Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Determination of Growth Characteristics
2.3. Photosynthetic Characteristics Measurements
2.4. Determination of H2O2 and Thiobarbituric Acid Reactive Substance (TBARS) Content
2.5. Abscisic Acid Content
2.6. Determination of NO Generation
2.7. Assay of Activity of Antioxidant Enzymes
2.8. Determination of Proline Content
2.9. Determination of Glycine Betaine Content
2.10. Estimation of Total Soluble Sugar and Trehalose Content
2.11. Quantitative RT-PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. Effect of NO and ABA on Growth Parameters under Heat Stress
3.2. Impact of Heat Stress on Photosynthetic Characteristics and Involvement of NO and ABA in Inhibiting Heat-Induced Photosynthetic Reduction
3.3. ABA Requires NO Action for Reducing Oxidative Stress in Wheat Plants Exposed to Heat Stress
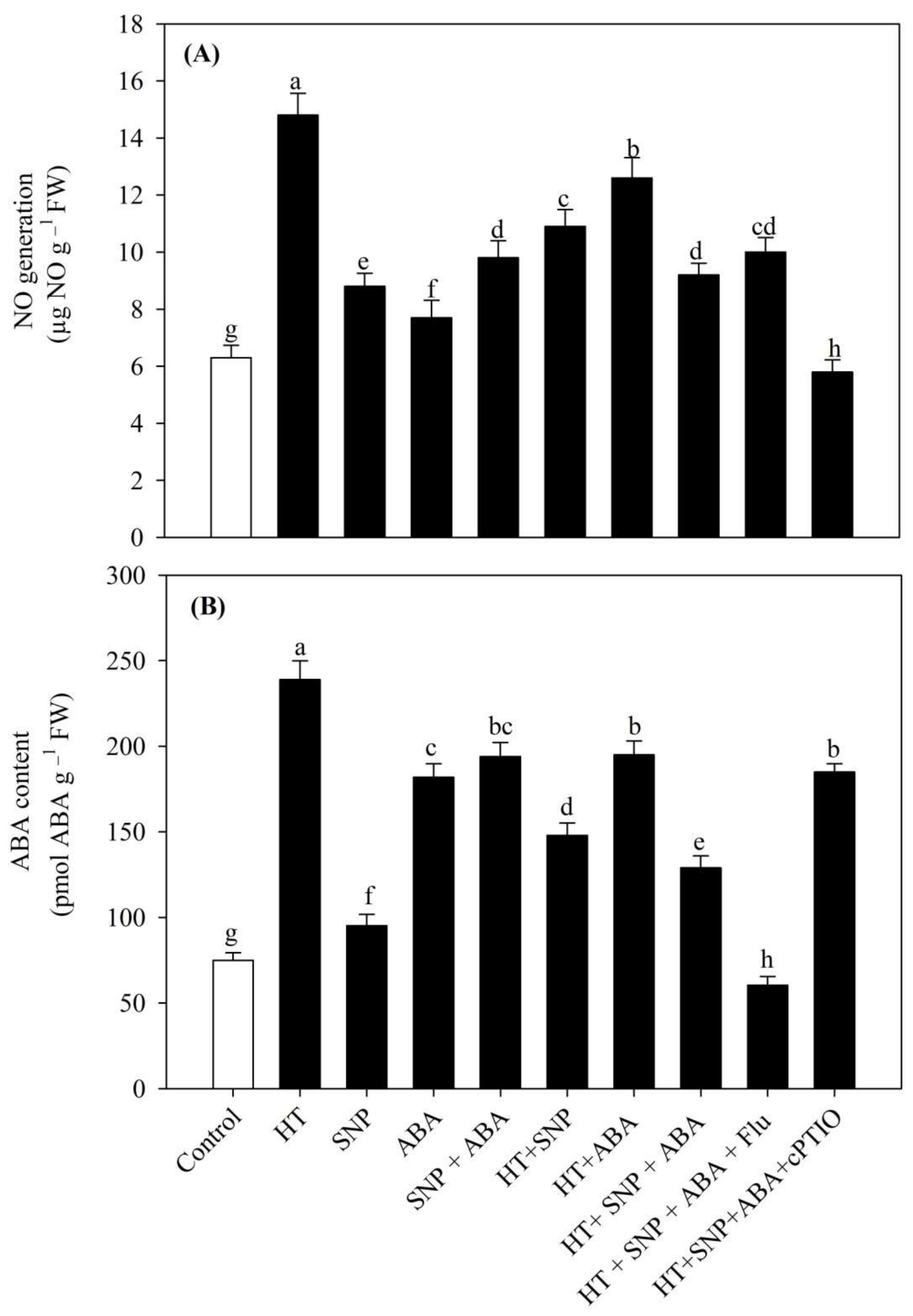
3.4. Impact of Heat Stress on NO and ABA Content
3.5. The Combined Application of NO and ABA Stimulated Antioxidant Enzyme Activity under Heat Stress
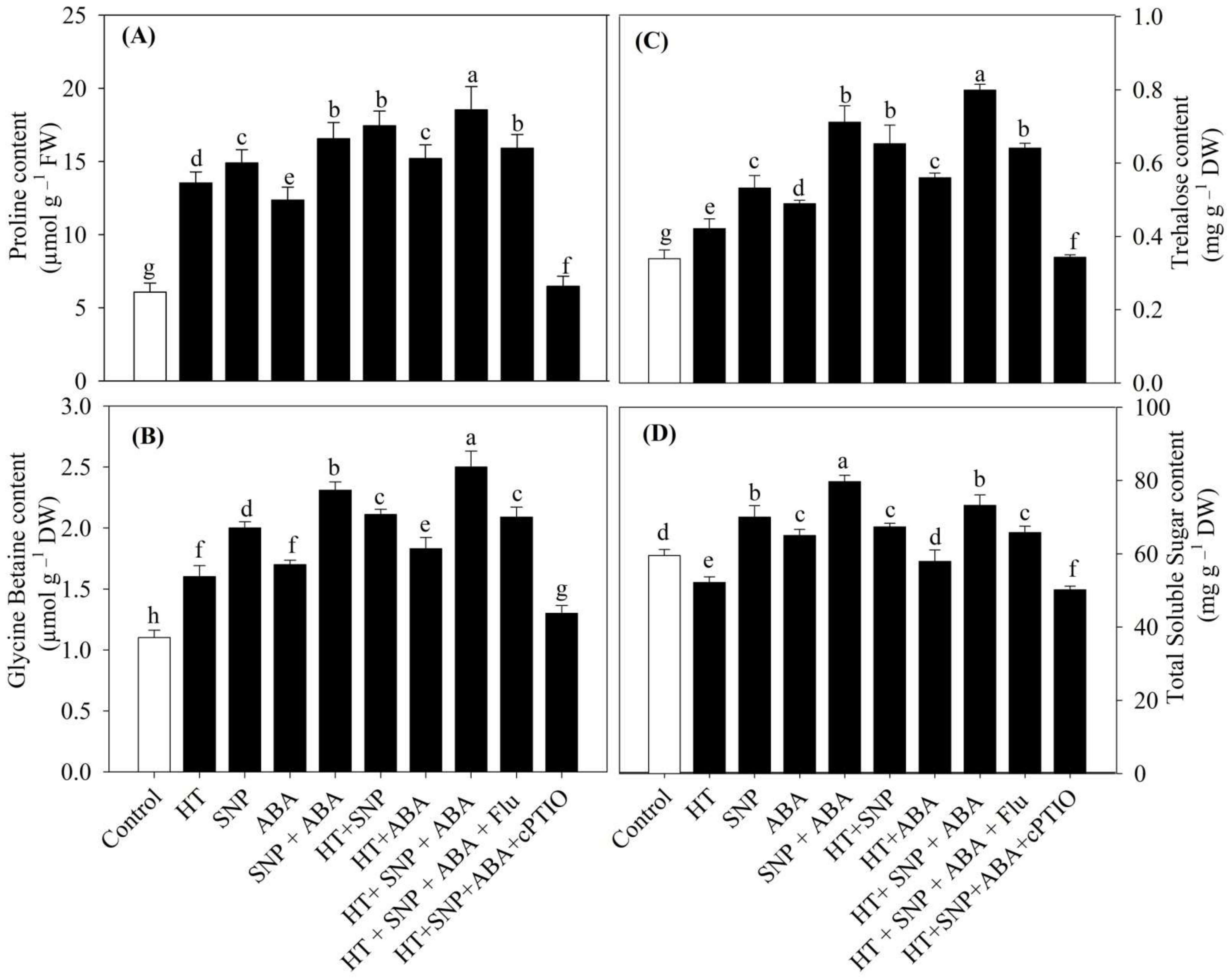
3.6. Osmolytes Accumulation Increased under Heat Stress Maximally with Combined NO and ABA Supplementation
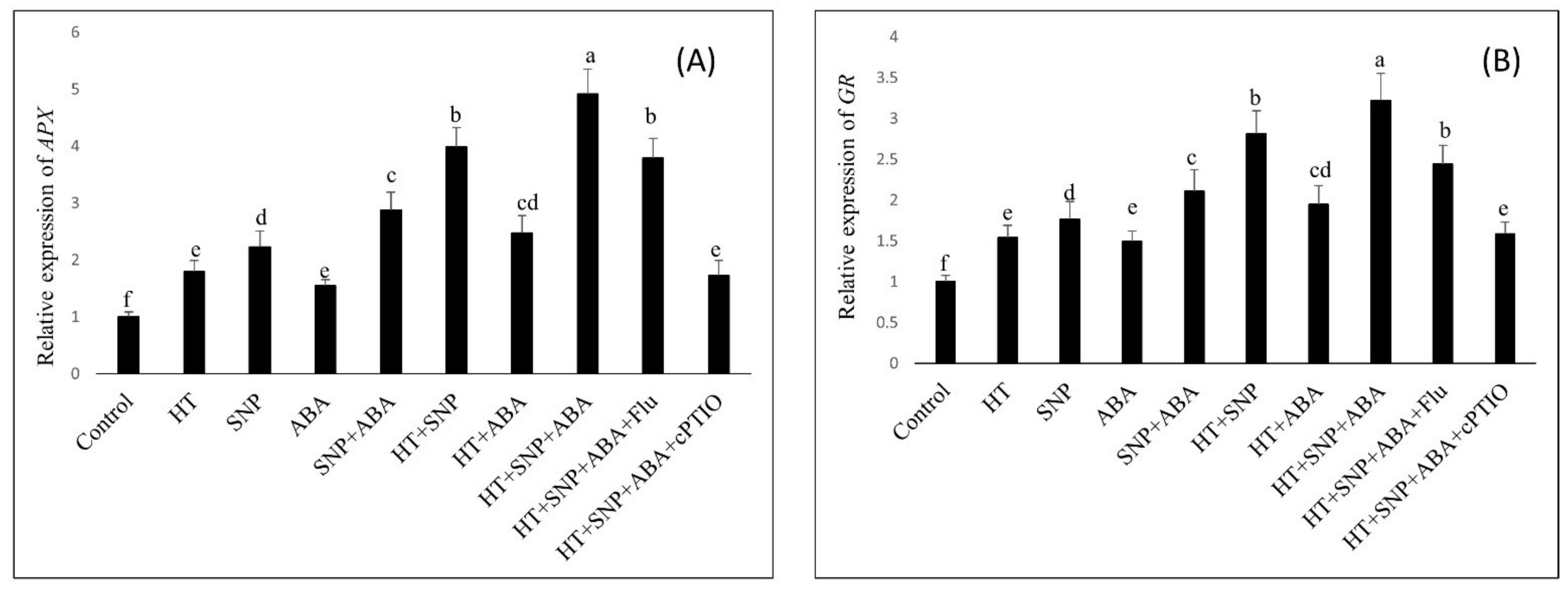
3.7. Application of NO and ABA on Antioxidant Enzyme Gene Expression under Heat Stress
4. Discussion
4.1. Role of NO and ABA in Reducing Photosynthesis Inhibition and Growth under Heat Stress
4.2. Nitric Oxide and ABA Increased Osmolytes Accumulation and Antioxidant Enzyme Activity and Expression to Reduce Heat-Induced Oxidative Stress
4.3. The Interaction of NO and ABA and Their Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef]
- Johkan, M.; Oda, M.; Maruo, T.; Shinohara, Y. Crop production and global warming. In Global Warming Impacts-Case Studies on the Economy, Human Health, and on Urban and Natural Environments; Stefano, C., Ed.; InTech: Rijeka, Croatia, 2011; pp. 139–152. [Google Scholar]
- IPCC. The Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2021. [Google Scholar]
- Teixeira, E.I.; Fischer, G.; Van Velthuizen, H.; Walter, C.; Ewert, F. Global hot-spots of heat stress on agricultural crops due to climate change. Agric. For. Meteorol. 2013, 170, 206–215. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum). Biochim. Biophys. Acta 2011, 1807, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Rising temperature in the changing environment: A serious threat to plants. Clim. Chang. Environ. Sustain. 2013, 1, 25–36. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Gautam, H.; Sehar, Z.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Khan, N.A. Nitric oxide enhances photosynthetic nitrogen and sulfur-use efficiency and activity of ascorbate-glutathione cycle to reduce high temperature stress-induced oxidative stress in rice (Oryza sativa L.) plants. Biomolecules 2021, 11, 305. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-sourced ethylene positively modulates photosynthesis, carbohydrate metabolism, and antioxidant defense to enhance heat tolerance in rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Ferretti, U.; Sedlářová, M.; Pospíšil, P. Singlet oxygen production in Chlamydomonas reinhardtii under heat stress. Sci. Rep. 2016, 6, 20094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smertenko, A.; Dráber, P.; Viklický, V.; Opatrný, Z. Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant Cell Environ. 1997, 20, 1534–1542. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Tokachichu, R.N.; Trethowan, R.M.; Tan, D.K. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Sharma, N.; Yadav, A.; Khetarpal, S.; Anand, A.; Sathee, L.; Kumar, R.R.; Singh, B.; Soora, N.K.; Pushkar, S. High day–night transition temperature alters nocturnal starch metabolism in rice (Oryza sativa L.). Acta Physiol. Plant 2017, 39, 74. [Google Scholar] [CrossRef]
- Hossain, M.A.; Fujita, M. Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait 2013, 4, 109–123. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ. Exp. Bot. 2015, 112, 44–54. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Ding, W.; Zhao, M.; Sun, B.; Zhang, L. Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed. Plant Sci. 2006, 171, 449–458. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Chan, Z.; Shi, H. Improved abiotic stress tolerance of bermudagrass by exogenous small molecules. Plant Signal. Behav. 2015, 10, e991577. [Google Scholar] [CrossRef] [Green Version]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant. 2015, 8, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Lamattina, L.; Spoel, S.H.; Loake, G.J. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol. 2014, 202, 1142–1156. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.; Jones, R.L. Nitric oxide in Seed Dormancy and Germination. In Annual Plant Reviews, Seed Development, Dormancy and Germination; Bradford, K.J., Nonogaki, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 27, pp. 153–175. [Google Scholar]
- Popova, L.; Tuan, T. Nitric oxide in plants: Properties, biosynthesis and physiological functions. Iran. J. Sci. Technol. 2010, 34, 173–183. [Google Scholar] [CrossRef]
- Mishina, T.E.; Lamb, C.; Zeier, J. Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant Cell Environ. 2007, 30, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehrawat, A.; Gupta, R.; Deswal, R. Nitric oxide-cold stress signalling cross-talk, evolution of a novel regulatory mechanism. Proteomics 2013, 13, 1816–1835. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, K.S.; Lamotte, O.; Klinguer, A.; Pugin, A.; Wendehenne, D. Nitric oxide production in tobacco leaf cells: A generalized stress response? Plant Cell Environ. 2003, 26, 1851–1862. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289. [Google Scholar] [CrossRef]
