound Healing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Miño, J.H.; Moscatelli, V.; Acevedo, C.; Ferraro, G. Psychopharmacological effects of Artemisia copa aqueous extract in mice. Pharm. Biol. 2010, 48, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, A.; Ravichandiran, V.; Prasad, K.V.D.; Kumar, A.K.; Rakesh, K.; Naidu, K.R.; Vardhan, K.S. Anticonvulsant activity of Artemisia nilagirica leaves. J. Pharm. Res. 2011, 4, 2881–2883. [Google Scholar]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Artemisia sieberi Besser essential oil and treatment of fungal infections. Biomed. Pharmacother. 2017, 89, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Artemisia annua (Qinghao): A pharmacological review. Int. J. Pharm. Sci. Res. 2012, 3, 4573–4577. [Google Scholar]
- Hijazi, A.M.; Salhab, A.S. Effects of Artemisia monosperma ethanolic leaves extract on implantation, mid-term abortion and parturition of pregnant rats. J. Ethnopharmacol. 2010, 128, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578–19850354. [Google Scholar]
- Willcox, M.L.; Burton, S.; Oyweka, R.; Namyalo, R.; Challand, S.; Lindsey, K. Evaluation and pharmacovigilance of projects promoting cultivation and local use of Artemisia annua for malaria. Malar. J. 2011, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, M.Q.; Khan, M.A.; Ashraf, M.; Jabeen, S. Ethnobotany of the genus Artemisia L. (Asteraceae) in Pakistan. Ethnobot. Res. Appl. 2009, 7, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, R.G.; Shafizadeh, F. Sesquiterpene lactones and systematics of the genus Artemisia. Phytochemistry 1979, 18, 1591–1611. [Google Scholar] [CrossRef]
- Martínez, M.J.A.; Del Olmo, L.M.B.; Ticona, L.A.; Benito, P.B. The Artemisia L. genus: A review of bioactive sesquiterpene lactones. Stud. Nat. Prod. Chem. 2012, 37, 43–65. [Google Scholar]
- Vouillamoz, J.F.; Carlen, C.; Taglialatela-Scafati, O.; Pollastro, F.; Appendino, G. The génépi Artemisia species. Ethnopharmacology, cultivation, phytochemistry, and bioactivity. Fitoterapia 2015, 106, 231–241. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; Al Borki, A.E.-N.S.; Elagami, S.A. Potential of wild plant Artemisia judaica L. as sustainable source of antioxidant and antimicrobial compounds. J. Exp. Sci. 2019, 10, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Benmansour, N.; Benmansour, A.; El Hanbali, F.; González-Mas, M.C.; Blázquez, M.A.; El Hakmaoui, A.; Akssira, M. Antimicrobial activity of essential oil of Artemisia judaica L. from Algeria against multi-drug resistant bacteria from clinical origin. Flavour Fragr. J. 2016, 31, 137–142. [Google Scholar] [CrossRef]
- Amin, S.M.; Hassan, H.M.; El Gendy, A.E.G.; El-Beih, A.A.; Mohamed, T.A.; Elshamy, A.I.; Bader, A.; Shams, K.A.; Mohammed, R.; Hegazy, M.F. Comparative chemical study and antimicrobial activity of essential oils of three Artemisia species from Egypt and Saudi Arabia. Flavour Fragr. J. 2019, 34, 450–459. [Google Scholar] [CrossRef]
- Guetat, A.; Al-Ghamdi, F.A.; Osman, A.K. The genus Artemisia L. in the northern region of Saudi Arabia: Essential oil variability and antibacterial activities. Nat. Prod. Res. 2017, 31, 598–603. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Helail, N.S.; El Ghanim, W. Genetic diversity of Artemisia populations in central and north Saudi Arabia based on morphological variation and RAPD polymorphism. Plant Syst. Evol. 2012, 298, 871–886. [Google Scholar] [CrossRef]
- Moharram, F.A.; Nagy, M.M.; El Dib, R.A.; El-Tantawy, M.M.; El Hossary, G.G.; El-Hosari, D.G. Pharmacological activity and flavonoids constituents of Artemisia judaica L. aerial parts. J. Ethnopharmacol. 2021, 270, 113777. [Google Scholar] [CrossRef]
