Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Basil Supercritical Fluid Extract (SFE)
2.2. Characterization of VOCs in F1-BEO
2.3. Content and Composition of Fatty Acids in F1-BEO
2.4. Determination of Bioactive Compounds in F1-BEO
2.4.1. Extract Preparation
2.4.2. Total Polyphenol Content
2.4.3. Total Flavan-3-ols Content
2.5. Spectrophotometric Evaluation of Antioxidant Power
2.5.1. Radical Scavenging Activity
2.5.2. Reducing Activity
2.6. Identification of Polyphenolic Compounds
2.7. Diet Formulation and Rainbow Trout Nutrition
2.8. Oxidative Stress Biomarkers
2.8.1. Preparation of Fish Tissue Extracts
2.8.2. Evaluation of SOD Activity
2.8.3. Evaluation of CAT Activity
2.8.4. Evaluation of GPx Activity
2.8.5. Evaluation of GST Activity
2.8.6. Evaluation of GR Activity
2.8.7. Evaluation of GI Activity
2.8.8. Evaluation of GII Activity
2.8.9. Evaluation of LDH Activity
2.8.10. Evaluation of GSH + 2GSSG Concentration
2.8.11. Determination of MDA Levels
2.9. Statistical Analysis
3. Results
3.1. Chemical Profiling of F1-BEO
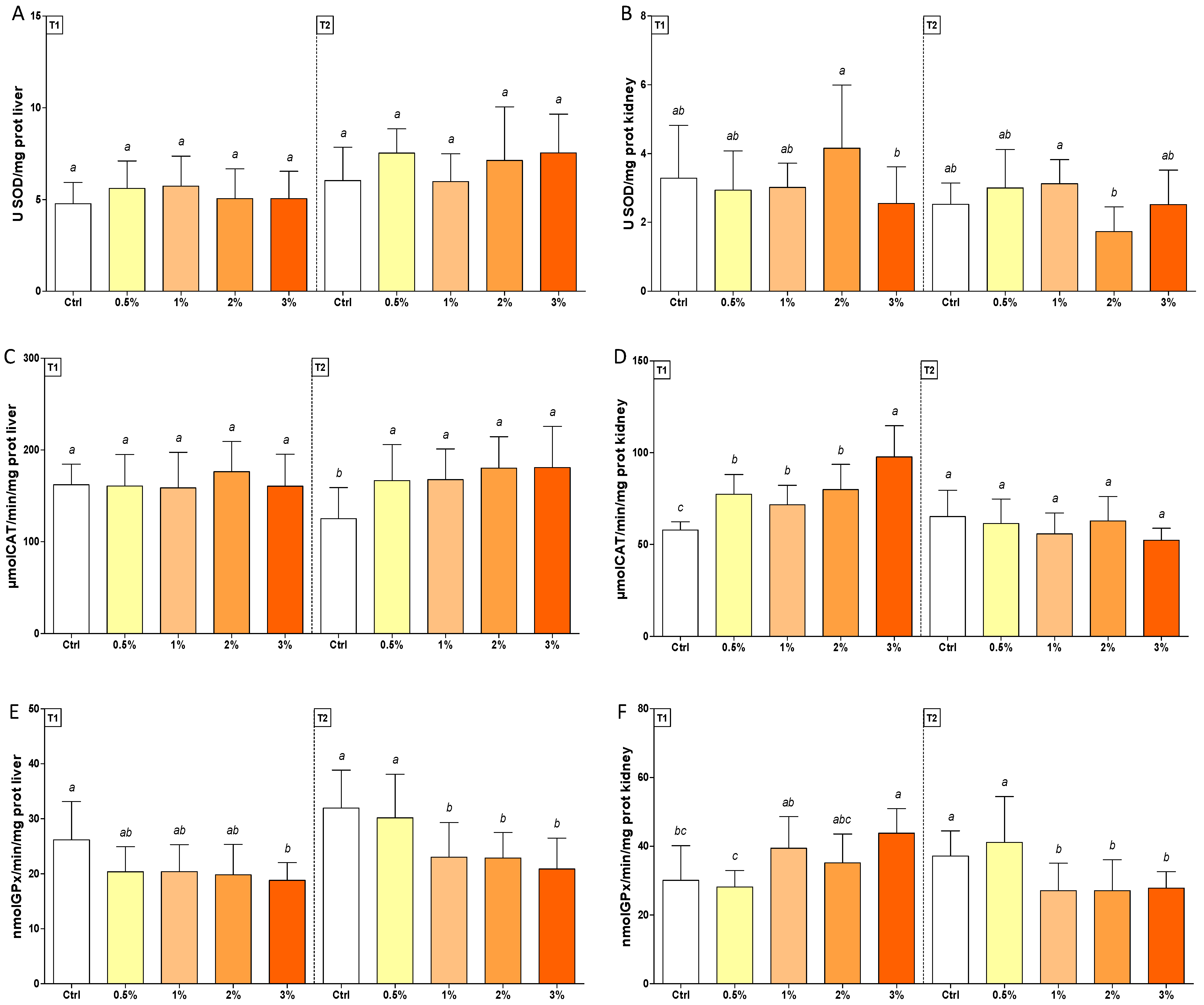
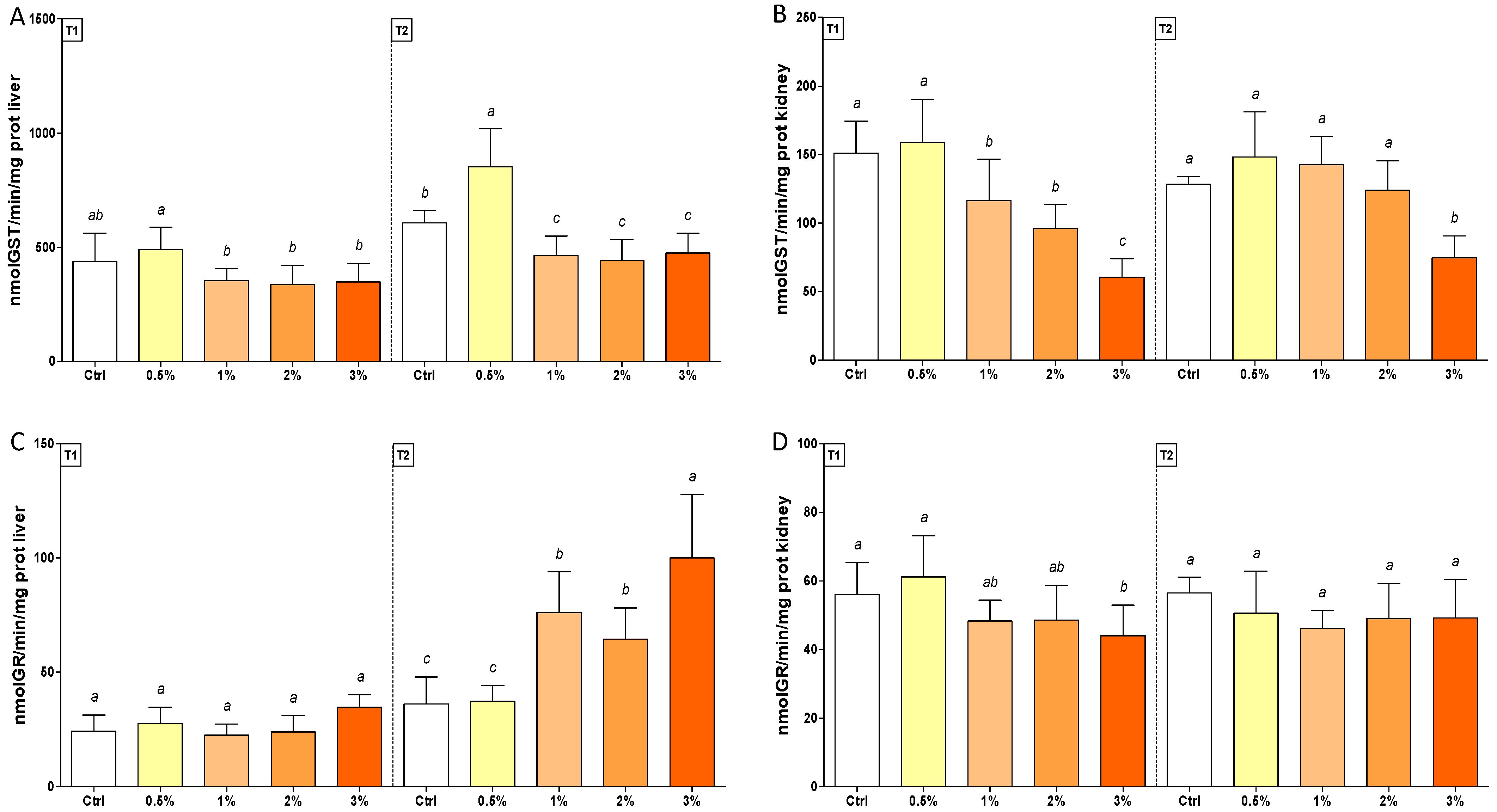
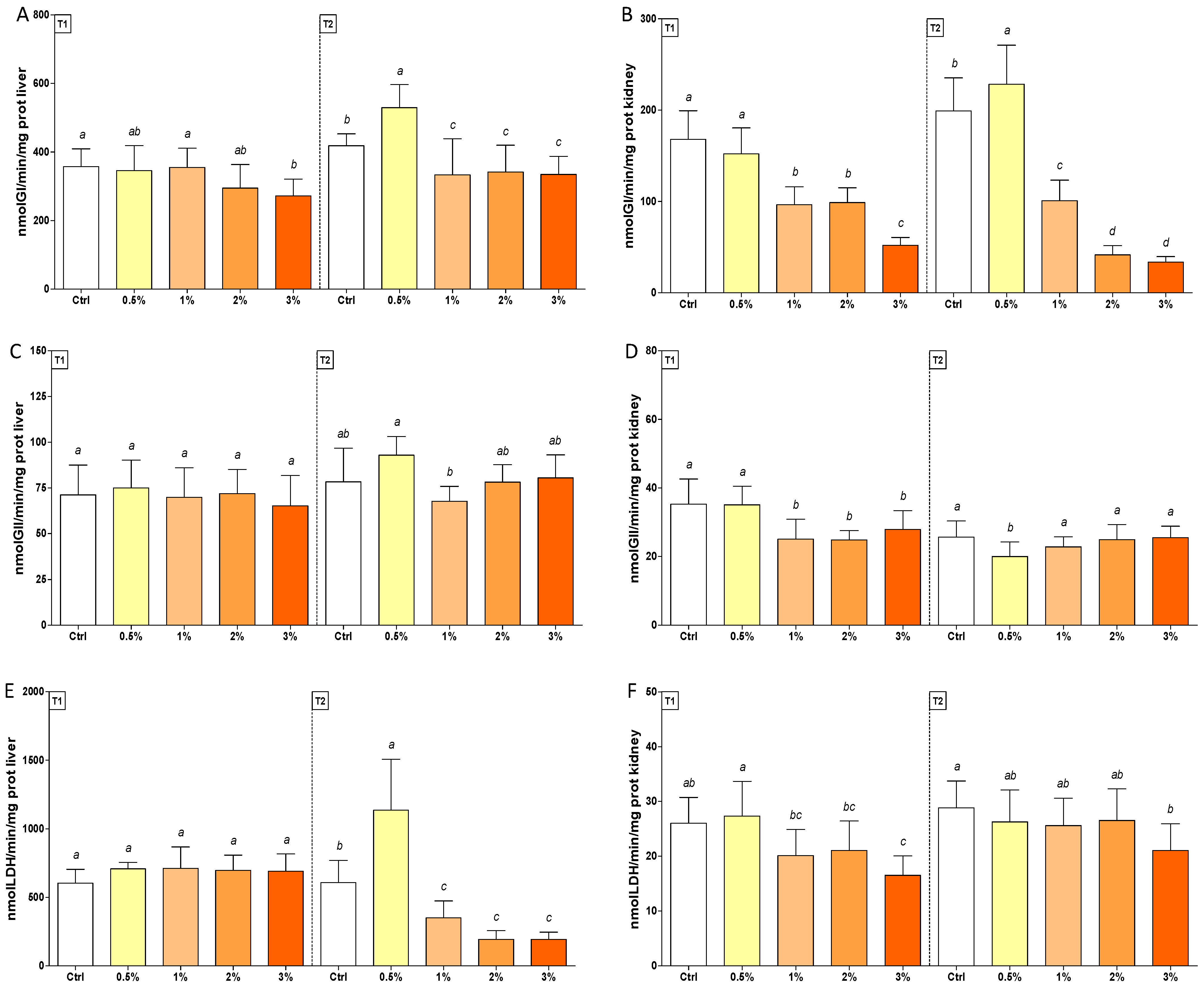
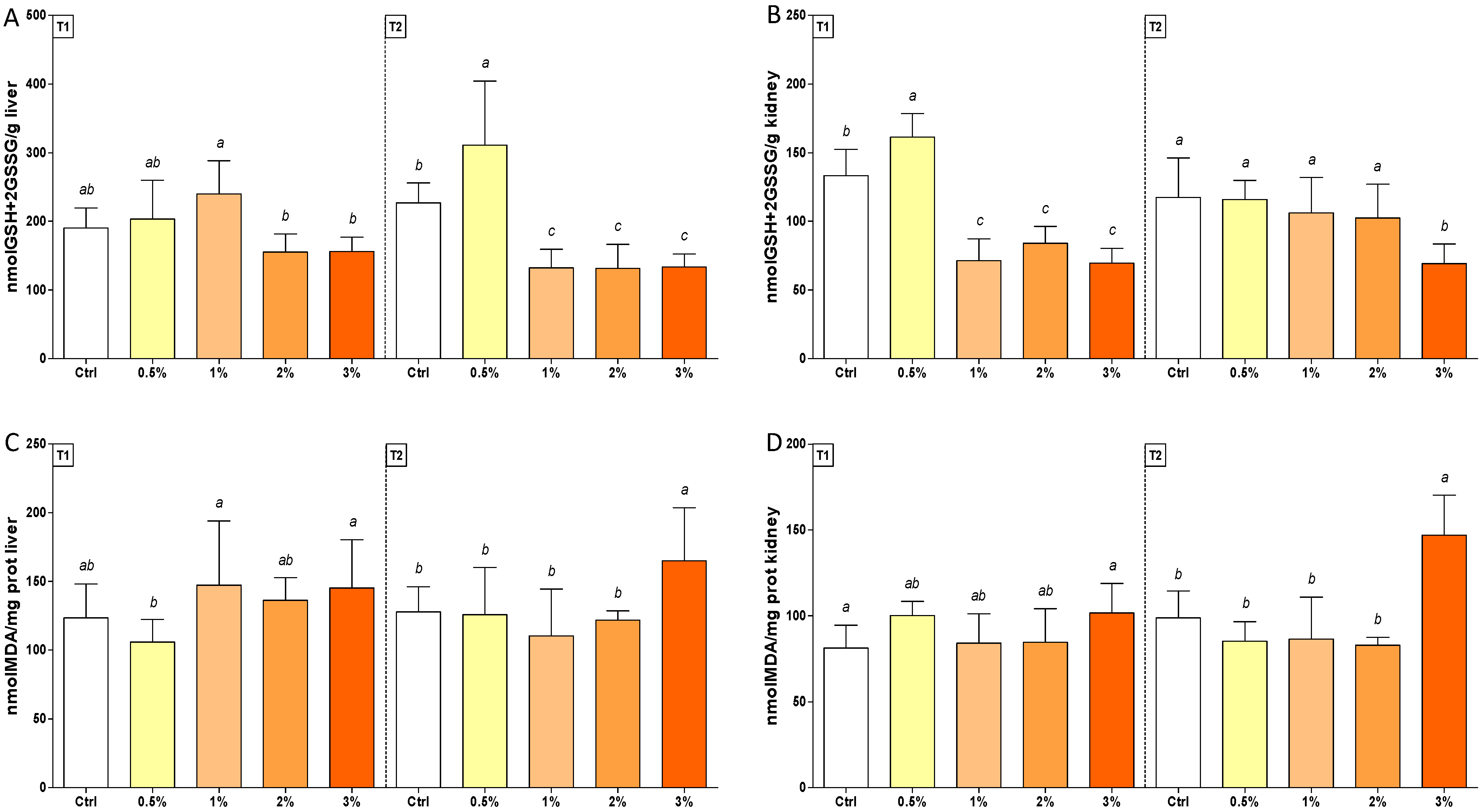
3.2. Changes in Oxidative Stress Biomarkers in Rainbow Trout after Feeding with F1-BEO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization Sustainability in Action. State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; Volume 200. [Google Scholar]
- Ahmed, N.; Thompson, S.; Glaser, M. Global Aquaculture Productivity, Environmental Sustainability, and Climate Change Adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Hwang, S.Y.; Hong, M.K.; Kwon, K.H. Use of Antimicrobial Agents in Aquaculture. Rev. Sci. Tech. (Int. Off. Epizoot.) 2012, 31, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicuro, B.; Pastorino, P.; Barbero, R.; Barisone, S.; Dellerba, D.; Menconi, V.; Righetti, M.; De Vita, V.; Prearo, M. Prevalence and Antibiotic Sensitivity of Bacteria Isolated from Imported Ornamental Fish in Italy: A Translocation of Resistant Strains? Prev. Vet. Med. 2020, 175, 104880. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.Y.; Teng, L.; Fateeva, T.V.; Brown, J.C.; Job, K.M.; Bortnikova, V.V.; Krepkova, L.V.; Gubarev, M.I.; Sherwin, C.M. Phytotherapy as an Alternative to Conventional Antimicrobials: Combating Microbial Resistance. Expert Rev. Clin. Pharmacol. 2017, 10, 1203–1214. [Google Scholar] [CrossRef]