- Diao, Q.N.; Song, Y.J.; Shi, D.M.; Qi, H.Y. Nitric oxide induced by polyamines involves antioxidant systems against chilling stress in tomato (Lycopersicon esculentum Mill) seedling. J. Zhejiang Univ. Sci. B 2016, 17, 916–930. [Google Scholar] [CrossRef] [Green Version]
- Gayatri, G.; Agurla, S.; Raghavendra, A. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 2013, 4, 425. [Google Scholar] [CrossRef] [Green Version]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic acid synthesis and response. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teplova, I.R.; Farkhutdinov, R.G.; Mitrichenko, A.N.; Ivanov, I.I.; Veselov, S.Y.; Valcke, R.L.; Kudoyarova, G.R. Response of tobacco plants transformed with the ipt gene to elevated temperature. Russ. J. Plant Physiol. 2000, 47, 367–369. [Google Scholar]
- Gong, M.; Li, Y.J.; Chen, S.Z. Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Chen, Y.; Li, H.; Xu, S.; Yang, T.; Zhang, X.; Su, X.; Xia, Z. Overexpression of NtDOG1L-T improves heat stress tolerance by modulation of antioxidant capability and defense-, heat-, and ABA-related gene expression in tobacco. Front. Plant Sci. 2020, 11, 568489. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Crosstalk between abscisic acid and nitric oxide under heat stress: Exploring new vantage points. Plant Cell Rep. 2021, 40, 1429–1450. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef] [Green Version]
- Alnusairi, G.S.; Mazrou, Y.S.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.; El Nahhas, N. Exogenous nitric oxide reinforces photosynthetic efficiency, osmolyte, mineral uptake, antioxidant, expression of stress-responsive genes and ameliorates the effects of salinity stress in wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef]
- He, H.; Oo, T.L.; Huang, W.; He, L.F.; Gu, M. Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Sci. Rep. 2019, 9, 9516. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel, L.A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.M.; Zhao, J.; Scandalios, J.G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000, 22, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Wen, F.; Yao, D.; Wang, L.; Guo, J.; Ni, L.; Zhang, A.; Tan, M.; Jiang, M. A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defence and oxidative stress tolerance in rice. J. Exp. Bot. 2014, 65, 5795–5809. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Li, C.; Yang, H.; Wang, W.; Lu, M. Characterization of small heat shock proteins associated with maize tolerance to combined drought and heat stress. J. Plant Growth Regul. 2010, 29, 455–464. [Google Scholar] [CrossRef]