- Janacković, P.; Novaković, J.; Soković, M.; Vujisić, L.; Giweli, A.A.; Dajić-Stevanović, Z.; Marin, P.D. Composition and antimicrobial activity of essential oils of Artemisia judaica, A. herba-alba and A. arborescens from Libya. Arch. Biol. Sci. 2015, 67, 455–466. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.N.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem. 2020, 13, 2053–2065. [Google Scholar] [CrossRef]
- Hussain, A.; Hayat, M.Q.; Sahreen, S.; Ain, Q.U.; Bokhari, S.A.I. Pharmacological promises of genus Artemisia (Asteraceae): A review. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2017, 54, 265–287. [Google Scholar]
- Nofal, S.M.; Mahmoud, S.S.; Ramadan, A.; Soliman, G.A.; Fawzy, R. Anti-diabetic effect of Artemisia judaica extracts. Res. J. Med. Med. Sci. 2009, 4, 42–48. [Google Scholar]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Nasr, F.A.; Noman, O.M.; Mothana, R.A.; Alqahtani, A.S.; Al-Mishari, A.A. Cytotoxic, antimicrobial and antioxidant activities and phytochemical analysis of Artemisia judaica and A. sieberi in Saudi Arabia. Afr. J. Pharm. Pharmacol. 2020, 14, 278–284. [Google Scholar]
- El-Sayed, M.A.; BaAbbad, R.; Balash, A.; Al-Hemdan, N.A.; Softah, A. The potential anti Helicobacter pylori and antioxidant effects of Artemisia judaica. Funct. Foods Health Dis. 2013, 3, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, H.I.; Aly, H.F.; Shalaby, N.M.M.; Albalawy, M.A.; Aboutabl, E.A. Hunting for renal protective phytoconstituents in Artemisia judaica L. and Chrysanthemum coronarium L. (Asteraceae). Egypt. Pharm. J. 2014, 13, 46. [Google Scholar] [CrossRef]
- Charchari, S. The essential oil of Artemisia judaica L. from Algeria. J. Essent. Oil Res. 2002, 14, 16–17. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 3528–3550. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.; El Ouassis Dahmane, H.M.; Rym, E.; Amira, S.; el Houda, H.N.; Selma, B.A.; Nadia, F. Chemical composition and bilogical effects of essential oil of Artemisia judaica an endemic plant from central Sahara of Algeria Hoggar. Int. J. Biosci. 2017, 10, 16–23. [Google Scholar]
- Abdallah Sallam, S.M.; Mohamed Abdelgaleil, S.A.; da Silva Bueno, I.C.; Abdelwahab Nasser, M.E.; Araujo, R.C.; Abdalla, A.L. Effect of some essential oils on in vitro methane emission. Arch. Anim. Nutr. 2011, 65, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Onizat, M.A.; Alshamari, A.K.; Al-Jaber, H.I.; Bdair, O.M.; Muhaidat, R.; Al Zoubi, M.; Al-Bataineh, N. Chemical composition and antioxidant activity of Jordanian Artemisia judaica L. as affected by different drying methods. Int. J. Food Prop. 2021, 24, 482–492. [Google Scholar] [CrossRef]
- Mengesha, B.; Mohammed, O.; Tessema, T.; Abate, S. Production, Processing and Utilization of Aromatic Plants; Ethiopian Institute of Agricultural Research: Addis Ababa, Ethiopia, 2010. [Google Scholar]
- Piesse, G.W.S. The Art of Perfumery and the Methods of Obtaining the Odors of Plants: With Instructions for the Manufacture of Perfumes for the Handkerchief, Scented Powders, Odorous Vinegars, Dentifrices, Pomatums, Cosmetics, Perfumed Soap, etc., to Which is Added an a; Lindsay & Blakiston: Philadelphia, PA, USA, 1867. [Google Scholar]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar]
- Zhi-ling, C.; Jian-ping, C.; Hui-lin, C.; Wei-tao, B.; Hai-yan, C.; Mo-lin, L. Research on the extraction of plant volatile oils. Procedia Environ. Sci. 2011, 8, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Sievers, A.F. Methods of Extracting Volatile Oils from Plant Material and the Production of Such Oils in the United States; US Department of Agriculture: Washington, DC, USA, 1952.
