Potassium (K+) Starvation-Induced Oxidative Stress Triggers a General Boost of Antioxidant and NADPH-Generating Systems in the Halophyte Cakile maritima
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Plant Crude Extracts
2.3. Water Content
2.4. Potassium Content and Cellular Potassium Concentration
2.5. Lipid Peroxidation and Histochemical Detection of Superoxide Radical (O2•−) in Leaves
2.6. Anthocyanins
2.7. In-Gel Isozyme Profile Analyses of Superoxide Dismutase (SOD), Peroxidase (POX), and Ascorbate Peroxidase (APX)
2.8. Determination of Enzyme Activities
2.9. Statistical Analysis
3. Results
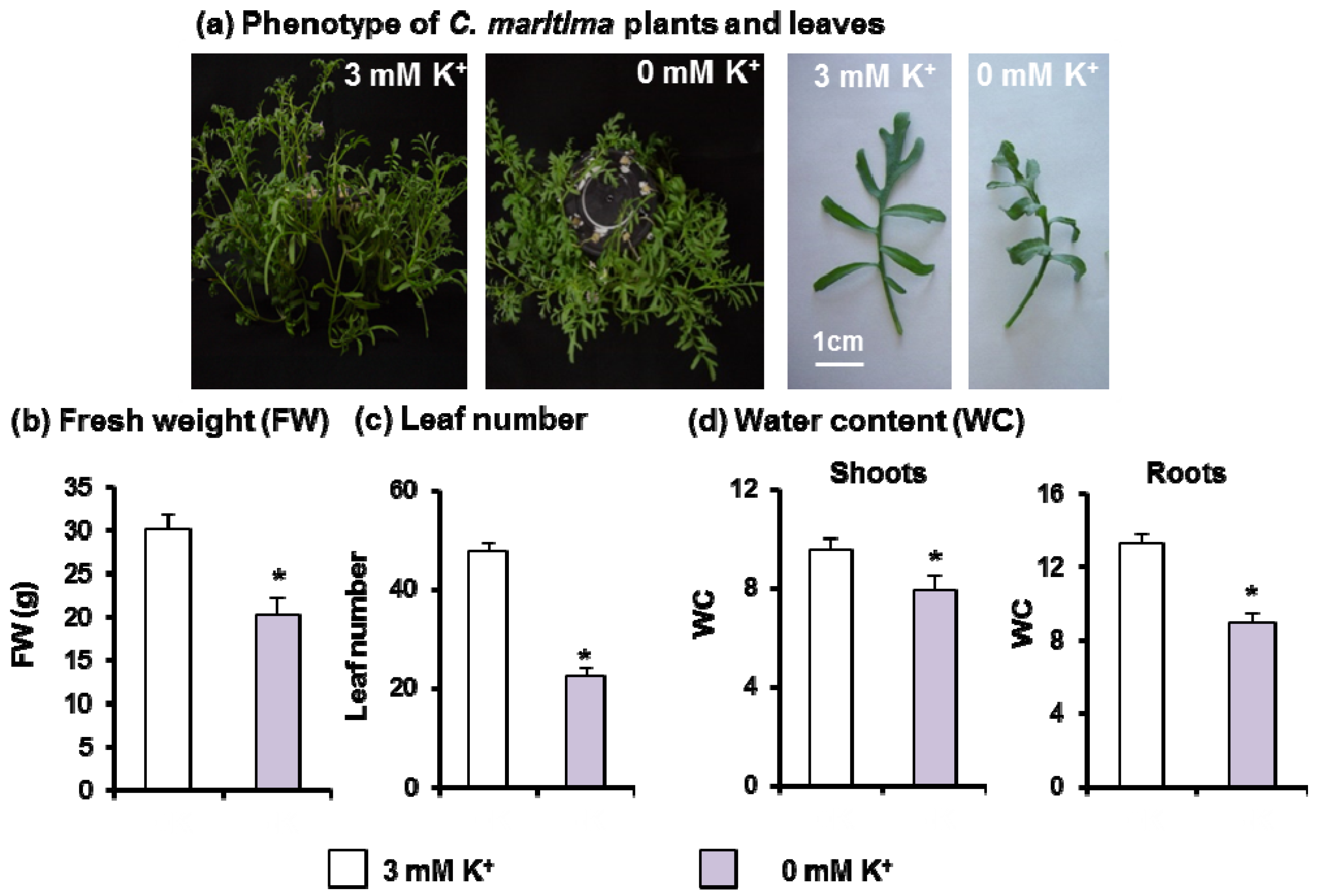
3.1. Effect of Potassium Starvation on C. maritima Growth Parameters
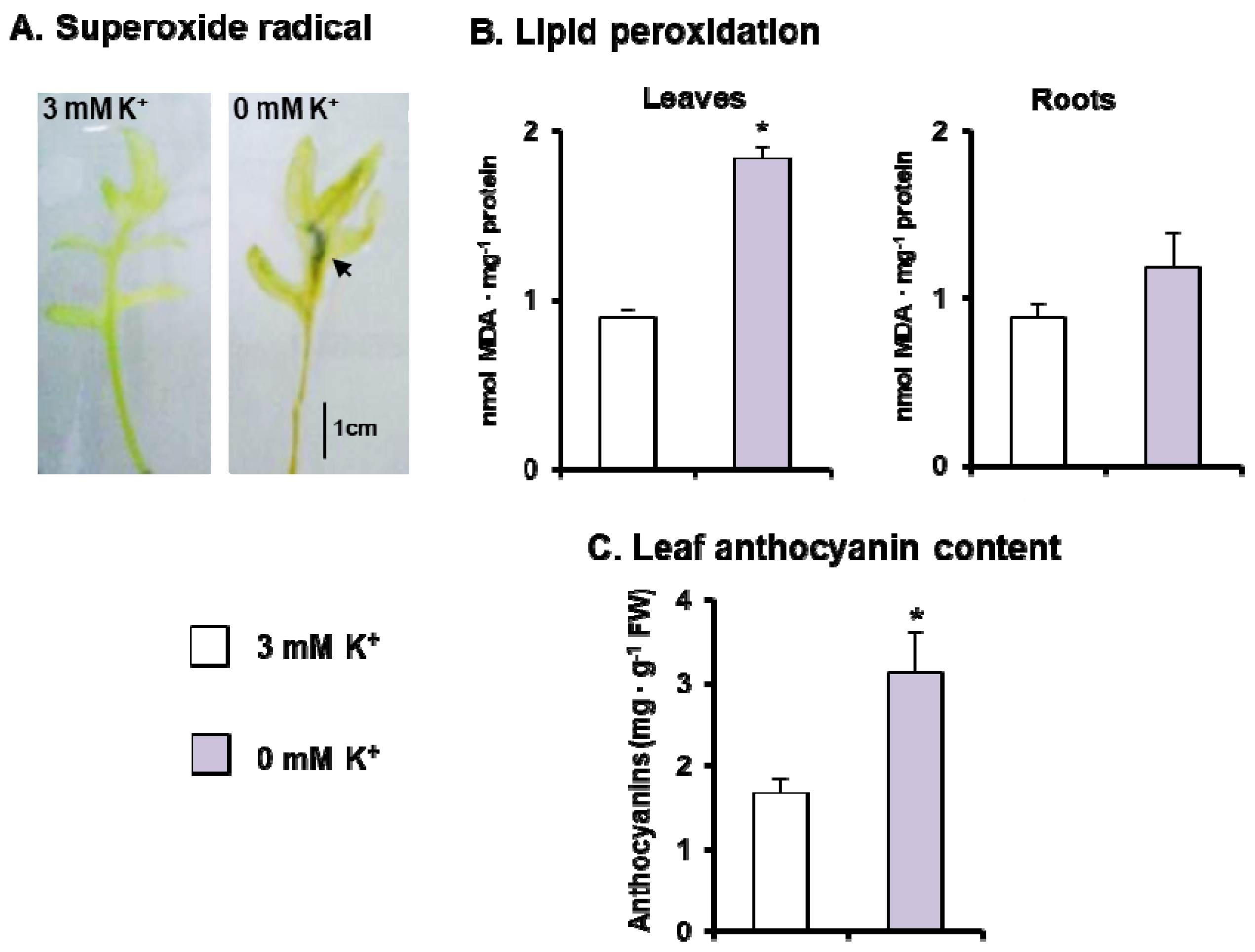
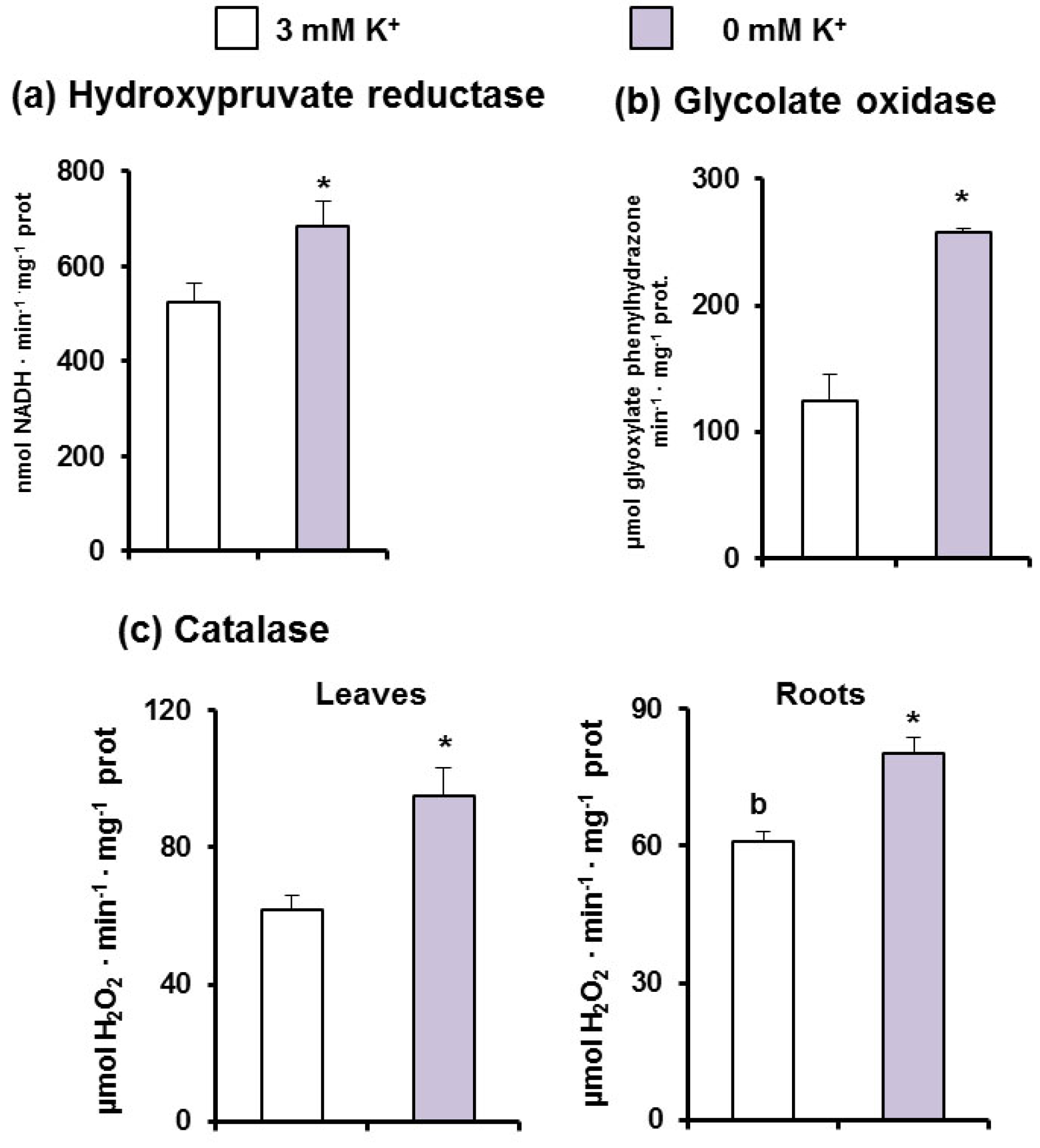
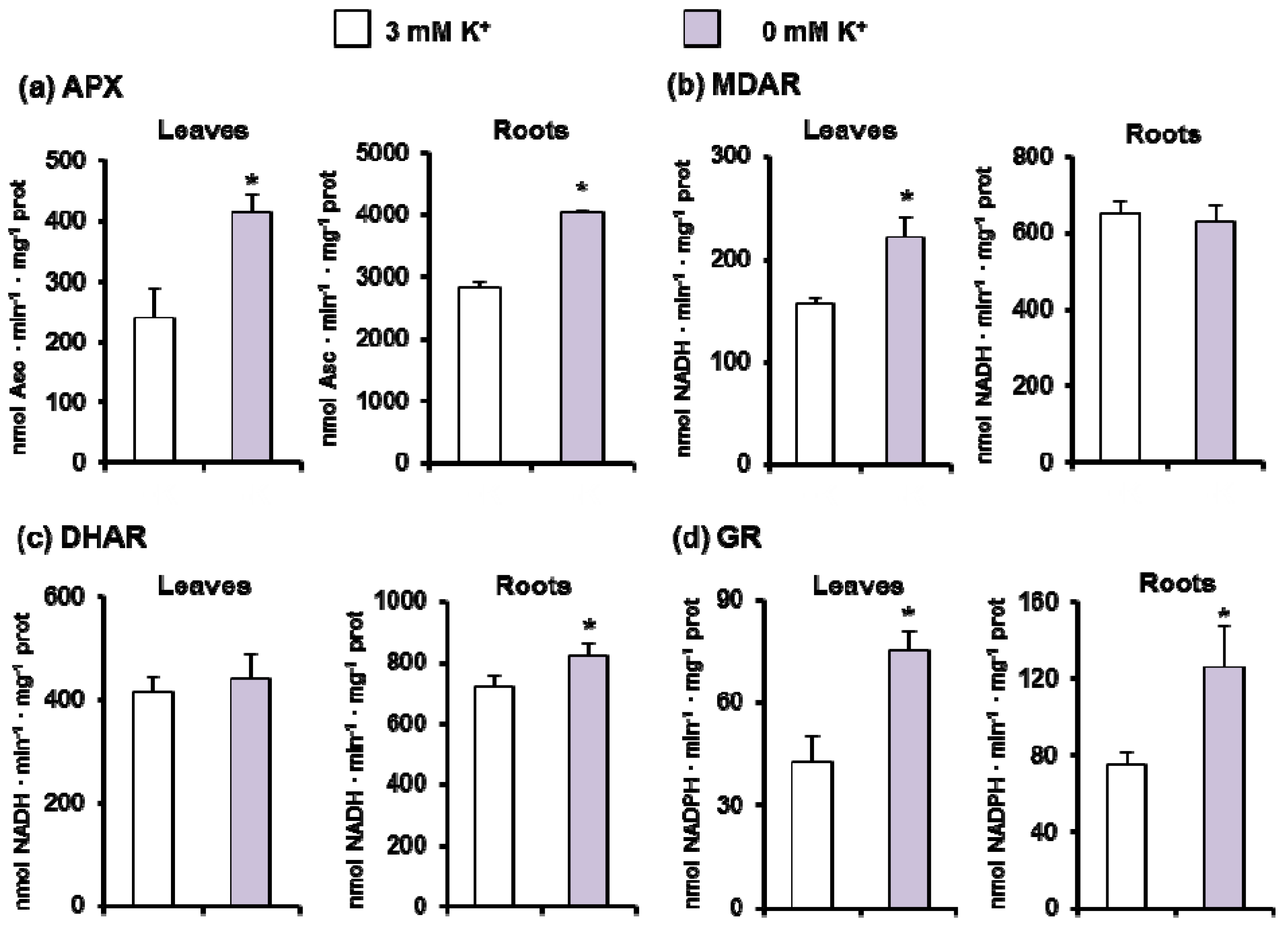
3.2. Metabolism of ROS and Photorespiration under K+ Deficiency in C. maritima
3.3. Metabolism of NADP-Dehydrogenases under K+ Deficiency in C. maritima
4. Discussion
4.1. K+ Deficiency Alters C. maritima Growth and Induces Oxidative Stress
4.2. Oxidative Stress Is Overcome by a General Induction of the Enzymatic Antioxidant System
4.3. K+ Deficiency Triggers a General Increase of the NADPH-Generating Systems
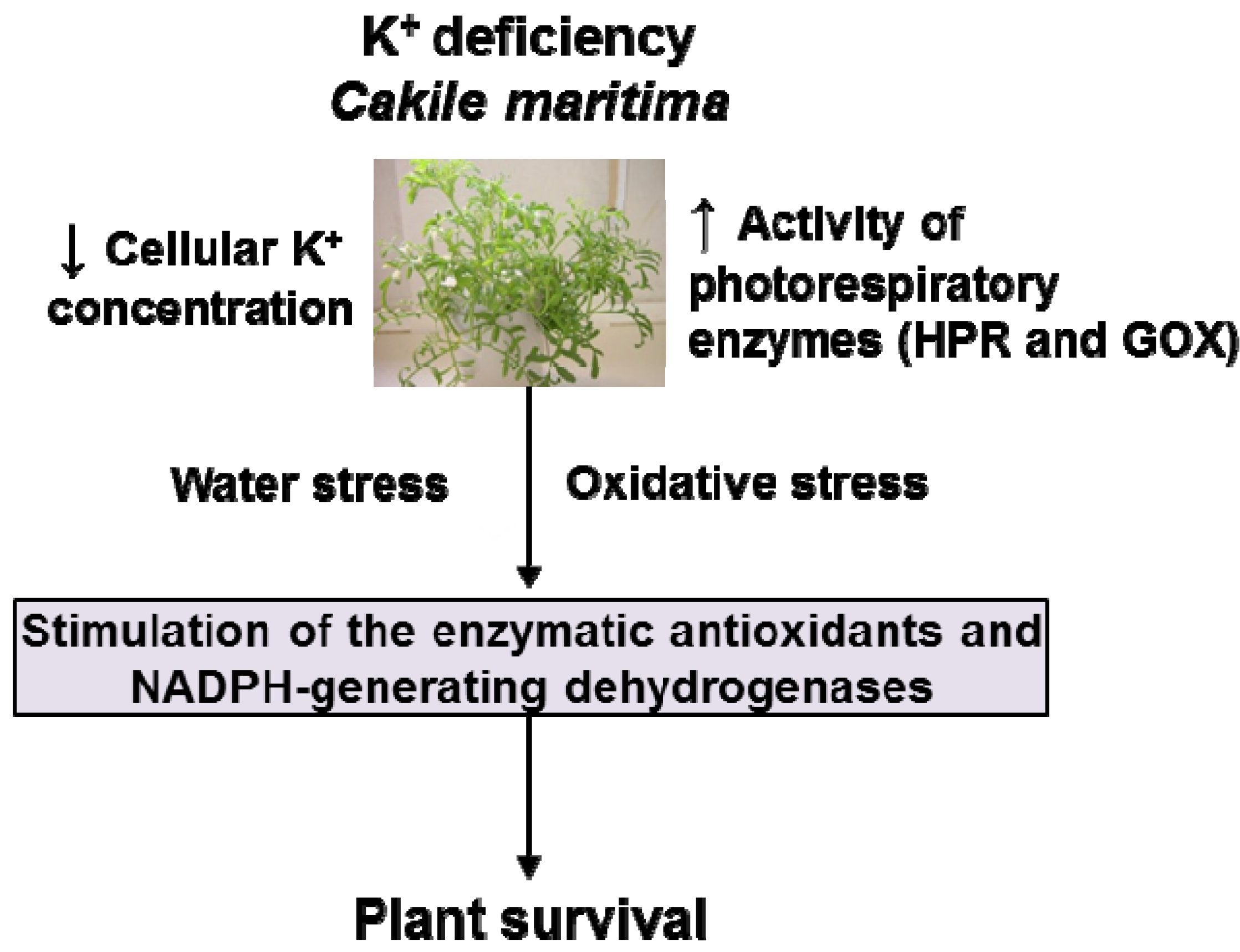
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ragel, P.T.; Raddatz, N.T.; Leidi, E.O.T.; Quintero, F.J.T.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.; Mahmud, J.; Hossen, M.; Masud, A.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Alberdi, M.; Arce-Johnson, P.; Romero, J.; Reyes-Díaz, M.; Rengel, Z.; Inostroza-Blancheteau, C. Role of potassium in governing photosynthetic processes and plant yield. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 191–203. [Google Scholar] [CrossRef]
- Lana, L.G.; de Araújo, L.M.; Silva, T.F.; Modolo, L.V. Interplay between gasotransmitters and potassium is a Key factor during plant response to abiotic stress. Plant Phys. Biochem. 2021, 169, 322–332. [Google Scholar] [CrossRef]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Yadav, B.K.; Sidhu, A.S. Dynamics of potassium and their bioavailability for plant nutrition. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 187–201. [Google Scholar] [CrossRef]
- Hafsi, C.; Romero-Puertas, M.C.; del Río, L.A.; Abdelly, C.; Sandalio, L.M. Antioxidative response of Hordeum martitimum L. to potassium deficiency. Acta Physiol. Plant. 2011, 33, 193–202. [Google Scholar] [CrossRef]
- Hafsi, C.; Falleh, H.; Saada, M.; Ksouri, R.; Abdelly, C. Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol. Biochem. 2017, 118, 609–617. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, A.-J.; Chen, X.G.; Jin, R.; Li, H.M.; Tang, Z.H. Effects of potassium deficiency on root morphology, ultrastructure and antioxidant enzyme system in sweet potato (Ipomoea batatas [L.] Lam.) during early growth. Acta Physiol. Plant. 2017, 39, 211. [Google Scholar] [CrossRef]
