Antioxidant Activity of Natural Hydroquinones
Abstract
:1. Introduction
2. Free Radical Scavenging and Related Antioxidant Chemical Analysis
2.1. Simple Hydroquinones
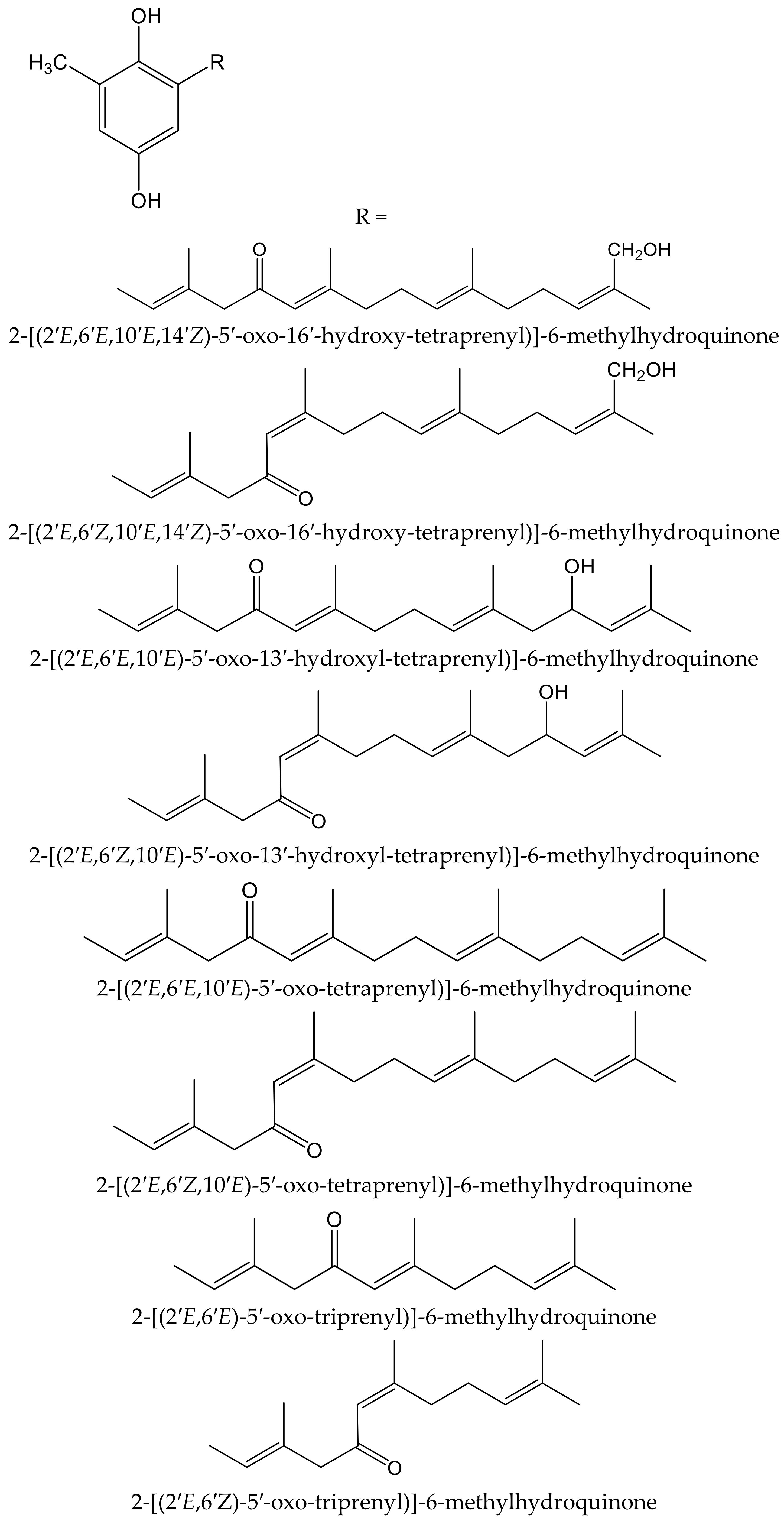
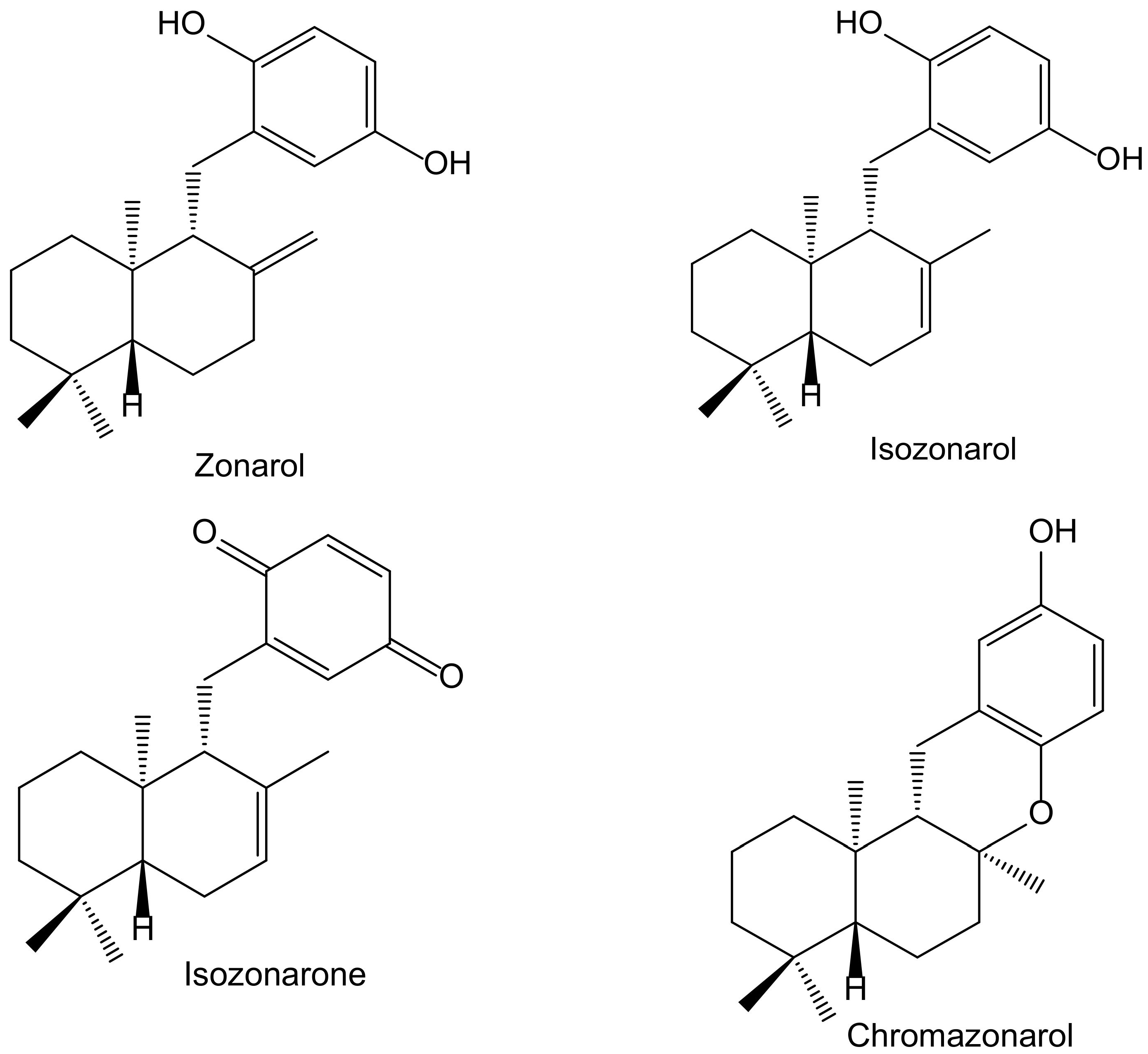
2.2. Terpenoid Hydroquinones
2.3. Other Alkyl-Hydroquinones
3. Interactions with Enzymes, Transcription Factors, and Other Proteins
4. Structure/Activity Relationship
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ABAP | 2,2′-azobis(2-amidinopropane) |
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) |
| BHT | butylated hydroxytoluene |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC50 | half maximal effective concentration |
| EDTA | ethylenediaminetetraacetic acid |
| FRAP | ferric reducing antioxidant power |
| HO-1 | heme oxygenase-1 |
| IC50 | half maximal inhibitory concentration |
| IL | interleukin |
| 15-LOX | 15-lipoxygenase |
| LXRα | liver X receptor α |
| NQO1 | NADPH quinone oxidoreductase 1 |
| Nrf2/ARE | Nuclear factor-erythroid 2-related factor 2/antioxidant-responsive element |
| O2•− | superoxide anion |
| OCl− | hypochlorite anion |
| ONOO− | peroxynitrite anion |
| PHB | p-hydroxybenzoate |
| ROS | reactive oxygen species |
| SC50 | scavenger concentration 50% |
| TBARS | thiobarbituric acid reacting species |
| TEAC | Trolox equivalent antioxidant capacity |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
References
- Shang, Y.; Wei, W.; Zhang, P.; Ye, B.C. Engineering Yarrowia lipolytica for enhanced production of arbutin. J. Agric. Food Chem. 2020, 68, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.E. Biogenetic relationships of bioactive sponge merotriterpenoids. Mar. Drugs 2017, 15, 285. [Google Scholar] [CrossRef] [Green Version]
- Lenaz, G. The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. IUBMB Life 2001, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L.; Hatefi, Y.; Lester, R.L.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 1957, 25, 220–221. [Google Scholar] [CrossRef]
- Ohsugi, M.; Fan, W.; Hase, K.; Xiong, Q.; Tezuka, Y.; Komatsu, K.; Namba, T.; Saitoh, T.; Tazawa, K.; Kadot, S. Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra. J. Ethnopharmacol. 1999, 67, 111–119. [Google Scholar] [CrossRef]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, İ. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.M.; Ji, X.C.; Luo, Q.W.; Zhang, Q.; Luo, B.; Liu, W.B.; Zhang, X.; Xu, Q.L.; Tan, J.W. Phenolics from Mikania micrantha and their antioxidant activity. Molecules 2017, 22, 1140. [Google Scholar] [CrossRef]