- Kruger, E.; Kowal, S.; Bilir, S.P.; Han, E.; Foster, K. Relationship between patient characteristics and number of procedures as well as length of stay for patients surviving severe burn injuries: Analysis of the American Burn Association National Burn Repository. J. Burn Care Res. 2020, 41, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Guaouguaou, F.-E.; Taghzouti, K.; Oukabli, M.; Es-Safi, N.E. The Effect of Salvia verbenaca Extracts for Healing of Second-Degree Burn Wounds in Rats. Curr. Bioact. Compd. 2018, 14, 419–427. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Tradit. Complement. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Fahimi, S.; Abdollahi, M.; Mortazavi, S.A.; Hajimehdipoor, H.; Abdolghaffari, A.H.; Rezvanfar, M.A. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran. Red Crescent Med. J. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Akhoondinasab, M.R.; Khodarahmi, A.; Akhoondinasab, M.; Saberi, M.; Iranpour, M. Assessing effect of three herbal medicines in second and third degree burns in rats and comparison with silver sulfadiazine ointment. Burns 2015, 41, 125–131. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal products for treatment of burn wounds. J. Burn Care Res. 2020, 41, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Aroua, L.M.; Almuhaylan, H.R.; Alminderej, F.M.; Messaoudi, S.; Chigurupati, S.; Al-Mahmoud, S.; Mohammed, H.A. A facile approach synthesis of benzoylaryl benzimidazole as potential α-amylase and α-glucosidase inhibitor with antioxidant activity. Bioorg. Chem. 2021, 114, 105073. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007; ISBN 1420014498. [Google Scholar]
- Qureshi, K.A.; Imtiaz, M.; Parvez, A.; Rai, P.K.; Jaremko, M.; Emwas, A.-H.; Bholay, A.D.; Fatmi, M.Q. In vitro and in silico approaches for the evaluation of antimicrobial activity, time-kill kinetics, and anti-biofilm potential of thymoquinone (2-Methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione) against selected human pathogens. Antibiotics 2022, 11, 79. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Bholay, A.D.; Rai, P.K.; Mohammed, H.A.; Khan, R.A.; Azam, F.; Jaremko, M.; Emwas, A.-H.; Stefanowicz, P.; Waliczek, M.; et al. Isolation, characterization, anti-MRSA evaluation, and in-silico multi-target anti-microbial validations of actinomycin X2 and actinomycin D produced by novel Streptomyces smyrnaeus UKAQ_23. Sci. Rep. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- The British Pharmacopoeia Commission. British Pharmacopoeia; Her Majesty’s Stationary Office: London, UK, 1998.
- Akhoondinasab, M.R.; Akhoondinasab, M.; Saberi, M. Comparison of healing effect of aloe vera extract and silver sulfadiazine in burn injuries in experimental rat model. World J. Plast. Surg. 2014, 3, 29–34. [Google Scholar]
- Manafi, A.; Kohanteb, J.; Mehrabani, D.; Japoni, A.; Amini, M.; Naghmachi, M.; Zaghi, A.H.; Khalili, N. Active immunization using exotoxin A confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. 2009, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.T.; Hughes, M.A.; Cherry, G.W. Enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin), an herbal remedy for treating wounds. Plast. Reconstr. Surg. 1998, 101, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Al-Omar, M.S.; Naz, M.; Mohammed, S.A.A.; Mansha, M.; Ansari, M.N.; Rehman, N.U.; Kamal, M.; Mohammed, H.A.; Yusuf, M.; Hamad, A.M.; et al. Pyrethroid-Induced Organ Toxicity and Anti-Oxidant-Supplemented Amelioration of Toxicity and Organ Damage: The Protective Roles of Ascorbic Acid and α-Tocopherol. Int. J. Environ. Res. Public Health 2020, 17, 6177. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics; Essentials; Wiley: Hoboken, NJ, USA, 2010; ISBN 9781444392845. [Google Scholar]