- Li, L.Q.; Lyu, C.C.; Li, J.H.; Wan, C.Y.; Liu, L.; Xie, M.Q.; Zuo, R.J.; Ni, S.; Liu, F.; Zeng, F.C.; et al. Quantitative proteomic analysis of alligator weed leaves reveals that cationic peroxidase 1 plays vital roles in the potassium deficiency stress response. Int. J. Mol. Sci. 2020, 21, 2537. [Google Scholar] [CrossRef]
- Houmani, H.; Debez, A.; Slatni, T.; Yousfi, S.; Jellali, N.; M’sehli, W.; Abdelly, C.; Gharsalli, M. Insights into physiological responses of the halophyte Suaeda fruticosa to simultaneous salinity and iron deficiency. CLEAN–Soil Air Water 2015, 43, 382–390. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Santini, R.; de Lima, J.P.; Gratão, P.L.; Camargo, A.F.M. Evaluation of growth and oxidative stress as indicative of salinity tolerance by the invasive tropical aquatic macrophyte tanner grass. Hydrobiologia 2022, 1–11. [Google Scholar] [CrossRef]
- Kharbech, O.; Houmani, H.; Chaoui, A.; Corpas, F.J. Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J. Plant Physiol. 2017, 219, 71–80. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. Mechanical wounding promotes local and long distance response in the halophyte Cakile maritima through the involvement of the ROS and RNS metabolism. Nitric Oxide. 2018, 74, 93–101. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Functional implications of peroxisomal nitric oxide (NO) in plants. Front. Plant Sci. 2014, 5, 97. [Google Scholar] [CrossRef]
- Corpas, F.J.; Aguayo-Trinidad, S.; Ogawa, T.; Yoshimura, K.; Shigeoka, S. Activation of NADPH-recycling systems in leaves and roots of Arabidopsis thaliana under arsenic-induced stress conditions is accelerated by knock-out of Nudix hydrolase 19 (AtNUDX19) gene. J. Plant Physiol. 2016, 192, 81–89. [Google Scholar] [CrossRef]
- de Freitas-Silva, L.; Rodríguez-Ruiz, M.; Houmani, H.; da Silva, L.C.; Palma, J.M.; Corpas, F.J. Glyphosate-induced oxidative stress in Arabidopsis thaliana affecting peroxisomal metabolism and triggers activity in the oxidative phase of the pentose phosphate pathway (OxPPP) involved in NADPH generation. J. Plant Physiol. 2017, 218, 196–205. [Google Scholar] [CrossRef]
- Ruíz-Torres, C.; Feriche-Linares, R.; Rodríguez-Ruíz, M.; Palma, J.M.; Corpas, F.J. Arsenic-induced stress activates sulfur metabolism in different organs of garlic (Allium sativum L.) plants accompanied by a general decline of the NADPH-generating systems in roots. J. Plant Physiol. 2017, 211, 27–35. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Palma, J.M.; Corpas, F.J. NADPH as a quality footprinting in horticultural crops marketability. Trends Food Sci. Technol. 2020, 103, 152–161. [Google Scholar] [CrossRef]