- Chantarudee, A.; Phuwapraisirisan, P.; Kimura, K.; Okuyama, M.; Mori, H.; Kimura, A.; Chanchao, C. Chemical constituents and free radical scavenging activity of corn pollen collected from Apis mellifera hives compared to floral corn pollen at Nan, Thailand. BMC Complement. Altern. Med. 2012, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Zhokhov, S.S.; Jastrebova, J.A.; Kenne, L.; Broberg, A. Antioxidant hydroquinones substituted by beta-1,6-linked oligosaccharides in wheat germ. J. Nat. Prod. 2009, 72, 656–661. [Google Scholar] [CrossRef]
- Adisa, R.A.; Olorunsogo, O.O. Robustaside B and para hydroxyphenol: Phenolic and antioxidant compounds purified from Cnestis ferruginea DC. induced membrane permeability transition in rat liver mitochondria. Mol. Med. Rep. 2013, 8, 1493–1498. [Google Scholar] [CrossRef]
- Karioti, A.; Milošević-Ifantis, T.; Pachopos, N.; Niryiannaki, N.; Hadjipavlou-Litina, D.; Skaltsa, H. Antioxidant, anti-inflammatory potential and chemical constituents of Origanum dubium Boiss., growing wild in Cyprus. J. Enzyme Inhib. Med. Chem. 2015, 30, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, L.F.; Lago, J.H.G.; Tanizaki, T.M.; Mascio, P.D.; Kato, M.J. Antioxidant activity of prenylated hydroquinone and benzoic acid derivatives from Piper crassinervium Kunth. Phytochemistry 2006, 67, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.M.; Böhm, V.; Wright, A.D.; König, G.M. Antioxidative meroterpenoids from the brown alga Cystoseira crinita. J. Nat. Prod. 2003, 66, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kamagai, M.; Nishikawa, K.; Matsuura, H.; Umezawa, T.; Matsuda, F.; Okino, T. Antioxidants from the brown alga Dictyopteris undulata. Molecules 2018, 23, 1214. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, I.; Ohkubo, K.; Ogawa, Y.; Matsumoto, K.I.; Ozawa, T.; Fukuzumi, S. Aluminium ion-promoted radical-scavenging reaction of methylated hydroquinone derivatives. Org. Biomol. Chem. 2016, 14, 7956–7961. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Wätjen, W.; Putzb, A.; Chovoloua, Y.; Kampköttera, A.; Totzkec, F.; Kubbutatc, M.H.G.; Prokschb, P.; Konuklugild, B. Hexa-, hepta- and nonaprenylhydroquinones isolated from marine sponges Sarcotragus muscarum and Ircinia fasciculata inhibit NF-κB signalling in H4IIE cells. J. Pharm. Pharmacol. 2009, 61, 919–924. [Google Scholar] [CrossRef]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef] [Green Version]