- Moisa, C.; Lupitu, A.; Pop, G.; Chambre, D.R.; Copolovici, L.; Cioca, G.; Bungau, S.; Copolovici, D.M. Variation of the chemical composition of Thymus vulgaris essential oils by phenological stages. Rev. Chim. 2019, 70, 633–637. [Google Scholar] [CrossRef]
- Fathi, E.; Sefidkon, F. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Eucalyptus sargentii. J. Agric. Sci. Technol. 2012, 14, 1035–1042. [Google Scholar]
- Bogdan, M.; Bungau, S.; Tit, D.M.; Copolovici, L.; Behl, T.; Otrisal, P.; Aleya, L.; Cioca, G.; Berescu, D.; Uivarosan, D. Variations in the chemical composition of the essential oil of Lavandula angustifolia Mill., Moldoveanca 4 Romanian variety. Rev. Chim. 2020, 71, 307–315. [Google Scholar] [CrossRef]
- Gil, A.; De La Fuente, E.B.; Lenardis, A.E.; López Pereira, M.; Suárez, S.A.; Bandoni, A.; Van Baren, C.; Di Leo Lira, P.; Ghersa, C.M. Coriander essential oil composition from two genotypes grown in different environmental conditions. J. Agric. Food Chem. 2002, 50, 2870–2877. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Aly, M.S.A.; Alsuqub, A.N.A.; Khan, R.A. Drying Induced Impact on Composition and Oil Quality of Rosemary Herb, Rosmarinus Officinalis Linn. Molecules 2020, 25, 2830. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Sajid, M.S.M.; Aly, M.S.A.; Ahmad, A.M.; Abdellatif, A.A.H.; Eid, S.Y. Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules 2021, 26, 5757. [Google Scholar] [CrossRef]
- Dob, T.; Chelghoum, C. Chemical composition of the essential oil of Artemisia judaica L. from Algeria. Flavour Fragr. J. 2006, 21, 343–347. [Google Scholar] [CrossRef]
- El-Massry, K.F.; El-Ghorab, A.H.; Farouk, A. Antioxidant activity and volatile components of Egyptian Artemisia judaica L. Food Chem. 2002, 79, 331–336. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential oil and phenolic compounds of Artemisia herba-alba (Asso.): Composition, antioxidant, antiacetylcholinesterase, and antibacterial activities. Int. J. Food Prop. 2016, 19, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Taherkhani, M. Chemical composition, antimicrobial, antioxidant activity, tyrosinase inhibition and chelating ability of the leaf essential oil of Artemisia diffusa. J. Essent. Oil Bear. Plants 2016, 19, 1600–1613. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six Algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef] [Green Version]
- De Araújo Couto, H.G.S.; Blank, A.F.; de Oliveira e Silva, A.M.; de Lima Nogueira, P.C.; de Fátima Arrigoni-Blank, M.; de Castro Nizio, D.A.; de Oliveira Pinto, J.A. Essential oils of basil chemotypes: Major compounds, binary mixtures, and antioxidant activity. Food Chem. 2019, 293, 446–454. [Google Scholar] [CrossRef]
- Hellali, N.; Mahammed, M.H.; Ramdane, F.; Talli, A. Antimicrobial and antioxidant activities of Cymbopogon schoenanthus (L.) spreng. essential oil, growing in Illizi-Algeria. J. Med. Plants Res. 2016, 10, 188–194. [Google Scholar]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćavar, S.; Maksimović, M. Antioxidant activity of essential oil and aqueous extract of Pelargonium graveolens L’Her. Food Control 2012, 23, 263–267. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef]
- Benderradji, L.; Ghadbane, M.; Messaoudi, N.; El Okki, L.E. In Vitro Multiple Solution Extracts from Leaves of Artemisia judaica L. Var. Sahariensis (L. Chevall.) Collected from the Algerian Sahara and Its Antimicrobial Activities Against Pathogenic Microorganisms; Springer International Publishing: Cham, Switzerland, 2021; ISBN 9783030512095. [Google Scholar]