- Cardi, M.; Castiglia, D.; Ferrara, M.; Guerriero, G.; Chiurazzi, M.; Esposito, S. The effects of salt stress cause a diversion of basal metabolism in barley roots: Possible different roles for glucose-6-phosphate dehydrogenase isoforms. Plant Physiol. Biochem. 2015, 86, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S. Nitrogen Assimilation, abiotic stress and glucose 6-phosphate dehydrogenase: The full circle of reductants. Plants 2016, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Mahato, A.K.; Sinha, S.K.; Dalal, M.; Singh, N.K.; Mandal, P.K. Homeologue specific gene expression analysis of two vital carbon metabolizing enzymes—Citrate synthase and NADP-isocitrate dehydrogenase—From wheat (Triticum aestivum L.) under nitrogen stress. Appl. Biochem. Biotechnol. 2019, 188, 569–584. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J. Exp. Bot. 2021, 72, 830–847. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Yan, H.; Jiang, X.; Ma, Y.; Qin, Y. Carbon and nitrogen metabolism under nitrogen variation affects flavonoid accumulation in the leaves of Coreopsis tinctoria. PeerJ 2021, 9, e12152. [Google Scholar] [CrossRef] [PubMed]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Abdelly, C.; Corpas, F.J. Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 2016, 3, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Debez, A.; Belghith, I.; Pich, A.; Taamalli, W.; Abdelly, C.; Braun, H.P. High salinity impacts germination of the halophyte Cakile maritima but primes seeds for rapid germination upon stress release. Physiol. Plant. 2018, 164, 134–144. [Google Scholar] [CrossRef]
- Arbelet-Bonnin, D.; Ben-Hamed-Louati, I.; Laurenti, P.; Abdelly, C.; Ben-Hamed, K.; Bouteau, F. Cakile maritima, a promising model for halophyte studies and a putative cash crop for saline agriculture. Adv. Agron. 2019, 155, 45–78. [Google Scholar] [CrossRef]
- Farhat, N.; Belghith, I.; Senkler, J.; Hichri, S.; Abdelly, C.; Braun, H.P.; Debez, A. Recovery aptitude of the halophyte Cakile maritima upon water deficit stress release is sustained by extensive modulation of the leaf proteome. Ecotoxicol. Environ. Saf. 2019, 179, 198–211. [Google Scholar] [CrossRef]
- Agudelo, A.; Carvajal, M.; Martinez-Ballesta, M.D.C. Halophytes of the Mediterranean basin-underutilized species with the potential to be nutritious crops in the scenario of the climate change. Foods 2021, 10, 119. [Google Scholar] [CrossRef]