- Chevalley, A. Utilisation de la Phytothérapie et de L’aromathérapie Dans le Cadre du Conseil Vétérinaire Chez le Chat, le Chien et le Cheval. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2016. [Google Scholar]
- Dawood, M.A.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.; Alagawany, M.; Abu-Elala, N.M. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef]
- Gholipourkanani, H.; Buller, N.; Lymbery, A. In Vitro Antibacterial Activity of Four Nano-encapsulated Herbal Essential Oils against Three Bacterial Fish Pathogens. Aquac. Res. 2019, 50, 871–875. [Google Scholar] [CrossRef]
- Jahazi, M.A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Van Doan, H.; Paolucci, M. Dietary Supplementation of Polyphenols Positively Affects the Innate Immune Response, Oxidative Status, and Growth Performance of Common Carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Lee, S.; Najiah, M.; Wendy, W.; Nadirah, M. Chemical Composition and Antimicrobial Activity of the Essential Oil of Syzygium aromaticum Flower Bud (Clove) against Fish Systemic Bacteria Isolated from Aquaculture Sites. Front. Agric. China 2009, 3, 332–336. [Google Scholar] [CrossRef]
- Majolo, C.; Pilarski, F.; Chaves, F.C.M.; Bizzo, H.R.; Chagas, E.C. Antimicrobial Activity of Some Essential Oils against Streptococcus agalactiae, an Important Pathogen for Fish Farming in Brazil. J. Essent. Oil Res. 2018, 30, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Meguro, S.; Hasumura, T.; Hase, T. Coffee Polyphenols Exert Hypocholesterolemic Effects in Zebrafish Fed a High-Cholesterol Diet. Nutr. Metab. 2013, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarrete, P.; Toledo, I.; Mardones, P.; Opazo, R.; Espejo, R.; Romero, J. Effect of Thymus vulgaris Essential Oil on Intestinal Bacterial Microbiota of Rainbow Trout, Oncorhynchus mykiss (Walbaum) and Bacterial Isolates. Aquac. Res. 2010, 41, e667–e678. [Google Scholar] [CrossRef]
- Schiewe, M.H.; Crosa, J.H. Vibrio ordalii sp. Nov.: A Causative Agent of Vibriosis in Fish. Curr. Microbiol. 1981, 6, 343–348. [Google Scholar] [CrossRef]
