Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects
Abstract
:1. Introduction
2. State of the Art
2.1. Autonomic Nervous System Network
2.2. The Autonomic Nervous System and Hypothalamic–Pituitary–Adrenal Axis
2.3. ANS and the Immune System
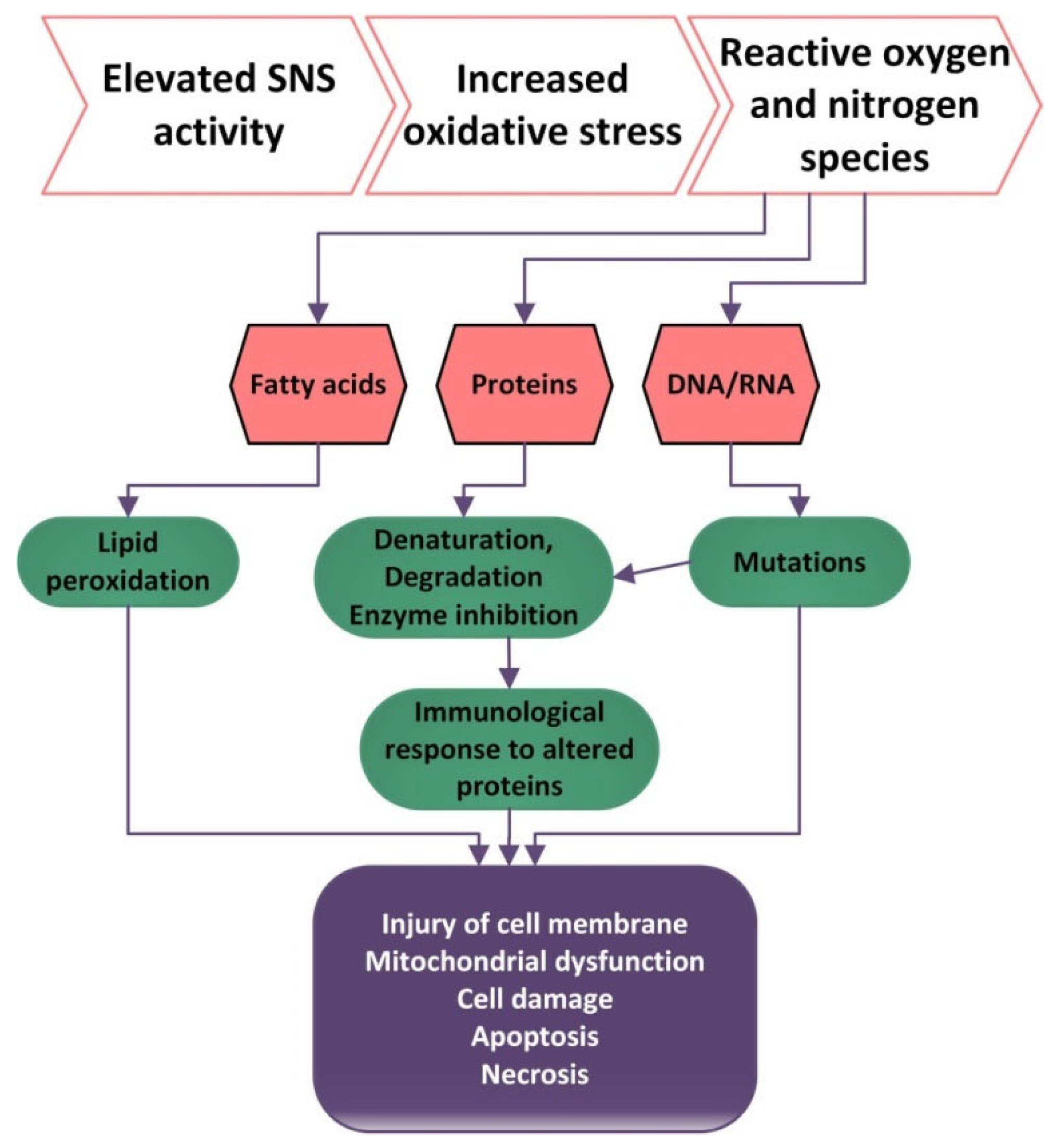
2.4. ANS and Oxidative Stress
2.5. Physical Exercise
2.6. Physical Exercise and Oxidative Stress
2.7. Physical Exercise and Anti-Inflammatory Effects
3. Concluding Remarks
Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benarroch, E.E. Central Autonomic Network; Oxford University Press: Oxford, UK, 2014; Volume 86, pp. 3–14. [Google Scholar]
- Kanji, M.; Shirai, M.; Murata, J.; Tsuchimochi, H.; Komine, H.; Ninomiya, I.; Shimizu, K. Sympathetic Cholinergic Vasodilation of Skeletal Muscle Small Arteries. Jpn. J. Pharmacol. 2002, 88, 14–18. [Google Scholar]
- Woolf, N.J.; Butcher, L.L. Cholinergic systems mediate action from movement to higher consciousness. Behav. Brain Res. 2011, 221, 488–498. [Google Scholar] [CrossRef]
- Séguéla, P.; Wadiche, J.; Dineley-Miller, K.; Dani, J.A.; Patrick, J.W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. 1993, 13, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Dampney, R.A.; Horiuchi, J. Functional organisation of central cardiovascular pathways: Studies using c-fos gene expression. Prog. Neurobiol. 2003, 71, 359–384. [Google Scholar] [CrossRef]
- Feldman, J.L.; Mitchell, G.S.; Nattie, E.E. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 2003, 26, 239–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travagli, R.A.; Hermann, G.E.; Browning, K.N.; Rogers, R.C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006, 68, 279–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, J.L. Free will versus survival: Brain systems that underlie intrinsic constraints on behavior. J. Comp. Neurol. 2005, 493, 132–139. [Google Scholar] [CrossRef]
- Morrison, S.F. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am. J. Physiol. 1999, 276 Pt 2, R962–R973. [Google Scholar] [CrossRef]
- Dampney, R.A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F.; Nakamura, K.; Madden, C.J. Central control of thermogenesis in mammals. Exp. Physiol. 2008, 93, 773–797. [Google Scholar] [CrossRef] [PubMed]
- Bandler, R.; Keay, K.A.; Floyd, N.; Price, J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res. Bull. 2000, 53, 95–104. [Google Scholar] [CrossRef]
- Corcoran, A.E.; Hodges, M.R.; Wu, Y.; Wang, W.; Wylie, C.J.; Deneris, E.S.; Richerson, G.B. Medullary serotonin neurons and central CO2 chemoreception. Respir. Physiol. Neurobiol. 2009, 168, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Price, C.J.; Hoyda, T.D.; Ferguson, A.V. The area postrema: A brain monitor and integrator of systemic autonomic state. Neuroscientist 2008, 14, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Periaqueductal gray: An interface for behavioral control. Neurology 2012, 78, 210–217. [Google Scholar] [CrossRef]
- Saper, C.B. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002, 25, 433–469. [Google Scholar] [CrossRef] [PubMed]
- Holstege, G. Micturition and the soul. J. Comp. Neurol. 2005, 493, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.L.; Valentino, R.J. Corticotropin-releasing factor neurotransmission in locus coeruleus: A possible site of antidepressant action. Brain Res. Bull. 1994, 35, 581–587. [Google Scholar] [CrossRef]
- Young, E.A.; Abelson, J.L.; Cameron, O.G. Interaction of brain noradrenergic system and the hypothalamic-pituitary-adrenal (HPA) axis in man. Psychoneuroendocrinology 2005, 30, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Ennis, M.; Aston-Jones, G. Activation of locus coeruleus from nucleus paragigantocellularis: A new excitatory amino acid pathway in brain. J. Neurosci. 1988, 8, 3644–3657. [Google Scholar] [CrossRef]
- Siever, L.J.; Uhde, T.W.; Insel, T.R.; Kaye, W.H.; Jimerson, D.C.; Lake, C.R.; Kafka, M.; Targum, S.; Murphy, D.L. Biological alterations in the primary affective disorders and other tricyclic-responsive disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatry 1985, 9, 15–24. [Google Scholar] [CrossRef]
- Chan-Palay, V. Alterations in the locus coeruleus in dementias of Alzheimer’s and Parkinson’s disease. Prog. Brain Res. 1991, 88, 625–630. [Google Scholar] [PubMed]
- Phelix, C.F.; Liposits, Z.; Paull, W.K. Catecholamine-CRF synaptic interaction in a septal bed nucleus: Afferents of neurons in the bed nucleus of the stria terminalis. Brain Res. Bull. 1994, 33, 109–119. [Google Scholar] [CrossRef]