- Strizhov, N.; Abrahám, E.; Okrész, L.; Blickling, S.; Zilberstein, A.; Schell, J.; Koncz, C.; Szabados, L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997, 12, 557–569. [Google Scholar]
- Sripinyowanich, S.; Klomsakul, P.; Boonburapong, B.; Bangyeekhun, T.; Asami, T.; Gu, H.; Buaboocha, T.; Chadchawan, S. Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): The role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 2013, 86, 94–105. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushal, N.; Harsh, N.; Gaur, P.M. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol. Plant. 2012, 34, 1651–1658. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.Y.; Al-Qutami, M.A.; Ali, H.M.; Khan, M.N. Nitric oxide and calcium induced physio-biochemical changes in tomato (Solanum lycopersicum) plant under heat stress. Fresenius Environ. Bull. 2017, 26, 1663–1672. [Google Scholar]
- Hai-Hua, R.; Wen-Biao, S.; Lang-Lai, X. Nitric oxide involved in the abscisic acid induced proline accumulation in wheat seedling leaves under salt stress. Acta Bot. Sin. 2004, 46, 1307–1315. [Google Scholar]
- Fan, H.F.; Du, C.X.; Guo, S.R. Effect of nitric oxide on proline metabolism in cucumber seedlings under salinity stress. J. Am. Soc. Hort. Sci. 2012, 137, 127–133. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, H.; Hong, J.; Han, Y.; Li, H.; Zhao, W. Effects of exogenous nitric oxide on photosynthesis, antioxidant capacity and proline accumulation in wheat seedlings subjected to osmotic stress. World J. Agric. Sci. 2008, 4, 307–313. [Google Scholar]
- Li, Z.G.; Luo, L.J.; Zhu, L.P. Involvement of trehalose in hydrogensulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot. Stud. 2014, 55, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- FAO. FAOSTAT; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Jaradat, A.A. Ecogeography, genetic diversity and breeding value of wild emmer wheat (Triticum dicoccoides Körn ex Asch. & Graebn). Thell.Austr. J. Crop. Sci. 2011, 5, 1072–1086. [Google Scholar]
- Poudel, P.B.; Poudel, M.R. Heat stress effects and tolerance in wheat: A review. J. Biol. Todays World 2020, 9, 217. [Google Scholar]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Zhao, X.; Xu, S.; Xia, Y.; Sun, X. Nitric oxide acts downstream of abscisic acid in molybdenum-induced oxidative tolerance in wheat. Plant Cell Rep. 2018, 37, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.A.M.E.L.A.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Hung, K.T.; Kao, C.H. Nitric oxide counteracts the senescence of rice leaves induced by abscisic acid. J. Plant Physiol. 