- Wei, L.S.; Wee, W. Chemical Composition and Antimicrobial Activity of Cymbopogon nardus Citronella Essential Oil against Systemic Bacteria of Aquatic Animals. Iran. J. Microbiol. 2013, 5, 147. [Google Scholar] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Dietary Inclusion of Chestnut (Castanea sativa) Polyphenols to Nile Tilapia Reared in Biofloc Technology: Impacts on Growth, Immunity, and Disease Resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef]
- Öntaş, C.; Baba, E.; Kaplaner, E.; Küçükaydin, S.; Öztürk, M.; Ercan, M.D. Antibacterial Activity of Citrus limon Peel Essential Oil and Argania spinosa Oil against Fish Pathogenic Bacteria. Kafkas Üniv. Vet. Fak. Derg. 2016, 22, 741–749. [Google Scholar]
- El-Ekiaby, W.T. Basil Oil Nanoemulsion Formulation and Its Antimicrobial Activity against Fish Pathogen and Enhance Disease Resistance against Aeromonas hydrophila in Cultured Nile Tilapia. Egypt. J. Aquac. 2019, 9, 13–33. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A. Dietary Origanum Essential Oil Improved Antioxidative Status, Immune-Related Genes, and Resistance of Common Carp (Cyprinus carpio L.) to Aeromonas hydrophila Infection. Fish Shellfish Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef]
- Diler, O.; Gormez, O.; Diler, I.; Metin, S. Effect of Oregano (Origanum onites L.) Essential Oil on Growth, Lysozyme and Antioxidant Activity and Resistance against Lactococcus garvieae in Rainbow Trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutr. 2017, 23, 844–851. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.W.; Liu, L.L.; Cao, Y.C.; Zhu, H. Dietary Oregano Essential Oil Improved the Immune Response, Activity of Digestive Enzymes, and Intestinal Microbiota of the Koi Carp, Cyprinus carpio. Aquaculture 2020, 518, 734781. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Tan, J.Y.; Liu, H.Y.; Zhou, X.H.; Xiang, X.; Wang, K.Y. Evaluation of Oregano Essential Oil (Origanum heracleoticum L.) on Growth, Antioxidant Effect and Resistance against Aeromonas hydrophila in Channel Catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E. Comparison of Extraction Methods for the Rapid Determination of Essential Oil Content and Composition of Basil. J. Am. Soc. Hortic. Sci. 1990, 115, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and Classical Methods for the Extraction of Mitragyna speciosa Leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Devani, B.M.; Jani, B.L.; Balani, P.C.; Akbari, S.H. Optimization of Supercritical CO2 Extraction Process for Oleoresin from Rotten Onion Waste. Food Bioprod. Process. 