- Cecchi, M.; Khoshbouei, H.; Javors, M.; Morilak, D.A. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience 2002, 112, 13–21. [Google Scholar] [CrossRef]
- Le Doux, J. The amygdala. Curr. Biol. 2007, 17, R868–R874. [Google Scholar] [CrossRef] [Green Version]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Harrison, N.A. Visceral influences on brain and behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef] [Green Version]
- Cechetto, D.F.; Saper, C.B. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J. Comp. Neurol. 1987, 262, 27–45. [Google Scholar] [CrossRef]
- Critchley, H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005, 493, 154–166. [Google Scholar] [CrossRef] [PubMed]
- De Vignemont, F.; Singer, T. The empathic brain: How, when and why? Trends Cogn. Sci. 2006, 10, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Jabbi, M.; Swart, M.; Keysers, C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage 2007, 34, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn. Sci. 2005, 9, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Beissner, F.; Meissner, K.; Bär, K.J.; Napadow, V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013, 33, 10503–10511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, B.A.; Vogt, L.; Farber, N.B.; Bush, G. Architecture and neurocytology of monkey cingulate gyrus. J. Comp. Neurol. 2005, 485, 218–239. [Google Scholar] [CrossRef] [Green Version]
- Harrison, N.A.; Brydon, L.; Walker, C.; Gray, M.A.; Steptoe, A.; Critchley, H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 2009, 66, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Radley, J.J.; Arias, C.M.; Sawchenko, P.E. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci. 2006, 26, 12967–12976. [Google Scholar] [CrossRef]
- Hoover, W.B.; Vertes, R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007, 212, 149–179. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J.; Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef]
- De Pascalis, V.; Ray, W.J.; Tranquillo, I.; D’Amico, D. EEG activity and heart rate during recall of emotional events in hypnosis: Relationships with hypnotizability and suggestibility. Int. J. Psychophysiol. 1998, 29, 255–275. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress hormones: Good and bad. Neurobiol. Dis. 2000, 7, 540–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasker, J.G.; Herman, J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011, 14, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riebe, C.J.; Wotjak, C.T. Endocannabinoids and stress. Stress 2011, 14, 384–397. [Google Scholar] [CrossRef]
- Culić, V. Acute risk factors for myocardial infarction. Int. J. Cardiol. 2007, 117, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Dimsdale, J.E. A new mechanism linking stress to coronary pathophysiology? Circulation 1991, 84, 2201–2202. [Google Scholar] [CrossRef] [Green Version]
- Vogt, C.J.; Schmid-Schönbein, G.W. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation 2001, 8, 129–139. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Walker, B.R. Human insulin resistance: The role of glucocorticoids. Diabetes Obes. Metab. 2003, 5, 5–12. [Google Scholar] [CrossRef]
- Dias-Ferreira, E.; Sousa, J.C.; Melo, I.; Morgado, P.; Mesquita, A.R.; Cerqueira, J.J.; Costa, R.M.; Sousa, N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 2009, 325, 621–625. [Google Scholar] [CrossRef]
- Heponiemi, T.; Keltikangas-Järvinen, L.; Kettunen, J.; Puttonen, S.; Ravaja, N. BIS-BAS sensitivity and cardiac autonomic stress profiles. Psychophysiology 2004, 41, 37–45. [Google Scholar] [CrossRef]
- Hayashi, T. Conversion of psychological stress into cellular stress response: Roles of the sigma-1 receptor in the process. Psychiatry Clin. Neurosci. 2014, 69, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Ochani, M.; Parrish, W.R.; Ochani, K.; Harris, Y.T.; Huston, J.M.; Chavan, S.; Tracey, K.J. Splenic nerve is required for cholinergic anti-inflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 2008, 105, 11008–11013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woody, A.; Figueroa, W.S.; Benencia, F.; Zoccola, P.M. Stress-induced parasympathetic control and its association with inflammatory reactivity. Psychosomat. Med. 2017, 79, 306–310. [Google Scholar] [CrossRef]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-kB, ERK and JNK signaling pathways. Sci. Rep. 2017, 7, 41411. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.M.; Ali, S.S.; Dugan, L.L. Interleukin-6 mediates the increase in NADPH-Oxidase in the ketamine model of schizophrenia. J. Neurosci. 2008, 28, 13957–13966. [Google Scholar] [CrossRef] [PubMed]