- Elazzouzi, H.; Khabbal, Y.; Bouachrine, M.; Zair, T.; Alaoui El Belghiti, M. Chemical composition and in vitro antibacterial activity of Artemisia ifranensis J. Didier essential oil Growing Wild in Middle Moroccan Atlas. J. Essent. Oil Res. 2018, 30, 142–151. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Alan, A.R.; Saxena, P.K. Evaluation of in vitro shoots of Artemisia judaica for allelopathic potential. Acta Physiol. Plant. 2009, 31, 1237–1248. [Google Scholar] [CrossRef]
- Kazemi, M.; Dakhili, M.; Dadkhah, A.; Fadaeian, M.; Shafizadeh, S. Composition, antimicrobial and antioxidant activities of artemisia deserti kracsh essential oil from Iran. Asian J. Chem. 2013, 25, 47–51. [Google Scholar] [CrossRef]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Aliabadi, A.; Valadaan, V.; Branch, K.; Kazerun, I. Comparison between the effect of silymarin and silver sulfadiazine on burned wound healing in rats. Bulg. J. Vet. Med. 2016, 19, 224–232. [Google Scholar] [CrossRef]
- Nasiri, E.; Hosseinimehr, S.J.; Akbari, J.; Azadbakht, M.; Azizi, S. The effects of Punica granatum flower extract on skin injuries induced by burn in rats. Adv. Pharmacol. Sci. 2017, 2017, 3059745. [Google Scholar]
- Ashrafi, M.; Baguneid, M.; Bayat, A. The role of neuromediators and innervation in cutaneous wound healing. Acta Derm. Venereol. 2016, 96, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadakis, M.A.; McPhee, S.J.; Rabow, M.C. Medical Diagnosis & Treatment; Mc Graw Hill: San Francisco, CA, USA, 2019. [Google Scholar]
- Phan, T.-T.; Wang, L.; See, P.; Grayer, R.J.; Chan, S.-Y.; Lee, S.T. Phenolic compounds of Chromolaena odorata protect cultured skin cells from oxidative damage: Implication for cutaneous wound healing. Biol. Pharm. Bull. 2001, 24, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.U.; Binti Aladdin, N.-A.; Jamal, J.A.; Shuid, A.N.; Mohamed, I.N. Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules 2021, 26, 228. [Google Scholar] [CrossRef]
- Pereira Beserra, F.; Sérgio Gushiken, L.F.; Vieira, A.J.; Augusto Bérgamo, D.; Luísa Bérgamo, P.; Oliveira de Souza, M.; Alberto Hussni, C.; Kiomi Takahira, R.; Henrique Nóbrega, R.; Monteiro Martinez, E.R. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Hussni, C.A.; Bastos, J.K.; Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Padovani, C.R.; Lemos, M.; Polizello Junior, M.; da Silva, J.J.M. Skin wound healing potential and mechanisms of the hydroalcoholic extract of leaves and oleoresin of Copaifera langsdorffii Desf. Kuntze in rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 6589270. [Google Scholar] [CrossRef] [Green Version]
- Bungau, S.; Vesa, C.M.; Abid, A.; Behl, T.; Tit, D.M.; Purza, A.L.; Pasca, B.; Todan, L.M.; Endres, L. Withaferin A—A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef]
- Kesarwani, A.; Nagpal, P.S.; Chhabra, H.S. Experimental animal modelling for pressure injury: A systematic review. J. Clin. Orthop. Trauma 2021, 17, 273–279. [Google Scholar] [CrossRef]
- Sanapalli, B.K.R.; Yele, V.; Singh, M.K.; Krishnamurthy, P.T.; Karri, V.V.S.R. Preclinical models of diabetic wound healing: A critical review. Biomed. Pharmacother. 2021, 142, 111946. [Google Scholar] [CrossRef]
- Hosseini, S.V.; Tanideh, N.; Kohanteb, J.; Ghodrati, Z.; Mehrabani, D.; Yarmohammadi, H. Comparison between Alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int. J. Surg. 2007, 5, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.V.; Niknahad, H.; Fakhar, N.; Rezaianzadeh, A.; Mehrabani, D. The healing effect of mixture of honey, putty, vitriol and olive oil in Pseudomonas aeroginosa infected burns in experimental rat model. Asian J. Anim. Vet. Adv. 2011, 6, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid.-Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltman, A.E. D-ribose-l-cysteine supplementation enhances wound healing in a rodent model. Am. J. Surg. 2015, 210, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, K.; Wang, I.; Stark, R.E. Potato wound-healing tissues: A rich source of natural antioxidant molecules with potential for food preservation. Food Chem. 2016, 210, 473–480. [Google Scholar] [CrossRef] [Green Version]