- Zitouni, M.; Wewer, V.; Dörmann, P.; Abdelly, C.; Ben Youssef, N. Quadrupole time-of-flight mass spectrometry analysis of glycerophospholipid molecular species in the two halophyte seed oils: Eryngium maritimum and Cakile maritima. Food Chem. 2016, 213, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Debez, A.; Rejeb, K.B.; Ghars, M.A.; Gandour, M.; Megdiche, W.; Hamed, K.B.; Ben Amor, N.; Brown, S.C.; Savouré, A.; Abdelly, C. Ecophysiological and genomic analysis of salt tolerance of Cakile maritima. Environ. Exp. Bot. 2013, 92, 64–72. [Google Scholar] [CrossRef]
- Debez, A.; Koyro, H.W.; Grignon, C.; Abdelly, C.; Huchzermeyer, B. Relationship between the photosynthetic activity and the performance of Cakile maritima after long-term salt treatment. Physiol. Plant. 2008, 133, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; Rehman, H.U.; Bilal, H.M. Synergistic consequences of salinity and potassium deficiency in quinoa: Linking with stomatal patterning, ionic relations and oxidative metabolism. Plant Physiol. Biochem. 2021, 159, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Vargas, W.A.; Martín, J.M.; Rech, G.E.; Rivera, L.P.; Benito, E.P.; Díaz-Mínguez, J.M.; Thon, M.R.; Sukno, S.A. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiol. 2012, 158, 1342–1358. [Google Scholar] [CrossRef]
- Gould, K.S.; Markham, K.R.; Smith, R.H.; Goris, J.J. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 2000, 51, 1107–1115. [Google Scholar] [CrossRef]
- Beauchamp, C.O.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Ádám, A.L.; Bestwick, C.S.; Barna, B.; Mansfield, J.W. Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. Phaseolicola. Planta 1995, 7, 240–249. [Google Scholar] [CrossRef]
- Bieker, S.; Riester, L.; Stahl, M.; Franzaring, J.; Zentgraf, U. Senescence specific-Alteration of hydrogen peroxide levels in Arabidopsis thaliana and oilseed rape spring variety Brassica napus L. cv. Mozart F. J. Integr. Plant Biol. 2012, 54, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.W.; Groves, D. Purification and properties of glycollate oxidase from Pisum sativum leaves. Phytochemistry 1975, 14, 359–362. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol. 1984, 25, 1285–1295. [Google Scholar] [CrossRef]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar] [CrossRef]
- Edwards, E.A.; Rawsthone, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Dalton, D.A.; Baird, L.M.; Langeberg, L.; Taugher, C.Y.; Anyan, W.R.; Vance, C.V.; Sarath, G. Subcellular localization of oxygen defense enzymes in soybean (Glycine max L. Merr) root nodules. Plant Physiol. 1993, 102, 481–489. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragón, J.; Contreras-Jurado, C.; García-Salguero, L.; Corpas, F.J.; Esteban, F.J.; Peinado, M.A.; De La Higuera, M.; Lupiáñez, J.A. Impact of starvation-refeeding on kinetics and protein expression of trout liver NADPH-production systems. Am. J. Physiol. 1998, 274, 1578–1587. [Google Scholar] [CrossRef]