2003, 160, 871–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthesguianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Syeed, S.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, N.A. Control of elevated ion accumulation, oxidative stress, and lipid peroxidation with salicylic acid-induced accumulation of glycine betaine in salinity-exposed Vigna radiata L. Appl. Biochem. Biotechnol. 2021, 193, 3301–3320. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; Hanumantha Rao, B.; Nair, R.M.; Vara Prasad, P.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [Green Version]
- Vollenweider, P.; Gunthardt-Goerg, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005, 137, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler., R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Rizwan, M.; Ali, S.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci. Rep. 2018, 8, 17086. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticumaestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Agric. Res. 2012, 6, 1314–1323. [Google Scholar]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishanya, M.Y.; Khan, M.N.; Ali, H.M.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Viciafaba L. Environ. Exp. Bot. 2019, 161, 290–302. [Google Scholar] [CrossRef]
- Planchet, E.; Verdu, I.; Delahaie, J.; Cukier, C.; Girard, C.; Morère-Le Paven, M.C.; Limami, A.M. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicago truncatula. J. Exp. Bot. 2014, 65, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Brestic, M.; Zivca, M.; Olsovaka, K.; Kalaji, H.M.; Shao, H.; Hakeem, K.R. Heat signaling and stress responses in photosynthesis. In Plant Signaling: Understanding the Molecular Crosstalk; Hakeem, K., Rehman, R., Tahir, I., Eds.; Springer: New York, NY, USA, 2014; pp. 241–256. [Google Scholar]
- Misra, A.N. Effect of temperature on chlorophyll degradation of senescing rice leaves. J. Sci. Res. 1981, 3, 9–10. [Google Scholar]
- Kong, J.; Dong, Y.; Xu, L.; Liu, S.; Bai, X. Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachishypogaea L. Bot. Stud. 2014, 55, 9. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; He, H.; Li, H.; Tian, H.; Zhang, J.; Zhai, L.; Chen, J.; Wu, H.; Yi, G.; He, Z.H.; et al. NOA1 functions in a temperature-dependent manner to regulate chlorophyll biosynthesis and RuBisCo formation in rice. PLoS ONE 2011, 6, e20015. [Google Scholar] [CrossRef]
- Wodala, B.; Deák, Z.; Vass, I.; Erdei, L.; Altorjay, I.; Horváth, F. In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol. 2008, 146, 1920–1927. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wollenweber, B.; Jiang, D.; Liu, F.; Zhao, J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J. Exp. Bot. 2008, 59, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, E.J.; Cheng, M.C.; Lin, T.P. Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant. Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, J.K.; Lang, Z. Nitric oxide suppresses the inhibitory effect of abscisic acid on seed germination by S-nitrosylation of SnRK2 proteins. Plant Signal. Behav. 2015, 10, e1031939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Song, L.; Wang, X.; Bi, Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol. Plant. 2010, 54, 607–613. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, M.; Zhang, J.; Ding, H.; Xu, S.; Hu, X.; Tan, M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007, 175, 36–50. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Pattanagul, W. Exogenous abscisic acid enhances sugar accumulation in rice (Oryza sativa L.) under drought stress. Asian J. Plant Sci. 2011, 10, 212–219. [Google Scholar] [CrossRef]
- Liu, L.; Cang, J.; Yu, J.; Wang, X.; Huang, R.; Wang, J.; Lu, B. Effects of exogenous abscisic acid on carbohydrate metabolism and the expression levels of correlative key enzymes in winter wheat under low temperature. Biosci. Biotechnol. Biochem. 2013, 77, 516–525. [Google Scholar] [CrossRef]
- Saeedipour, S. Exogenous abscisic acid enhances sugar accumulation in rice (Oryza sativa L.) under salinity. Int. J. Bio Sci. 2014, 4, 249–257. [Google Scholar]
- Robertson, A.J.; Ishikawa, M.; Gusta, L.V.; MacKenzie, S.L. Abscisic acid-induced heat tolerance in Bromus inermis leyss cell-suspension cultures (heat-stable, abscisic acid-responsive polypeptides in combination with sucrose confer enhanced thermostability). Plant Physiol. 1994, 105, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Ahmad, M.; Ahmed, M.; Hussain, M.I. Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of Glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Los, D.A.; Mohanty, P.; Nishiyama, Y.; Murata, N. Glycinebetaine alleviates the inhibitory effect of moderate heat stress on the repair of photosystem II during photoinhibition. Biochim. Biophys. Acta 2007, 1767, 1363–1371. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, F.S.G.; Ismail, M.R.; Rafii, M.Y.; Aslani, F.; Miah, G.; Muharam, F.M. Critical multifunctional role of the betaine aldehyde dehydrogenase gene in plants. Biotechnol. Biotechnol. Equip. 2018, 32, 815–829. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Egbichi, K.I.; Keyster, M.; Ludidi, N. Nitric oxide influences glycine betaine content and ascorbate peroxidase activity in maize. S. Afr. J. Bot. 2016, 105, 218–225. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Cameron, S.K.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperaturestress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Li, F.; Wang, G.P.; Yang, X.H.; Wang, W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant. 2010, 54, 495–501. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Kong, W.W.; Huang, C.Y.; Chen, Q.; Zou, Y.J.; Zhao, M.R.; Zhang, J.X. Nitric oxide is involved in the regulation of trehalose accumulation under heat stress in Pleurotuseryngii var. tuoliensis. Biotechnol. Lett. 2012, 34, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Tan, L.; An, L.; Zhao, Z.; Wang, S.; Zhang, C. Evidence for the involvement of nitric oxide and reactive oxygen species in osmotic stress tolerance of wheat seedlings: Inverse correlation between leaf abscisic acid accumulation and leaf water loss. Plant Growth Regul. 2004, 42, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Guo, Z.; Lu, S.; He, S.; Shu, W.; Zhou, B. Increased abscisic acid level in transgenic tobacco overexpressing 9-cis epoxycarotenoiddioxegenase influences H2O2 and NO production and antioxidant defences. Plant Cell Environ. 2009, 32, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.; Desikan, R.; Bright, J.; Confraria, A.; Harrison, J.; Hancock, J.T.; Barros, R.S.; Neill, S.J.; Wilson, I.D. Differential requirement for NO during ABA-induced stomatal closure in turgid and wilted leaves. Plant Cell Environ. 2009, 32, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007, 30, 1320–1325. [Google Scholar] [CrossRef]
- de Pinto, M.C.; Locato, V.; Sgobba, A.; Romero-Puertas, M.D.C.; Gadaleta, C.; Delledonne, M.; De Gara, L. S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol. 2013, 163, 1766–1775. [Google Scholar] [CrossRef] [Green Version]
- Romero-Puertas, M.C.; Rodríguez-Serrano, M.; Sandalio, L.M. Protein S-nitrosylation in plants under abiotic stress: An overview. Front. Plant Sci. 2013, 4, 373. [Google Scholar] [CrossRef] [Green Version]


| Treatments | Plant Length (cm) | Leaf Area (cm2 Plant−1) | Plant Fresh Weight | Plant Dry Weight |
|---|---|---|---|---|
| (g Plant−1) | ||||
| Control | 47 ± 2.35d | 27.2 ± 1.2d | 2.8 ± 0.14d | 0.91 ± 0.042d |
| HT | 37 ± 1.82g | 18.3 ± 0.84g | 2.1 ± 0.11g | 0.44 ± 0.017g |
| SNP | 52 ± 1.02b | 33.1 ± 1.28b | 3.2 ± 0.16b | 1.29 ± 0.060b |
| ABA | 50 ± 2.40c | 29.4 ± 1.72c | 3.1 ± 0.15c | 1.09 ± 0.051c |
| SNP + ABA | 55 ± 1.11a | 38.8 ± 1.99a | 3.6 ± 0.18a | 1.44 ± 0.072a |
| HT + SNP | 48 ± 2.36cd | 28.2 ± 1.65cd | 2.6 ± 0.13d | 0.98 ± 0.054d |
| HT + ABA | 44 ± 2.21e | 25.3 ± 1.23e | 2.4 ± 0.12e | 0.73 ± 0.044e |
| HT + SNP + ABA | 51 ± 2.49b | 32.2 ± 1.54b | 2.9 ± 0.14b | 1.22 ± 0.065b |
| HT + SNP + ABA + Flu | 47 ± 2.32d | 27.3 ± 1.11d | 2.7 ± 0.13cd | 1.03 ± 0.046cd |
| HT + SNP + ABA + cPTIO | 40 ± 1.98f | 19.6 ± 0.98f | 2.2 ± 0.11f | 0.53 ± 0.033f |
| Treatments | Net Photosynthesis (µmol CO2 m−2 s−1) | Stomatal Conductance (mmol CO2 m−2 s−1) | Intercellular CO2 Concentration (µmol mol−1) | Chlorophyll Content (SPAD Value) | Maximum Quantum Yield Efficiency of PSII (Fv/Fm) | Rubisco (µmol CO2 mg−1 Protein min−1) |