- Streltsova, L.I.; Tkacheva, Î.V.; Plokhova, E.V.; Akasheva, D.U.; Strazhesko, I.D.; Dudinskaya, E. Age-related changes in heart rate variability and their relation with leucocyte telomere length. Cardiovasc. Ther. Prevent. 2017, 16, 54–60. [Google Scholar] [CrossRef]
- Weber, C.S.; Thayer, J.F.; Rudat, M.; Wirtz, P.H.; Zimmermann-Viehoff, F.; Thomas, A.; Perschel, F.H.; Arck, P.C.; Deter, H.C. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine and immune markers. Eur. J. Appl. Physiol. 2010, 109, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, R.H.; Herfarth, H.; Falk, W.; Andus, T.; Scholmerich, J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J. Neuroimmunol. 2002, 126, 116–125. [Google Scholar] [CrossRef]
- Maldonado-Ruiz, R.; Fuentes-Mera, L.; Camacho, A. Central modulation of neuroinflammation by neuropeptides and energy-sensing hormones during obesity. BioMed Res. Int. 2017, 2017, 7949582. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Chobanyan-Jürgens, K.; Jordan, J. Autonomic nervous system activity and inflammation: Good ideas, good treatments, or both? Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1999–H2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, N.; Banks, W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007, 21, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Understanding immunity requires more than immunology. Nat. Immunol. 2010, 11, 561–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. The biological properties of interleukin-1. Eur. Cytokine Netw. 1994, 5, 517–531. [Google Scholar]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Pennica, D.; Nedwin, G.E.; Hayflick, J.S. Human tumor necrosis factor: Precursor structure, expression, and homology to lymphotoxin. Nature 1984, 312, 724–729. [Google Scholar] [CrossRef]
- Billiau, A. Interferon gamma: Biology and role in pathogenesis. Adv. Immunol. 1996, 62, 61–130. [Google Scholar]
- Mantovani, A.; Dejana, E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol. Today 1989, 10, 370–375. [Google Scholar] [CrossRef]
- Baggiolini, M.; Dewald, B.; Moser, B. Human chemokines: An update. Annu. Rev. Immunol. 1997, 15, 675–705. [Google Scholar] [CrossRef]
- Greenberg, M.J.; Streiter, R.M.; Kunkel, S.L.; Danforth, J.M.; Laichalk, L.L.; McGillicuddy, D.C.; Standiford, T.J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 1996, 173, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Kluth, D.C.; Rees, A.J. Inhibiting inflammatory cytokines. Semin. Nephrol. 1996, 16, 576–582. [Google Scholar] [PubMed]
- Te Velde, A.; Huijbens, R.J.F.; de Vries, J.E. IL-4 increases FcRc membrane expression and FcRc-mediated cytotoxic activity of human monocytes. J. Immunol. 1990, 144, 3046–3051. [Google Scholar] [PubMed]
- Joyce, D.A.; Gibbons, D.P.; Green, P. Two inhibitors of inflammatory cytokine release, interleukin 10 and interleukin 4, have contrasting effects on the release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur. J. Immunol. 1994, 24, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.L.; Millar, B.A.; Perez, S.; Carter, J.; Wood, C.; ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sympathetic modulation of immunity: Relevance to disease. Cell. Immunol. 2008, 252, 27–56. [Google Scholar] [CrossRef] [Green Version]
- Sanders, V.M.; Munson, A.E. Norepinephrine and the antibody response. Pharmacol. Rev. 1985, 37, 229–248. [Google Scholar] [PubMed]
- Calcagni, E.; Elenkov, I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann. N. Y. Acad. Sci. 2006, 1069, 62–76. [Google Scholar] [CrossRef]
- Greenfeld, K.; Avraham, R.; Benish, M.; Goldfarb, Y.; Rosenne, E.; Shapira, Y.; Rudich, T.; Ben-Eliyahu, S. Immune suppression while awaiting surgery and following it: Dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav. Immun. 2007, 21, 503–513. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Kin, N.W.; Sanders, V.M. It takes nerve to tell T and B cells what to do. J. Leukoc. Biol. 2006, 79, 1093–1104. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. Basic Clin. 2014, 182, 15–41. [Google Scholar] [CrossRef]