- Iwashima, M.; Mori, J.; Ting, X.; Matsunaga, T.; Hayashi, K.; Shinoda, D.; Saito, H.; Sankawa, U.; Hayashi, T. Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones. Biol. Pharm. Bull. 2005, 28, 374–377. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Li, L.; Wang, X.; Zhu, G.; Li, Z.; Qiu, M. Antioxidant farnesylated hydroquinones from Ganoderma capense. Fitoterapia 2016, 111, 18–23. [Google Scholar] [CrossRef]
- Ammar, S.; Mahjoub, M.A.; Charfi, N.; Skandarani, I.; Chekir-Ghedira, L.; Mighri, Z. Mutagenic, antimutagenic and antioxidant activities of a new class of β-glucoside hydroxyhydroquinone from Anagallis monelli growing in Tunisia. Chem. Pharm. Bull. 2007, 55, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.L.; Lin, S.B.; Huang, C.P.; Chiou, R.Y.Y. Antioxidative and cytotoxic compounds extracted from the sap of Rhus succedanea. J. Nat. Prod. 2002, 65, 1719–1721. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Issa, R.A.; Afifi, F.U.; Amor, B.I. Studying the anti-tyrosinase effect of Arbutus andrachne L. extracts. Int. J. Cosmet. Sci. 2008, 30, 271–276. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Jang, H.S.; Jeong, B.; Choi, S.Y.; Kim, J.; Kwon, Y.S.; Yang, H. α-Glucosidic hydroquinone derivatives from Viburnum erosum. Phytochemistry 2021, 187, 112782. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, Z.; Li, K.; Yang, B.; Liu, Y.; Fang, W.; Tang, L.; Zhou, X. Ene-yne hydroquinones from a marine-derived strain of the fungus Pestalotiopsis neglecta with effects on liver X receptor alpha. J. Nat. Prod. 2020, 83, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, M.K.; Luo, J.F.; Tu, Z.C.; Cheng, Y.X. COX-2 and JAK3 inhibitory meroterpenoids from the mushroom Ganoderma theaecolum. Tetrahedron 2018, 74, 4259–4265. [Google Scholar] [CrossRef]
- Yan, Y.M.; Ai, J.; Zhou, L.L.; Chung, A.C.K.; Li, R.; Nie, J.; Fang, P.; Wang, X.L.; Luo, J.; Hu, Q.; et al. Lingzhiols, unprecedented rotary door-shaped meroterpenoids as potent and selective inhibitors of p-Smad3 from Ganoderma lucidum. Org. Lett. 2013, 15, 5488–5491. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Tozawa, T.; Sugamoto, K.; Matsushita, Y.; Satoh, T. A novel diterpene para-hydroquinone compound derived from cryptoquinone protects neuronal cells against oxidative stress and activates the Nrf2/ARE pathway. Neurosci. Lett. 2013, 548, 132–136. [Google Scholar] [CrossRef]