- Leterrier, M.; Barroso, J.B.; Valderrama, R.; Palma, J.M.; Corpas, F.J. NADP-dependent isocitrate dehydrogenase from Arabidopsis roots contributes in the mechanism of defence against the nitro-oxidative stress induced by salinity. Sci. World J. 2012, 2012, 694740. [Google Scholar] [CrossRef]
- Mateos, R.M.; Bonilla-Valverde, D.; del Río, L.A.; Palma, J.M.; Corpas, F.J. NADP-dehydrogenases from pepper fruits: Effect of maturation. Physiol. Plant. 2009, 135, 130–139. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef]
- Tavakol, E.; Jákli, B.; Cakmak, I.; Dittert, K.; Karlovsky, P.; Pfohl, K.; Senbayram, M. Optimized potassium nutrition improves plant-water-relations of barley under PEG-induced osmotic stress. Plant Soil 2018, 430, 23–35. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Arquero, O.; Fournier, J.M.; Barranco, D.; Benlloch, M. K+ starvation inhibits water-stress-induced stomatal closure. J. Plant Physiol. 2008, 165, 623–630. [Google Scholar] [CrossRef]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiol. 2011, 31, 1142–1151. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 74452. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Aranda-Sicilia, M.N.; Aboukila, A.; Armbruster, U.; Cagnac, O.; Schumann, T.; Kunz, H.H.; Jahns, P.; Rodríguez-Rosales, M.P.; Sze, H.; Venema, K. Envelope K+/H+ ntiporters AtKEA1 and AtKEA2 function in plastid development. Plant Physiol. 2016, 172, 441–449. [Google Scholar] [CrossRef]
- Sánchez-McSweeney, A.; González-Gordo, S.; Aranda-Sicilia, M.N.; Rodríguez-Rosales, M.P.; Venema, K.; Palma, J.M.; Corpas, F.J. Loss of function of the chloroplast membrane K+/H+ antiporters AtKEA1 and AtKEA2 alters the ROS and NO metabolism but promotes drought stress resilience. Plant Physiol. Biochem. 2021, 160, 106–119. [Google Scholar] [CrossRef]
- Ma, T.L.; Wu, W.H.; Wang, Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012, 12, 161. [Google Scholar] [CrossRef]
- Mahiwal, S.; Pandey, G.K. Potassium: An emerging signal mediator in plants? In Plant Nutrition and Food Security in the Era of Climate Change; Academic Press: Cambridge, MA, USA, 2022; pp. 97–118. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Plant sensing and signaling in response to K+-deficiency. Mol. Plant 2010, 3, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.U.; Zhao, X.H.; Le, X.I.A.; Jiang, C.J.; Wang, X.G.; Yi, H.A.N.; Jing, W.A.N.G.; Yu, H.Q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Hernández, M.; Fernández-García, N.; García-Garma, J.; Rubio-Asensio, J.S.; Rubio, F.; Olmos, E. Potassium starvation induces oxidative stress in Solanum lycopersicum L. roots. J. Plant Physiol. 2012, 169, 1366–1374. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Hu, Z.; Wang, S.; Zhang, J.; Wang, X.; Wang, Q.; Zhang, B. Lack of K-dependent oxidative stress in cotton roots following coronatine-induced ROS accumulation. PLoS ONE 2015, 10, e0126476. [Google Scholar] [CrossRef]