- Martelli, D.; Yao, S.T.; McKinley, M.J.; McAllen, R.M. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol. 2014, 592, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol. Sci. 2004, 25, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Rothman, V.L.; L’Heureux, D.Z.; Tuszynski, G. Reduction of angiocidin expression in human umbilical vein endothelial cells via siRNA silencing inhibits angiogenesis. Exp. Mol. Pathol. 2006, 81, 108–114. [Google Scholar] [CrossRef]
- Nilsson, M.B.; Langley, R.R.; Fidler, I.J. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005, 65, 10794–10800. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Allavena, P.; Mantovani, A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008, 267, 204–215. [Google Scholar] [CrossRef]
- Buijs, R.M.; van der Vliet, J.; Garidou, M.L.; Huitinga, I.; Escobar, C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS ONE 2008, 3, e3152. [Google Scholar] [CrossRef]
- Gaykema, R.P.; Chen, C.C.; Goehler, L.E. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: Evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007, 1130, 130–145. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.K.; Conroy, W.G. Nicotinic alpha 7 receptors: Synaptic options and downstream signaling in neurons. J. Neurobiol. 2002, 53, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- King, J.R.; Gillevet, T.C.; Kabbani, N. A G protein-coupled alpha7 nicotinic receptor regulates signaling and TNF-alpha release in microglia. FEBS Open Bio. 2017, 7, 1350–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Matthay, M.A.; Malik, A. Requisite role of the cholinergic 7 nicotinic acetylcholine receptor pathway in suppressing gram-negative sepsisinduced acute lung inflammatory injury. J. Immunol. 2009, 184, 401–410. [Google Scholar] [CrossRef]
- Vida, G.; Peña, G.; Deitch, E.A.; Ulloa, L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 2011, 186, 4340–4346. [Google Scholar] [CrossRef] [Green Version]
- Marrero, M.B.; Bencherif, M. Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for antiapoptosis and anti-inflammation: Central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res. 2009, 1256, 1–7. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, P.H.; Ahn, Y.W.; Choi, Y.J.; Lee, G.; Lee Da Chung, E.S.; Jin, B.K. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur. J. Neurosci. 2007, 26, 79–89. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; León, R.; Lopez, M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef]
- Cedillo, J.L.; Arnalich, F.; Martín-Sánchez, C.; Quesada, A.; Rios, J.J.; Maldifassi, M.C.; Atienza, G.; Renart, J.; Fernández-Capitán, C.; García-Rio, F. Usefulness of α7 nicotinic receptor messenger RNA levels in peripheral blood mono-nuclear cells as a marker for cholinergic anti inflammatory pathway activity in septic patients: Results of a pilot study. J. Infect. Dis. 2015, 211, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Abumrad, N.; Eaton JWTracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Bernik, T.R.; Friedman, S.G.; Ochani, M.; di Raimo, R.; Susarla, S.; Czura, C.J.; Tracey, K.J. Cholinergic anti-inflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg. 2002, 36, 1231–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin. Pharmacol. Toxicol. 2012, 110, 87–94. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.H.; Gonsebatt, M.E. The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.L.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [Green Version]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Yan, G.; You, B.; Chen, S.; Liao, J.K.; Sun, J. Tumor necrosis factor-α downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-α 1. Circ. Res. 2008, 103, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Mäki-Petäjä, K.M.; Hall, F.C.; Booth, A.D.; Wallace, S.M.; Yasmin; Bearcroft, P.W.; Harish, S.; Furlong, A.; McEniery, C.M.; Brown, J.; et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-α therapy. Circulation 2006, 114, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirooka, Y.; Kishi, T.; Sakai, K.; Takeshita, A.; Sunagawa, K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R818–R826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Ebadi, S.A.; Laher, I. Antioxidants in the treatment of diabetes. Curr. Diabetes Rev. 2011, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.F.; Matthews, K.A. Interactions between autonomic nervous system activity and endothelial function: A model for the development of cardiovascular disease. Psychosom. Med. 2004, 66, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Isogawa, A.; Yamakado, M.; Yano, M.; Shiba, T. Serum superoxide dismutase activity correlates with the components of metabolic syndrome or carotid artery intima-media thickness. Diabetes Res. Clin. Pract. 2009, 86, 213. [Google Scholar]