2020, 119, 287–295. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Orlić, S.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Composition and Antibacterial Activities of Essential Oils of Seven Ocimum Taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Lachowicz, K.J.; Jones, G.P.; Briggs, D.R.; Bienvenu, F.E.; Wan, J.; Wilcock, A.; Coventry, M.J. The Synergistic Preservative Effects of the Essential Oils of Sweet Basil (Ocimum basilicum L.) against Acid-Tolerant Food Microflora. Lett. Appl. Microbiol. 1998, 26, 209–214. [Google Scholar] [CrossRef]
- Tenore, G.C.; Campiglia, P.; Ciampaglia, R.; Izzo, L.; Novellino, E. Antioxidant and Antimicrobial Properties of Traditional Green and Purple “Napoletano” Basil Cultivars (Ocimum basilicum L.) from Campania Region (Italy). Nat. Prod. Res. 2017, 31, 2067–2071. [Google Scholar] [CrossRef]
- Umerie, S.C.; Anaso, H.U.; Anyasoro, L.J.C. Insecticidal Potentials of Ocimum basilicum Leaf-Extract. Bioresour. Technol. 1998, 64, 237–239. [Google Scholar] [CrossRef]
- Vieira, R.F.; Simon, J.E. Chemical Characterization of Basil (Ocimum Spp.) Found in the Markets and Used in Traditional Medicine in Brazil. Econ. Bot. 2000, 54, 207–216. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical Composition and Antioxidant Capacity of Free Volatile Aglycones from Basil (Ocimum basilicum L.) Compared with Its Essential Oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Washburn, P.C.; Wenning, R.J.; Winston, G.W.; Jewell, C.S. Biochemical Responses in Aquatic Animals: A Review of Determinants of Oxidative Stress. Environ. Toxicol. Chem. Int. J. 1989, 8, 1103–1123. [Google Scholar] [CrossRef]
- Ji, L.L. Exercise and Oxidative Stress: Role of the Cellular Antioxidant Systems. Exerc. Sport Sci. Rev. 1995, 23, 135–166. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; He, Y.; Zhang, L.; Lelong, C.; Jouaux, A. Immune and Stress Responses in Oysters with Insights on Adaptation. Fish Shellfish Immunol. 2015, 46, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Pacini, N.; Fioravanti, M.L.; Dörr, A.J.M.; Zaccaroni, A.; Parmeggiani, A.M.; Gustinelli, A.; Mordenti, O.; Abete, M.C.; Prearo, M. Assessment of Detoxifying Markers for Florfenicol in Rainbow Trout Liver. J. Aquat. Anim. Health 2016, 28, 258–265. [Google Scholar] [CrossRef]