|---|---|---|---|---|---|---|
| Control | 11.2 ± 0.78d | 362 ± 16.1d | 211 ± 10.7d | 30.3 ± 1.6d | 0.72 ± 0.040d | 38.9 ± 2.1d |
| HT | 06.2 ± 0.64g | 259 ± 14.3g | 119 ± 6.47g | 19.1 ± 1.4g | 0.60 ± 0.030g | 25.2 ± 1.6g |
| NO | 16.1 ± 1.09b | 433 ± 18.9b | 354 ± 16.9b | 44.2 ± 2.8b | 0.88 ± 0.050b | 53.4 ± 2.6b |
| ABA | 14.6 ± 0.84c | 416 ± 17.8c | 319 ± 12.8c | 40.4 ± 2.1c | 0.75 ± 0.480c | 47.8 ± 2.4c |
| NO + ABA | 18.2 ± 0.99a | 456 ± 20.6a | 376 ± 17.19a | 49.1 ± 3.4a | 0.97 ± 0.060a | 57.7 ± 2.8a |
| HT + NO | 12.5 ± 0.87d | 356 ± 16.8d | 228 ± 11.7d | 31.8 ± 1.8d | 0.73 ± 0.041d | 40.2 ± 2.3d |
| HT + ABA | 09.8 ± 0.84e | 328 ± 15.2e | 191 ± 10.8e | 28.6 ± 2.1e | 0.66 ± 0.036e | 32.7 ± 2.2e |
| HT + NO + ABA | 15.4 ± 0.94c | 409 ± 18.2c | 326 ± 13.3c | 39.3 ± 1.9c | 0.87 ± 0.050c | 48.1 ± 2.4c |
| HT + NO + ABA + Flu | 11.9 ± 0.83d | 357 ± 15.4d | 203 ± 09.6d | 31.2 ± 1.8d | 0.77 ± 0.440d | 40.6 ± 2.2d |
| HT + NO + ABA + cPTIO | 07.3 ± 0.77f | 302 ± 13.9f | 152 ± 07.3f | 25.4 ± 1.1f | 0.66 ± 0.035f | 32.5 ± 1.8f |
| Treatments | SOD | CAT | APX | GR |
|---|---|---|---|---|
| (U min−1 mg−1 protein) | ||||
| Control | 7.69 ± 0.41f | 122 ± 09.2g | 2.2 ± 0.23f | 2.29 ± 0.18f |
| HT | 11.9 ± 0.61e | 146 ± 09.9f | 3.7 ± 0.33e | 3.72 ± 0.21e |
| SNP | 12.9 ± 0.84d | 161 ± 10.4d | 4.4 ± 0.48d | 4.11 ± 0.27d |
| ABA | 12.3 ± 0.69d | 142 ± 10.7e | 3.3 ± 0.31e | 3.71 ± 0.24e |
| SNP + ABA | 15.1 ± 1.21c | 174 ± 11.2c | 5.7 ± 0.55c | 4.90 ± 0.34c |
| HT + SNP | 18.3 ± 0.99b | 191 ± 12.3b | 6.7 ± 0.63b | 5.80 ± 0.43b |
| HT + ABA | 15.4 ± 1.29c | 169 ± 11.9c | 5.3 ± 0.43c | 4.70 ± 0.45c |
| HT + SNP + ABA | 20.7 ± 1.38a | 206 ± 13.8a | 8.2 ± 0.71a | 6.40 ± 0.42a |
| HT + SNP + ABA + Flu | 17.7 ± 1.17b | 181 ± 12.4b | 6.1 ± 0.39b | 5.70 ± 0.51b |
| HT + SNP + ABA + cPTIO | 12.0 ± 0.72de | 149 ± 11.5f | 3.4 ± 0.29e | 3.89 ± 0.14e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Sehar, Z.; Fatma, M.; Umar, S.; Sofo, A.; Khan, N.A. Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants 2022, 11, 372. https://doi.org/10.3390/antiox11020372
Iqbal N, Sehar Z, Fatma M, Umar S, Sofo A, Khan NA. Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants. 2022; 11(2):372. https://doi.org/10.3390/antiox11020372
Chicago/Turabian StyleIqbal, Noushina, Zebus Sehar, Mehar Fatma, Shahid Umar, Adriano Sofo, and Nafees A. Khan. 2022. "Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants" Antioxidants 11, no. 2: 372. https://doi.org/10.3390/antiox11020372
APA StyleIqbal, N., Sehar, Z., Fatma, M., Umar, S., Sofo, A., & Khan, N. A. (2022). Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants, 11(2), 372. https://doi.org/10.3390/antiox11020372