- El-Ferjani, R.M.; Ahmad, M.; Dhiyaaldeen, S.M.; Harun, F.W.; Ibrahim, M.Y.; Adam, H.; Yamin, B.M.; Al-Obaidi, M.M.J.; Al Batran, R. In vivo assessment of antioxidant and wound healing improvement of a new schiff base derived Co (ii) complex in rats. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.S.; Bloom, S.M.; Norian, L.A.; Geske, M.J.; Flavell, R.A.; Stappenbeck, T.S.; Allen, P.M. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Med. 2008, 5, e41. [Google Scholar] [CrossRef]
- Sellon, R.K.; Tonkonogy, S.; Schultz, M.; Dieleman, L.A.; Grenther, W.; Balish, E.D.; Rennick, D.M.; Sartor, R.B. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998, 66, 5224–5231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Elshibani, F.; Alshalmani, S.; Mohammed, H.A. Pituranthos tortuosus Essential Oil from Libya: Season Effect on the Composition and Antioxidant Activity. J. Essent. Oil Bear. Plants 2020, 23, 1095–1104. [Google Scholar] [CrossRef]
- Andjić, M.; Božin, B.; Draginić, N.; Kočović, A.; Jeremić, J.N.; Tomović, M.; Milojević Šamanović, A.; Kladar, N.; Čapo, I.; Jakovljević, V. Formulation and evaluation of helichrysum italicum essential oil-based topical formulations for wound healing in diabetic rats. Pharmaceuticals 2021, 14, 813. [Google Scholar] [CrossRef]
- Genčić, M.S.; Aksić, J.M.; Stošić, M.Z.Ž.; Randjelović, P.J.; Stojanović, N.M.; Stojanović-Radić, Z.Z.; Radulović, N.S. Linking the antimicrobial and anti-inflammatory effects of immortelle essential oil with its chemical composition–The interplay between the major and minor constituents. Food Chem. Toxicol. 2021, 158, 112666. [Google Scholar] [CrossRef]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1, 8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. (Stuttg.) 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Juergens, L.J.; Racké, K.; Tuleta, I.; Stoeber, M.; Juergens, U.R. Anti-inflammatory effects of 1, 8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma? Synergy 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Suzuki, S.; Fujisawa, S. Cytotoxicity and pro-/anti-inflammatory properties of cinnamates, acrylates and methacrylates against RAW264. 7 cells. In Vivo (Brooklyn) 2018, 32, 1309–1322. [Google Scholar]

| Locations | Major Constituents | Y% | Ref. |
|---|---|---|---|
| Saudi Arabia | cis-Thujone (2.5%), thymol (3.5%), trans-sabinyl acetate (3.3%), carvacrol (3.5%), b-eudesmol (13.1%), eudesma-4 (15), 7-dien-1-b-ol (3.5%), and hexadecanoic acid (5.7%) | 0.18% (v/w) | [24] |
| Algeria | Piperitone (66.17%), ethyl cinnamate isomer (6.11%), spathulenol (2.34%), E-longipinane (2.55%) | 1.7% (w/w) | [33] |
| Egypt | Piperitone (49.1%) and camphor (34.5%), borneol (3.90%) | [34] | |
| Sinai, Egypt | Camphor (31.4%), endo-borneol (5.72%), piperitone (29.9%) | 0.28% | [18] |
| Jordan | Artemisia ketone (9–24%), chrysanthenone (4–31%), piperitone (3–15%), camphor (0.3–16%), cinnamate (11.0%) | 0.4–0.9% (w/w) | [20,35] |
| Libya | cis-Chrysanthenol (9.1%), piperitone (30.2%), ethyl cinnamate (3.8%). | 0.62% (w/w) | [23] |
| RT | Chemical Compounds | Area Mean | RIcal | RIrep | m/z | Weight g/100 g of the Plant |
|---|---|---|---|---|---|---|
| 12.096 | (Z)-3-Hexenol | 0.50 ± 0.08 | 845 | 845 | 0.0085 | |