- Elia, A.C.; Ciccotelli, V.; Pacini, N.; Dörr, A.J.M.; Gili, M.; Natali, M.; Gasco, L.; Prearo, M.; Abete, M.C. Transferability of Oxytetracycline (OTC) from Feed to Carp Muscle and Evaluation of the Antibiotic Effects on Antioxidant Systems in Liver and Kidney. Fish Physiol. Biochem. 2014, 40, 1055–1068. [Google Scholar] [CrossRef]
- Elia, A.C.; Fanetti, A.; Dörr, A.J.M.; Taticchi, M.I. Effects of Concentrated Drinking Water Injection on Glutathione and Glutathione-Dependent Enzymes in Liver of Cyprinus carpio L. Chemosphere 2008, 72, 791–796. [Google Scholar] [CrossRef]
- Khessiba, A.; Hoarau, P.; Gnassia-Barelli, M.; Aissa, P.; Roméo, M. Biochemical Response of the Mussel Mytilus galloprovincialis from Bizerta (Tunisia) to Chemical Pollutant Exposure. Arch. Environ. Contam. Toxicol. 2001, 40, 222–229. [Google Scholar] [CrossRef]
- Magara, G.; Elia, A.C.; Dörr, A.J.M.; Abete, M.C.; Brizio, P.; Caldaroni, B.; Righetti, M.; Pastorino, P.; Scoparo, M.; Prearo, M. Metal Load and Oxidative Stress Driven by Organotin Compounds on Rainbow Trout. Environ. Sci. Pollut. Res. 2021, 28, 35012–35022. [Google Scholar] [CrossRef]
- Magara, G.; Sangsawang, A.; Pastorino, P.; Oddon, S.B.; Caldaroni, B.; Menconi, V.; Kovitvadhi, U.; Gasco, L.; Meloni, D.; Dörr, A.J.M. First Insights into Oxidative Stress and Theoretical Environmental Risk of Bronopol and Detarox® AP, Two Biocides Claimed to Be Ecofriendly for a Sustainable Aquaculture. Sci. Total Environ. 2021, 778, 146375. [Google Scholar] [CrossRef]
- Torricelli, P.; Elia, A.C.; Magara, G.; Feriotto, G.; Forni, C.; Borromeo, I.; De Martino, A.; Tabolacci, C.; Mischiati, C.; Beninati, S. Reduction of Oxidative Stress and Ornithine Decarboxylase Expression in a Human Prostate Cancer Cell Line PC-3 by a Combined Treatment with α-Tocopherol and Naringenin. Amino Acids 2021, 53, 63–72. [Google Scholar] [CrossRef]
- Elia, A.C.; Prearo, M.; Dörr, A.J.M.; Pacini, N.; Magara, G.; Brizio, P.; Gasco, L.; Abete, M.C. Effects of Astaxanthin and Canthaxanthin on Oxidative Stress Biomarkers in Rainbow Trout. J. Toxicol. Environ. Health Part A 2019, 82, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Burioli, E.; Magara, G.; Pastorino, P.; Caldaroni, B.; Menconi, V.; Dörr, A.J.M.; Colombero, G.; Abete, M.C.; Prearo, M. Oxidative Stress Ecology on Pacific Oyster Crassostrea gigas from Lagoon and Offshore Italian Sites. Sci. Total Environ. 