- Lu, A.L.; Li, X.; Gu, Y.; Wright, P.M.; Chandy, D.Y. Repair of oxidative DNA damage: Mechanisms and functions. Cell. Biochem. Biophys. 2001, 35, 141–170. [Google Scholar] [CrossRef]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Liu, Y.; Varma, N.R.S.; Arbab, A.S.; Gautam, S.C. Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent. Molecules 2013, 18, 3250–3265. [Google Scholar] [CrossRef]
- Epel, E.S.; Daubenmier, J.; Moskowitz, J.T.; Folkman, S.; Blackburn, E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann. N. Y. Acad. Sci. 2009, 1172, 34–53. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Epel, E.; Adler, N.; Bush, N.R.; Obradovic, J.; Lin, J.; Blackburn, E.; Stamperdahl, J.L.; Boyce, W.T. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom. Med. 2011, 73, 533–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Saso, L.; Firuzi, O. Pharmacological applications of antioxidants: Lights and shadows. Curr. Drug Targets 2014, 15, 1177–1199. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Budde, H.; Schwarz, R.; Velasques, B.; Ribeiro, P.; Holzweg, M.; Machado, S.; Brazaitis, M.; Staack, F.; Wegner, M. The need for differentiating between exercise, physical activity, and training. Autoimmun. Rev. 2016, 15, 110–111. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Djordjevic, D.; Cubrilo, D.; Macura, M.; Barudzic, N.; Djuric, D.; Jakovljevic, V. The influence of training status on oxidative stress in young male handball players. Mol. Cell. Biochem. 2011, 351, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.M.; Husain, K. Influence of exercise-induced oxidative stress on the central nervous system. In Handbook of Oxidants and Antioxidants in Exercise; Sen, C.K., Packer, L., Hanninen, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; art IX, Chapter 26. [Google Scholar]
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; la Torre, A.; Porcelli, S. Time-Course Changes of Oxidative Stress Response to High-Intensity Discontinuous Training versus Moderate-Intensity Continuous Training in Masters Runners. PLoS ONE 2014, 9, e87506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.-J.; Hung, C.-H.; Tsai, W.-M.; Cheng, H.-C.; Yang, H.-L.; Lu, Y.-J.; Tsai, K.-L. Effect of Exercise Training on Exercise Tolerance and Level of Oxidative Stress for Head and Neck Cancer Patients Following Chemotherapy. Front. Oncol. 2020, 10, 1536. [Google Scholar] [CrossRef]
- Finkler, M.; Lichtenberg, D.; Pinchuk, I. The relationship between oxidative stress and exercise. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 1–11. [Google Scholar] [CrossRef]
- González-Bartholin, R.; Mackay, K.; Valladares, D.; Zbinden-Foncea, H.; Nosaka, K.; Peñailillo, L. Changes in oxidative stress, inflammation and muscle damage markers following eccentric versus concentric cycling in older adults. Eur. J. Appl. Physiol. 2019, 119, 2301–2312. [Google Scholar] [CrossRef]
- Dantas de Lucas, R.; Caputob, F.; Mendes de Souza, K.; Sigwalt, A.R.; Ghisoni, K.; Silveira, P.C.L.; Remor, A.P.; Scheffer, D.d.L.; Guglielmo, L.G.A.; Latini, A. Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. J. Sports Sci. 2014, 32, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.T.; Kong, Z.; Lin, H.; Lippi, G.; Zhang, H.; Nie, J. Serum Oxidant and Antioxidant Status Following an All-Out 21-km Run in Adolescent Runners Undergoing Professional Training—A One-Year Prospective Trial. Int. J. Mol. Sci. 2013, 14, 15167–15178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Levine, B.D. Exercise and the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 147–160. [Google Scholar] [PubMed]
- Hautala AJKiviniemi, A.M.; Tulppo, M.P. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009, 33, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, J.-Y.; Zhang, H.-X.; Li, Q.; Zhang, S.W. Exercise Training Attenuates Sympathetic Activation and Oxidative Stress in Diet-Induced Obesity. Physiol. Res. 2015, 64, 355–367. [Google Scholar] [CrossRef]