| 12.209 | 2-Methyl-ethylbutanoate | 0.4 ± 0.06 | 850 | 853 | 0.0068 | |
| 16.403 | Sabinene | 0.13 ± 0.11 | 953 | 954 | 59.04 (100%), 81.05 (96.37%), 96.07 (83.12%) | 0.0022 |
| 18.449 | α-Phellandrene | 1.30 ± 0.16 | 1000 | 999 | 68.04 (100%), 79.03 (42.69%), 93.04 (93.23%) | 0.0222 |
| 19.795 | Limonene | 0.72 ± 0.01 | 1029 | 1028 | 85.04 (100%), 55.03 (12.29%), 70.07 (7.28%) | 0.0123 |
| 20.239 | 1,8-Cineole | 2.56 ± 0.05 | 1038 | 1040 | 69.04 (100%), 110.08 (70.37%), 95.06 (46.98%) | 0.0437 |
| 21.359 | γ-Terpinene | 3.58 ± 0.18 | 1062 | 1063 | 135.05 (100%), 91.03 (20.36%), 107.03 (11.26%) | 0.0612 |
| 23.548 | Linalool | 1.21 ± 0.04 | 1108 | 1104 | 91.04 (100%), 92.04 (98.97%), 55.04 (47.04%) | 0.0207 |
| 23.739 | α-Thujone | 5.94 ± 0.09 | 1112 | 1112 | 95.06 (100%), 81.04 (68.49%), 109.04 (35.48%) | 0.1016 |
| 24.24 | β-Thujone | 3.61 ± 0.03 | 1123 | 1124 | 84.0 (100%), 55.02 (80.71%), 126.05 (47.17%) | 0.0617 |
| 24.49 | α-Campholenal | 0.91 ± 0.02 | 1128 | 82.04 (100%), 110.06 (91.66%), 95.04 (43.11%) | 0.0156 | |
| 24.639 | Terpinene-4-ol | 6.42 ± 0.17 | 1132 | 1140 | 70.04 (100%), 83.03 (71.89%), 71.03 (29.17) | 0.1098 |
| 25.443 | Isothujol | 0.27 ± 0.47 | 1149 | 1145 | 0.0046 | |
| 25.651 | Camphor | 1.92 ± 0.09 | 1154 | 1155 | 68.01 (100%), 81.02 (26.97%), 55.04 (18.33%) | 0.0328 |
| 26.454 | Borneol | 0.47 ± 0.01 | 1170 | 1170 | 95.04 (100%), 110.04 (48.36%), 54.06 (25.12%) | 0.0080 |
| 27.156 | p-Cymene-8-ol | 0.08 ± 0.14 | 1186 | 1185 | 135.06 (100%), 150.08 (40.42), 91.03 (32.05) | 0.0014 |
| 29.403 | Neral | 0.36 ± 0.00 | 1235 | 1236 | 69.02 (100%), 68.01 (17.21%), 83.01 (11.68%) | 0.0062 |
| 30.034 | Linalyl acetate | 0.10 ± 0.17 | 1249 | 1250 | 107.06 (100%), 95.04 (34.18), 55.03 (14.79%) | 0.0017 |
| 30.845 | Piperitone | 31.99 ± 0.50 | 1268 | 1260 | 82.04 (100%), 110.02 (37.44%), 95.06 (19.20%) | 0.5470 |
| 31.485 | Phellandral | 0.38 ± 0.02 | 1281 | 0.0065 | ||
| 31.965 | p-Cymen-7-ol | 0.08 ± 0.13 | 1292 | 1290 | 0.0014 | |
| 32.146 | Thymol | 1.81 ± 0.03 | 1296 | 1297 | 135.06 (100%), 107.03 (11.26), 77.01 (10.75%) | 0.0309 |
| 33.406 | Carvacrol | 0.10 ± 0.12 | 1325 | 1324 | 0.0017 | |
| 33.643 | Citronellyl acetate | 0.89 ± 0.02 | 1330 | 1334 | 107.06 (100%), 91.04 (39.11), 122.08 (15.51) | 0.0152 |
| 34.649 | (E)-Methyl cinnamate | 0.35 ± 0.02 | 1354 | 1355 | 131.02 (100%), 103.03 (61.07), 162.04 (49.31%) | 0.0060 |
| 35.894 | Cis-Ethyl cinnamate | 4.02 ± 0.06 | 1383 | 1376 | 131.04 (100%), 103.04 (48.29%), 77.03 (30.40%) | 0.0687 |
| 36.303 | Jasmone | 0.71 ± 0.02 | 1392 | 1396 | 91.03 (100%), 95.03 (66.88%), 79.03 (60.65%) | 0.0121 |
| 36.658 | β-Bourbounene | 4.06 ± 0.17 | 1401 | 1401 | 111.02 (100%), 137.07 (41.99%), 180.09 (21.39%) | 0.0694 |
| 37.754 | β-Caryophyllene | 0.43 ± 0.08 | 1427 | 1429 | 161.12 (100%), 105.04 (57.31%), 93.05 (27.09%) | 0.0075 |
| 39.75 | Trans-Ethyl cinnamate | 13.67 ± 0.55 | 1477 | 1455 | 131.04 (100%), 103.04 (48.29%), 77.03 (30.40%) | 0.2337 |
| 40.542 | Valencene | 3.24 ± 0.09 | 1497 | 1497 | 161.12 (100%), 105.04 (57.31%), 91.04 (53.35%) | 0.0554 |
| 41.138 | γ-Cadinene | 0.79 ± 0.14 | 1511 | 1513 | 161.11 (100%), 133.07 (30.58%), 120.07 (27.73%) | 0.0135 |
| 41.968 | σ-Cadinene | 2.37 ± 0.09 | 1532 | 1526 | 91.04 (100%), 205.11 (86.11%), 77.02 (46.25%) | 0.04052476 |
| 44.321 | Spathulenol | 3.33 ± 0.07 | 1593 | 1575 | 91.04 (100%), 93.05 (73.39), 77.02 (46.25%) | 0.0569 |
| 47.198 | β-Eudesmol | 0.22 ± 0.19 | 1671 | 1672 | 59.04 (100%), 149.11 (67.04%), 146.14 (33.10%) | 0.0038 |
| 48.344 | a-Caryophylene acetate | 1.11 ± 0.31 | 1702 | 1696 | 67.04 (100%), 95.06 (62.38%), 96.07 (41.92%) | 0.0190 |
| Total | 100 | 1.71 | ||||
| Monoterpene hydrocarbons | 5.74 | |||||