- Gong, X.; Chao, L.; Zhou, M.; Hong, M.; Luo, L.; Wang, L.; Ying, W.; Jingwei, C.; Songjie, G.; Fashui, H. Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 2011, 340, 443–452. [Google Scholar] [CrossRef]
- Perveen, S.; Shahbaz, M.; Ashraf, M. Is pre-sowing seed treatment with triacontanol effective in improving some physiological and biochemical attributes of wheat (Triticum aestivum L.) under salt stress? J. Appl. Bot. Food Qual. 2012, 85, 418. [Google Scholar]
- Akram, N.A.; Ashraf, M.; Al-Qurainy, F. Aminolevulinic acid-induced regulation in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) under saline regimes. Sci. Hort. 2012, 142, 1438. [Google Scholar] [CrossRef]
- Miao, B.H.; Han, X.G.; Zhang, W.H. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 2010, 105, 967–973. [Google Scholar] [CrossRef]
- Ferreira, R.M.B. The Metabolic Responses of Plants to Stress, with Particular Reference to Protein Turnover. Ph.D. Thesis, University of East Anglia, Norwich, UK, 1987. [Google Scholar]
- Malnoë, A. Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 2018, 154, 123–133. [Google Scholar] [CrossRef]
- Singh, P.; Blanke, M. Deficiency of potassium but not phosphorus enhances root respiration. Plant Growth Regul. 2000, 32, 77–81. [Google Scholar] [CrossRef]
- Gould, K.S.; McKelvie, J.; Markham, K.R. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ. 2002, 25, 1261–1269. [Google Scholar] [CrossRef]
- Li, Y.C.; Lin, T.C.; Martin, C.E. Leaf anthocyanin, photosynthetic light-use efficiency, and ecophysiology of the South African succulent Anacampseros rufescens (Anacampserotaceae). S. Afr. J. Bot. 2015, 99, 122–128. [Google Scholar] [CrossRef]
- Corpas, F.J. What is the role of hydrogen peroxide in plant peroxisomes? Plant Biol. 2015, 17, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Palma, J.M.; Corpas, F.J. Editorial: Subcellular Compartmentalization of plant antioxidants and ROS generating systems. Front. Plant Sci. 2021, 12, 643239. [Google Scholar] [CrossRef]
- Liu, C.H.; Chao, Y.Y.; Kao, C.H. Effect of potassium deficiency on antioxidant status and cadmium toxicity in rice seedlings. Bot. Stud. 2013, 54, 2. [Google Scholar] [CrossRef][Green Version]