- Conti, F.F.; de Oliveira Brito, J.; Bernardes, N.; Dias, D.d.S.; Malfitano, C.; Morris, M.; Llesuy, S.F.; Irigoyen, M.-C.; de Angelis, K. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: Autonomic, inflammatory, and oxidative stress evaluations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1532–R1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cikrikcioglu, M.A.; Hursitoglu, M.; Erkal, H.; Kınas, B.E.; Sztajzel, J.; Cakirca, M.; Arslan, A.G.; Erek, A.; Halac, G.; Tukek, T. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur. J. Clin. Investig. 2011, 41, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Van Oosterwijck, J.; Nijs, J.; Marusic, U.; De Wandele, I.; Paul, L.; Meeus, M.; Moorkens, G.; Lambrecht, L.; Ickmans, K. Relationship Between Exercise-induced Oxidative Stress Changes and Parasympathetic Activity in Chronic Fatigue Syndrome: An Observational Study in Patients and Healthy Subjects. Clin. Ther. 2019, 41, 641–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobley, J.N. How exercise induces oxidative eustress. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 2020; pp. 447–462. [Google Scholar]
- Pedersen, B.K.; Ostrowski, K.; Rohde, T.; Bruunsgaard, H. The cytokine response to strenuous exercise. Can. J. Physiol. Pharmacol. 1998, 76, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Lexell, J.; Deierborg, T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior. Neurorehabil. Neural Repair 2015, 29, 577–589. [Google Scholar]
- Fischer, C.P.; Hiscock, N.; Basu, S.; Vessby, B.; Kallner, A.; Sjöberg, L.B.; Febbraio, M.A.; Pedersen, B.K. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J. Physiol. 2004, 558, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2009, 2008, 109502. [Google Scholar]
- LuzScheffer, D.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 10. [Google Scholar]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508 Pt 3, 949–953. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin 6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Alizaei Yousefabadi, H.; Niyazi, A.; Alaee, S.; Fathi, M.; Mohammad Rahimi, G.R. Anti-Inflammatory Effects of Exercise on Metabolic Syndrome Patients: A Systematic Review and Meta-Analysis. Biol. Res. Nurs. 2020, 23, 280–292. [Google Scholar] [CrossRef]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; Fernando, F.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Letizia, C.; Jimenez, A. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 608–617. [Google Scholar] [CrossRef]
- Dadrass, A.; Mohamadzadeh Salamat, K.; Hamidi, K.; Azizbeigi, K. Anti-inflammatory effects of vitamin D and resistance training in men with type 2 diabetes mellitus and vitamin D deficiency: A randomized, double-blinded, placebo-controlled clinical trial. J. Diabetes Metab. Disord. 2019, 18, 323–331. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Nonas, C.; Weil, R.; Horlick, M.; Fennoy, I.; Vargas, I.; Kringas, P. Camino Diabetes Prevention Group. School-based intervention acutely improves insulin sensitivity and decreases inflammatory markers and body fatness in junior high school students. J. Clin. Endocrinol. Metab. 2007, 92, 504–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamat, K.M.; Azarbayjani, M.A.; Yusof, A.; Dehghan, F. The response of pre-inflammatory cytokines factors to different exercises (endurance, resistance, concurrent) in overweight men. Alex. J. Med. 2016, 52, 367–370. [Google Scholar] [CrossRef]
- Chen, C.-W.; Kuo, Y.-C.; How, C.-K.; Juan, C.-C. Long-term aerobic exercise training-induced anti-inflammatory response and mechanisms: Focusing on the toll-like receptor 4 signaling pathway. Chin. J. Physiol. 2020, 63, 250–255. [Google Scholar]
- Pedersen, B.K.; Rohde, T.; Zacho, M. Immunity in athletes. J. Sports Med. Phys. Fit. 1996, 3, 236–245. [Google Scholar]
- Goh, J.; Lim, C.L.; Suzuki, K. Effects of Endurance, Strength, and Concurrent Training on Cytokines and Inflammation. In Concurrent Aerobic and Strength Training; Schumann, M., Rønnestad, B.R., Eds.; Springer: Basel, Switzerland, 2019; pp. 125–138. [Google Scholar]
- Pedersen, B.K.; Ullum, H. NK cell response to physical activity: Possible mechanisms of action. Med. Sci. Sports Exerc. 1994, 26, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.M.; Radom-Aizik, S.; Schwindt, C.; Zaldivar, F., Jr. Dangerous exercise: Lessons learned from dysregulated inflammatory responses to physical activity. J. Appl. Physiol. 2007, 103, 700–709. [Google Scholar] [CrossRef] [Green Version]