2020, 739, 139886. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The Application of a Plant Biostimulant Based on Seaweed and Yeast Extract Improved Tomato Fruit Development and Quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Gentile, C.; Mannino, G.; Palazzolo, E.; Gianguzzi, G.; Perrone, A.; Serio, G.; Farina, V. Pomological, Sensorial, Nutritional and Nutraceutical Profile of Seven Cultivars of Cherimoya (Annona cherimola Mill). Foods 2021, 10, 35. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, P.; Vela Alonso, A.I.; Colussi, S.; Cavazza, G.; Menconi, V.; Mugetti, D.; Righetti, M.; Barbero, R.; Zuccaro, G.; Fernández-Garayzábal, J.F. A Summer Mortality Outbreak of Lactococcosis by Lactococcus garvieae in a Raceway System Affecting Farmed Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis). Animals 2019, 9, 1043. [Google Scholar] [CrossRef] [Green Version]
- Elia, A.C.; Magara, G.; Righetti, M.; Dörr, A.J.M.; Scanzio, T.; Pacini, N.; Abete, M.C.; Prearo, M. Oxidative Stress and Related Biomarkers in Cupric and Cuprous Chloride-Treated Rainbow Trout. Environ. Sci. Pollut. Res. 2017, 24, 10205–10219. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Akerboom, T.P.; Sies, H. Assay of Glutathione, Glutathione Disulfide, and Glutathione Mixed Disulfides in Biological Samples. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1981; Volume 77, pp. 373–382. ISSN 0076-6879. [Google Scholar]
- Pacini, N.; Prearo, M.; Abete, M.C.; Brizio, P.; Dörr, A.J.M.; Reimschuessel, R.; Andersen, W.; Gasco, L.; Righetti, M.; Elia, A.C. Antioxidant Responses and Renal Crystal Formation in Rainbow Trout Treated with Melamine Administered Individually or in Combination with Cyanuric Acid. J. Toxicol. Environ. Health Part A 2013, 76, 491–508. [Google Scholar] [CrossRef]
- Lopes, F.C.M.; Benzatti, F.P.; Jordão Junior, C.M.; Moreira, R.R.D.; Carlos, I.Z. Effect of the Essential Oil of Achillea millefolium L. in the Production of Hydrogen Peroxide and Tumor Necrosis Factor-Alpha in Murine Macrophages. Rev. Bras. Ciênc. Farm. 2005, 41, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of Dietary Intake of Phenolic Compounds from Mango Peel Extract on Growth, Lipid Peroxidation and Antioxidant Enzyme Activities in Zebrafish (Danio rerio). Lat. Am. J. Aquat. Res. 2019, 47, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, P.; Das, S. Deltamethrin Induced Alteration of Biochemical Parameters in Channa punctata, Bloch and Its Amelioration by Quercetin. Bull. Environ. Contam. Toxicol. 