| Oxygenated monoterpenes | 57.20 | |||||
| Sesquiterpene hydrocarbons | 10.88 | |||||
| Oxygenated sesquiterpenes | 4.66 | |||||
| Phenolics | 1.87 | |||||
| Cinnamic acid derivatives | 18.03 | |||||
| Microorganisms | Zone of Inhibition (mm) | |
|---|---|---|
| ArJ Essential Oil | Control Drugs | |
| S. aureus ATCC 29213 | 7.7 ± 0.20 | 14.2 ± 0.20 |
| S. saprophyticus ATCC 43867 | 8.8 ± 0.20 | 12.8 ± 0.20 |
| S. pyogenes (A) ATCC 27736 | 7.4 ± 0.30 | 11.7 ± 0.10 |
| S. pneumoniae ATCC 49619 | 7.2 ± 0.17 | 11.7 ± 0.20 |
| E. faecalis ATCC 29212 | 8.7 ± 0.17 | 11.9 ± 0.10 |
| B. cereus ATCC 10876 | 12.9 ± 0.10 | 19.6 ± 0.35 |
| E. coli ATCC 25922 | 6.4 ± 0.10 | 23.1 ± 0.20 |
| K. pneumoniae ATCC 27736 | 6.2 ± 0.10 | 21.1 ± 0.10 |
| S. typhimurium ATCC 13311 | 10.0 ± 0.20 | 16.3 ± 0.30 |
| S. flexneri ATCC 12022 | 6.2 ± 0.10 | 17.9 ± 0.17 |
| P. vulgaris ATCC 6380 | 8.1 ± 0.17 | 16.2 ± 0.35 |
| P. mirabilis ATCC 29906 | 7.7 ± 0.20 | 18.7 ± 0.20 |
| C. albicans ATCC 10231 | 25.2 ± 0.20 | 25.0 ± 0.20 |
| A. niger ATCC 6275 | 15.0 ± 0.20 | 13.1 ± 0.35 |
| Microorganisms | MIC | MBC | MBIC | MBEC |
|---|---|---|---|---|
| S. aureus ATCC 29213 | 50 | 100 | 50 | 100 |
| S. saprophyticus ATCC 43867 | 50 | 100 | 50 | 100 |
| S. pyogenes (A) ATCC 27736 | 100 | >100 | 100 | 200 |
| S. pneumoniae ATCC 49619 | 100 | >100 | 100 | 200 |
| E. faecalis ATCC 29212 | 100 | >100 | 100 | 200 |
| B. cereus ATCC 10876 | 6.25 | 12.5 | 6.25 | 12.5 |
| E. coli ATCC 25922 | 50 | 100 | 50 | 100 |
| K. pneumoniae ATCC 27736 | 25 | 50 | 25 | 50 |
| S. typhimurium ATCC 13311 | 12.5 | 25 | 12.5 | 25 |
| S. flexneri ATCC 12022 | 12.5 | 25 | 12.5 | 25 |
| P. vulgaris ATCC 6380 | 25 | 50 | 25 | 50 |
| P. mirabilis ATCC 29906 | 100 | >100 | 100 | 200 |
| C. albicans ATCC 10231 | 6.25 | 12.5 | NT | NT |
| A. niger ATCC 6275 | 3.125 | 6.25 | NT | NT |
| Groups | CAT | SOD | LP |
|---|---|---|---|
| ng/g | |||
| I. Intact control | 1.35 ± 0.05 A,B | 0.04 ± 0.00 A | 836.9 ± 37.75 A |
| II. Negative control (skin burn without treatment) | 1.11 ± 0.06 A | 0.04 ± 0.01 A | 1214 ± 51.46 B |
| III. Silver sulfadiazine | 1.79 ± 0.204 B | 0.19 ± 0.06 A,B | 1197 ± 30.30 B |
| IV. Artemisia judaica | 1.82 ± 0.17 B | 0.37 ± 0.13 B | 1291 ± 18.85 B |
| Groups | IL-1b | IL-6 | TNF-α | TGF-b1 | IL-10 |
|---|---|---|---|---|---|
| ng/g | |||||
| I. Intact control | 20.77 ±1.95 A | 806.1 ±10.20 A | 9.57 ±0.55 A | 4.19 ±0.24 A | 3.54 ±0.19 A |
| II. Negative control (skin burn without treatment) | 19.37 ± 2.33 A | 776.2 ±32.77 A | 15.54 ± 0.92 B | 3.87 ± 0.09 A | 2.99 ± 0.25 A |
| III. Sulfadiazine | 23.55± 0.88 A | 789.4 ±18.02 A | 12.85± 0.26 C | 19.28± 0.30 B | 12.68± 0.15 B |
| IV. Artemisia judaica | 19.55 ± 1.34 A | 869.2 ±51.91 A | 11.96 ± 0.34 C | 19.18 ± 0.33 B | 13.39 ± 0.35 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.A.; Qureshi, K.A.; Ali, H.M.; Al-Omar, M.S.; Khan, O.; Mohammed, S.A.A. Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants 2022, 11, 332. https://doi.org/10.3390/antiox11020332
Mohammed HA, Qureshi KA, Ali HM, Al-Omar MS, Khan O, Mohammed SAA. Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants. 2022; 11(2):332. https://doi.org/10.3390/antiox11020332
Chicago/Turabian StyleMohammed, Hamdoon A., Kamal A. Qureshi, Hussein M. Ali, Mohsen S. Al-Omar, Omar Khan, and Salman A. A. Mohammed. 2022. "Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils" Antioxidants 11, no. 2: 332. https://doi.org/10.3390/antiox11020332
APA StyleMohammed, H. A., Qureshi, K. A., Ali, H. M., Al-Omar, M. S., Khan, O., & Mohammed, S. A. A. (2022). Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants, 11(2), 332. https://doi.org/10.3390/antiox11020332