- Tidball, J.G. Inflammatory processes in muscle injury and repair. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Rokitzki, L.; Logemann, E.; Sagredos, A.N.; Murphy, M.; Wetzel-Roth, W.; Keul, J. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiol. Scand. 1994, 151, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Dufaux, B.; Order, U. Plasma elastase-1-antitrypsin, neopterin, tumor necrosis factor, and soluble interleukin-2 receptor after prolonged exercise. Int. J. Sports Med. 1989, 10, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Hellsten, Y.; Frandsen, U.; Orthenblad, N.; Sjodin, N.; Richter, E.A. Xanthine oxidase in human skeletal muscle following eccentric exercise: A role of inflammation. J. Physiol. 1997, 498, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Nybo, L.; Nielsen, B.; Pedersen, B.K.; Moller, K.; Secher, N.H. Interleukin-6 release from the human brain during prolonged exercise. J. Physiol. 2002, 542, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, N.J.; Benveniste, E.N. Interleukin-6 expression and regulation in astrocytes. J. Neuroimmunol. 1999, 100, 124–139. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Kim, S.-C.; Hong, H.-P.; Park, C.-Y.; Shin, M.-S.; Kim, C.-J.; Seo, J.-H.; Kim, C.-Y.; Kim, D.-J.; Cho, H.-J. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson’s disease mice. Life Sci. 2012, 91, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.H.; Schlesinger, L.; Terwilliger, R.; Russell, D.S.; Newton, S.S.; Duman, R.S. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 2009, 198, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Wu, H.M.; Chen, S.H.; Zhang, D.; Ali, S.F.; Peterson, L.; Wilson, B.; Lu Ru Hong, J.-S.; Flood, P.M. Beta2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. J. Immunol. 2011, 186, 4443–4454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragala, M.S.; Kraemer, W.J.; Mastro, A.M.; Denegar, C.R.; Volek, J.S.; Häkkinen, K.; Anderson, J.M.; Lee, E.C.; Maresh, C.M. Leukocyte beta2-adrenergic receptor expression in response to resistance exercise. Med. Sci. Sports Exerc. 2011, 43, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C.; Schilling, B.K.; Weiss, L.W.; Chiu, L.Z. Beta2-adrenergic receptor downregulation and performance decrements during high-intensity resistance exercise overtraining. J. Appl. Physiol. 2006, 101, 1664–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radom-Aizik, S.; Zaldivar, F.P., Jr.; Haddad, F.; Cooper, D.M. Impact of brief exercise on circulating monocyte gene and microRNA expression: Implications for atherosclerotic vascular disease. Brain Behav. Immun. 2014, 39, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Schmolesky, M.T.; Webb, D.L.; Hansen, R.A. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J. Sports Sci. Med. 2013, 12, 502–511. [Google Scholar] [PubMed]
- Pluchino, N.; Russo, M.; Santoro, A.N.; Litta, P.; Cela, V.; Genazzani, A.R. Sterioid hormones and BDNF. Neuroscience 2013, 239, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.G. 60 years of POMC: Lipotropin and beta-endorphin: A perspective. J. Mol. Endocrinol. 2016, 56, T13–T25. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, C.H. Endorphins: The basis of pleasure? J. Neurol. Neurosurg. Psychiatry 1992, 55, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, L.; Kindermann, W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992, 13, 25–36. [Google Scholar] [CrossRef]
- Siswantoyo; Aman, M.S. The Effects of Breathing Exercise Toward IgG, Beta Endorphin and Blood Glucose Secretion. Asia Pac. J. Educ. Arts Sci. 2014, 1, 27–32. [Google Scholar]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Stensson, N.; Gerdle, B.; Ernberg, M.; Mannerkorpi, K.; Kosek, E.; Ghafouri, B. Increased Anandamide and Decreased Pain and Depression after Exercise in Fibromyalgia. Med. Sci. Sports Exerc. 2020, 52, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.J.; dos Lacerda, J.R.M.; Cristina-Souza, G.; Lopes Filho, B.J.P.; Camilo, B.d.F. Effect of resistance training in women with fibromyalgia: A review study. Res. Soc. Dev. 2021, 10, e29410514674. [Google Scholar] [CrossRef]
- Marin Bosch, B.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Thomas, A.; Ferretti, G.; Schwartz, S.; Igloi, K. Effect of acute physical exercise on motor sequence memory. Sci. Rep. 2020, 10, 15322. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Johnsen, L.K.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Intensity. PLoS ONE 2016, 11, e0159589. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.; Teixeira, A.M.; Serrano, J.; Santos, H.; Campos, M.J.; Oliveiros, B.; Silva, F.M.; Cascante-Rusenhack, M.; Luís, P.; Ferreira, J.P. Impact of Different Aquatic Exercise Programs on Body Composition, Functional Fitness and Cognitive Function of Non-Institutionalized Elderly Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 8963. [Google Scholar] [CrossRef]