- Özgüruzilda, R.; Uzilday, B.; Yalçinkaya, T.; Türkan, I. Mg deficiency changes the isoenzyme pattern of reactive oxygen species-related enzymes and regulates NADPH oxidase-mediated ROS signaling in cotton. Turk. J. Biol. 2017, 41, 868–880. [Google Scholar] [CrossRef]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Nixon, P.J. Photosynthesis solutions to enhance productivity. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 3–6. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Ding, Y.C.; Chang, C.R.; Luo, W.; Wu, Y.S.; Ren, X.L.; Wang, P.; Xu, G.H. High potassium aggravates the oxidative stress induced by magnesium deficiency in rice leaves. Pedosphere 2008, 18, 316–327. [Google Scholar] [CrossRef]
- Ahmad, I.; Maathuis, F.J. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Singh, A.; Kanwar, P.; Srivastava, A.K.; Pandey, A.; Suprasanna, P.; Kapoor, S.; Pandey, G.K. Gene expression analysis of rice seedling under potassium deprivation reveals major changes in metabolism and signalling components. PLoS ONE 2013, 8, e70321. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lyu, C.; Huang, L.; Chen, Q.; Zhuo, W.; Wang, X.; Lu, Y.; Zeng, F.; Lu, L. Physiology and proteomic analysis reveals root, stem and leaf responses to potassium deficiency stress in alligator weed. Sci. Rep. 2019, 9, 17366. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; del Río, L.A.; Palma, J.M. Impact of Nitric Oxide (NO) on the ROS Metabolism of Peroxisomes. Plants 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Breitling, R.; Amtmann, A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Vaahtera, L.; Brosche, M.; Wrzaczek, M.; Kangasjarvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal. 2014, 21, 1422–1441. [Google Scholar] [CrossRef]
- Considine, M.J.; Sandalio, L.M.; Foyer, C.H. Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 2015, 116, 469–473. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idanheimo, N.; Hoeberichts, F.A.; Muhlenbock, P.; Brosche, M.; Van Breusegem, F.; Kangasjarvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; López-Delacalle, M.; Ródenas, R.; Martínez, V.; Rubio, F.; Rivero, R.M. Critical responses to nutrient deprivation: A comprehensive review on the role of ROS and RNS. Environ. Exp. Bot. 2019, 161, 74–85. [Google Scholar] [CrossRef]
- Jung, J.Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Kim, M.J.; Ciani, S.; Schachtman, D.P.A. peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol. Plant 2010, 3, 420–427. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Gómez-Rodríguez, M.V.; Chaki, M.; Pedrajas, J.R.; Fernández-Ocaña, A.; del Río, L.A.; Barroso, J.B. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 2006, 29, 1449–1459. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013, 201, 137–146. [Google Scholar] [CrossRef]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef]