2017, 98, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Yoo, J.H.; Min, T.S.; Lee, K.Y.; Choi, C.Y. The Effects of Quercetin on Physiological Characteristics and Oxidative Stress Resistance in Olive Flounder, Paralichthys olivaceus. Asian-Australas. J. Anim. Sci. 2010, 23, 588–597. [Google Scholar] [CrossRef]
- Shin, H.S.; Yoo, J.H.; Min, T.S.; Lee, J.; Choi, C.Y. Effect of Quercetin on the Activity and MRNA Expression of Antioxidant Enzymes and Physiological Responses in Olive Flounder (Paralichthys olivaceus) Exposed to Cadmium. Asian-Australas. J. Anim. Sci. 2010, 23, 742–749. [Google Scholar] [CrossRef]
- Li, E.; Wang, Y.; Li, Q.; Li, L.; Wei, L. Protective Effects of Sal B on Oxidative Stress-Induced Aging by Regulating the Keap1/Nrf2 Signaling Pathway in Zebrafish. Molecules 2021, 26, 5239. [Google Scholar] [CrossRef]
- Pês, T.S.; Saccol, E.M.; Ourique, G.M.; Londero, É.P.; Gressler, L.T.; Golombieski, J.I.; Glanzner, W.G.; Llesuy, S.F.; Gonçalves, P.B.; Neto, J.R. Quercetin in the Diet of Silver Catfish: Effects on Antioxidant Status, Blood Parameters and Pituitary Hormone Expression. Aquaculture 2016, 458, 100–106. [Google Scholar] [CrossRef]
- Pês, T.S.; Saccol, E.M.; Ourique, G.M.; Londero, É.P.; Gressler, L.T.; Finamor, I.A.; Rotili, D.A.; Golombieski, J.I.; Glanzner, W.G.; Llesuy, S.F. Effect of Diets Enriched with Rutin on Blood Parameters, Oxidative Biomarkers and Pituitary Hormone Expression in Silver Catfish (Rhamdia quelen). Fish Physiol. Biochem. 2016, 42, 321–333. [Google Scholar] [CrossRef]
- Gressler, L.T.; Riffel, A.P.K.; Parodi, T.V.; Saccol, E.M.H.; Koakoski, G.; da Costa, S.T.; Pavanato, M.A.; Heinzmann, B.M.; Caron, B.; Schmidt, D. Silver Catfish Rhamdia quelen Immersion Anaesthesia with Essential Oil of Aloysia triphylla (L’Hérit) Britton or Tricaine Methanesulfonate: Effect on Stress Response and Antioxidant Status. Aquac. Res. 2014, 45, 1061–1072. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Saccol, E.M.H.; Pês, T.S.; Salbego, J.; Koakoski, G.; dos Santos, A.C.; Heinzmann, B.M.; da Cunha, M.A.; Barcellos, L.J.G.; Pavanato, M.A. Aloysia triphylla Essential Oil as Food Additive for Rhamdia quelen–Stress and Antioxidant Parameters. Aquac. Nutr. 2017, 23, 1362–1367. [Google Scholar] [CrossRef]