- Pesce, M.; La Fratta, I.; Paolucci, T.; Grilli, A.; Patruno, A.; Agostini, F.; Bernetti, A.; Mangone, M.; Paoloni, M.; Invernizzi, M.; et al. From Exercise to Cognitive Performance: Role of Irisin. Appl. Sci. 2021, 11, 7120. [Google Scholar] [CrossRef]
- Muller, P.; Taubert, M.; Muller, N.G. Physical exercise as personalized medicine for dementia prevention? Front. Physiol. 2019, 10, 672. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhao, X.; Hou, Y.Y.; Liu, T.; Wu, Q.; Huang, Y.H.; Wang, X.H. Meta-analysis of effects of voluntary slow breathing exercises for control of heart rate and blood pressure in patients with cardiovascular diseases. Am. J. Cardiol. 2017, 120, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Porta, C.; Spicuzza, L.; Bellwon, J.; Spadacini, G.; Frey, A.W.; Yeung, L.Y.C.; Sanderson, J.E.; Pedretti, R.; Tramarin, R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002, 105, 143–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straznicky, N.E.; Nestel, P.J.; Esler, M. Autonomic Nervous System: Metabolic Function. Encycl. Neurosci. 2010, 951–959. [Google Scholar] [CrossRef]
- Johnson, M.S.; DeMarco, V.G.; Whaley-Connell, A.; Sowers, J.R. Insuline resistance and the autonomic nervous system. In Primer on the Autonomic Nervous System, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Robertson, D.; Biaggioni, I.; Burnstock, G.; Low, P.A.; Paton, J.F.R. (Eds.) Primer on the Autonomic Nervous System; Mayo Clinic, Elsevier Inc.: Rochester, MN, USA, 2012; ISBN 978-0-12-386525-0. [Google Scholar]
- Borer, K.T. Counter regulation of insulin by leptin as key component of autonomic regulation of body weight. World J. Diabetes 2014, 5, 606–629. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Sousa, M.; Lourenço Reis, P.; Leão, C.; Cardoso-Marinho, B.; Massada, M.; Afonso, J. Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review. Nutrients 2021, 13, 438. [Google Scholar] [CrossRef]
- Roberts, L.; Suzuki, K. Exercise and Inflammation. Antioxidants 2019, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Machefer, G.; Groussard, C.; Vincent, S.; Zouhal, H.; Faure, H.; Cillard, J.; Radák, Z.; Gratas Delamarche, A. Multivitamin-mineral supplementation prevents lipid peroxidation during “the Marathon des Sables”. J. Am. Coll. Nutr. 2007, 26, 111–120. [Google Scholar] [CrossRef]
- Taherkhani, S.; Suzuki, K.; Castell, L. A Short Overview of Changes in Inflammatory Cytokines and Oxidative Stress in Response to Physical Activity and Antioxidant Supplementation. Antioxidants 2020, 9, 886. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.A.; Lee, D.H.; Cho, D.-Y.; Lee, Y.-J. Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at theWorkplace-Drinking Electrolyzed-ReducedWater (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane Protects Cells against Lipopolysaccharide-Stimulated Inflammation in Murine Macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Andreeva-Gateva, P.; Traikov, L.; Sabit, Z.; Bakalov, D.; Tafradjiiska-Hadjiolova, R. Antioxidant Effect of Alpha-Lipoic Acid in 6-Hydroxydopamine Unilateral Intrastriatal Injected Rats. Antioxidants 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.A.; Suzuki, K. Anti-Inflammatory and Antioxidant Effects of Dietary Supplementation and Lifestyle Factors. Antioxidants 2021, 10, 371. [Google Scholar] [CrossRef]
- Steckhan, N.; Hohmann, C.D.; Kessler, C.; Dobos, G.; Michalsen, A.; Cramer, A. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 338–348. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. https://doi.org/10.3390/antiox11020350
Daniela M, Catalina L, Ilie O, Paula M, Daniel-Andrei I, Ioana B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants. 2022; 11(2):350. https://doi.org/10.3390/antiox11020350
Chicago/Turabian StyleDaniela, Matei, Luca Catalina, Onu Ilie, Matei Paula, Iordan Daniel-Andrei, and Buculei Ioana. 2022. "Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects" Antioxidants 11, no. 2: 350. https://doi.org/10.3390/antiox11020350
APA StyleDaniela, M., Catalina, L., Ilie, O., Paula, M., Daniel-Andrei, I., & Ioana, B. (2022). Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants, 11(2), 350. https://doi.org/10.3390/antiox11020350