- Kofujita, H.; Ota, M.; Takahashi, K.; Kawai, Y.; Hayashi, Y. A diterpene quinone from the bark of Cryptomeria japonica. Phytochemistry 2002, 61, 895–898. [Google Scholar] [CrossRef]
- Sasaki, S.; Tozawa, T.; van Wagoner, R.M.; Ireland, C.M.; Harper, M.K.; Satoh, T. Strongylophorine-8, a pro-electrophilic compound from the marine sponge Petrosia (Strongylophora) corticata, provides neuroprotection through Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2011, 415, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Tsurukawa, Y.; Kamisuki, S.; Shibasaki, H.; Iguchi, K.; Murakami, H.; Uchiyama, J.; Kuramochi, K.J. Novel neuroprotective hydroquinones with a vinyl alkyne from the fungus, Pestalotiopsis microspora. J. Antibiot. 2019, 72, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Shindo, T.; Kasanuki, N.; Hasegawa, K. Antioxidant metabolites from the tunicate Amaroucium multiplicatum. J. Nat. Prod. 1989, 52, 975–981. [Google Scholar] [CrossRef]
- Olmos, A.; Máñez, S.; Giner, R.M.; Recio, M.C.; Ríos, J.L. Isoprenylhydroquinone glucoside: A new non-antioxidant inhibitor of peroxynitrite-mediated tyrosine nitration. Nitric Oxide 2005, 12, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Giner, R.M.; Recio, M.C.; Máñez, S. Phenylpropanoid and phenylisoprenoid metabolites from Asteraceae species as inhibitors of protein carbonylation. Phytochemistry 2011, 72, 1821–1825. [Google Scholar] [CrossRef]
- Chen, X.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. J. Am. Oil Chem. Soc. 1998, 75, 1717–1721. [Google Scholar] [CrossRef]




















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giner, R.M.; Ríos, J.L.; Máñez, S. Antioxidant Activity of Natural Hydroquinones. Antioxidants 2022, 11, 343. https://doi.org/10.3390/antiox11020343
Giner RM, Ríos JL, Máñez S. Antioxidant Activity of Natural Hydroquinones. Antioxidants. 2022; 11(2):343. https://doi.org/10.3390/antiox11020343
Chicago/Turabian StyleGiner, Rosa M., José Luis Ríos, and Salvador Máñez. 2022. "Antioxidant Activity of Natural Hydroquinones" Antioxidants 11, no. 2: 343. https://doi.org/10.3390/antiox11020343