- Leterrier, M.; Barroso, J.B.; Valderrama, R.; Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Luque, F.; Viñegla, B.; Palma, J.M.; Corpas, F.J. Peroxisomal NADP-isocitrate dehydrogenase is required for Arabidopsis stomatal movement. Protoplasma 2016, 253, 403–415. [Google Scholar] [CrossRef]
- Manai, J.; Gouia, H.; Corpas, F.J. Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J. Plant Physiol. 2014, 171, 1028–1035. [Google Scholar] [CrossRef]
- Bouthour, D.; Kalai, T.; Chaffei, H.C.; Gouia, H.; Corpas, F.J. Differential response of NADP-dehydrogenases and carbon metabolism in leaves and roots of two durum wheat (Triticum durum Desf.) cultivars (Karim and Azizi) with different sensitivities to salt stress. J. Plant Physiol. 2015, 179, 56–63. [Google Scholar] [CrossRef]
- Li, L.Q.; Liu, L.; Zhuo, W.; Chen, Q.; Hu, S.; Peng, S.; Wang, X.Y.; Lu, Y.F.; Lu, L.M. Physiological and quantitative proteomic analyses unraveling potassium deficiency stress response in alligator weed (Alternanthera philoxeroides L.) root. Plant Mol. Biol. 2018, 97, 265–278. [Google Scholar] [CrossRef]



| Organs | 3 mM K+ | 0 mM K+ |
|---|---|---|
| Shoot K content (mg g−1 dry weight) | 4.05 ± 0.14 | 0.46 ± 0.04 * |
| Root K content (mg g−1 dry weight) | 3.89 ± 0.57 | 0.56 ± 0.09 * |
| Shoot K concentration (mg mL−1) | 0.43 ± 0.02 | 0.065 ± 0.003 * |
| Root K concentration (mg mL−1) | 0.29 ± 0.02 | 0.063 ± 0.010 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houmani, H.; Debez, A.; Freitas-Silva, L.d.; Abdelly, C.; Palma, J.M.; Corpas, F.J. Potassium (K+) Starvation-Induced Oxidative Stress Triggers a General Boost of Antioxidant and NADPH-Generating Systems in the Halophyte Cakile maritima. Antioxidants 2022, 11, 401. https://doi.org/10.3390/antiox11020401
Houmani H, Debez A, Freitas-Silva Ld, Abdelly C, Palma JM, Corpas FJ. Potassium (K+) Starvation-Induced Oxidative Stress Triggers a General Boost of Antioxidant and NADPH-Generating Systems in the Halophyte Cakile maritima. Antioxidants. 2022; 11(2):401. https://doi.org/10.3390/antiox11020401
Chicago/Turabian StyleHoumani, Hayet, Ahmed Debez, Larisse de Freitas-Silva, Chedly Abdelly, José M. Palma, and Francisco J. Corpas. 2022. "Potassium (K+) Starvation-Induced Oxidative Stress Triggers a General Boost of Antioxidant and NADPH-Generating Systems in the Halophyte Cakile maritima" Antioxidants 11, no. 2: 401. https://doi.org/10.3390/antiox11020401
APA StyleHoumani, H., Debez, A., Freitas-Silva, L. d., Abdelly, C., Palma, J. M., & Corpas, F. J. (2022). Potassium (K+) Starvation-Induced Oxidative Stress Triggers a General Boost of Antioxidant and NADPH-Generating Systems in the Halophyte Cakile maritima. Antioxidants, 11(2), 401. https://doi.org/10.3390/antiox11020401