- Saccol, E.M.; Toni, C.; Pês, T.S.; Ourique, G.M.; Gressler, L.T.; Silva, L.V.; Mourão, R.H.; Oliveira, R.B.; Baldisserotto, B.; Pavanato, M.A. Anaesthetic and Antioxidant Effects of Myrcia sylvatica (G. Mey.) DC. and Curcuma longa L. Essential Oils on Tambaqui (Colossoma macropomum). Aquac. Res. 2017, 48, 2012–2031. [Google Scholar] [CrossRef]
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbiç, O.S.; Yılmaz, S. Evaluation of Citrus limon Peels Essential Oil on Growth Performance, Immune Response of Mozambique Tilapia Oreochromis mossambicus Challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- De Freitas Souza, C.; Baldissera, M.D.; Bianchini, A.E.; da Silva, E.G.; Mourão, R.H.V.; da Silva, L.V.F.; Schmidt, D.; Heinzmann, B.M.; Baldisserotto, B. Citral and Linalool Chemotypes of Lippia alba Essential Oil as Anesthetics for Fish: A Detailed Physiological Analysis of Side Effects during Anesthetic Recovery in Silver Catfish (Rhamdia quelen). Fish Physiol. Biochem. 2018, 44, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Mirghaed, A.T.; Hoseini, S.M.; Ghelichpour, M. Effects of Dietary 1, 8-Cineole Supplementation on Physiological, Immunological and Antioxidant Responses to Crowding Stress in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 81, 182–188. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, O. Effects of Dietary Limonene and Thymol on the Growth and Nutritional Physiology of Nile Tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Köprücü, K.; Yonar, M.E.; Özcan, S. Effect of Dietary N-3 Polyunsaturated Fatty Acids on Antioxidant Defense and Sperm Quality in Rainbow Trout (Oncorhynchus mykiss) under Regular Stripping Conditions. Anim. Reprod. Sci. 2015, 163, 135–143. [Google Scholar] [CrossRef]
- Saccol, E.M.; Uczay, J.; Pês, T.S.; Finamor, I.A.; Ourique, G.M.; Riffel, A.P.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; Llesuy, S.F. Addition of Lippia alba (Mill) NE Brown Essential Oil to the Diet of the Silver Catfish: An Analysis of Growth, Metabolic and Blood Parameters and the Antioxidant Response. Aquaculture 2013, 416, 244–254. [Google Scholar] [CrossRef]
- Azambuja, C.R.; Mattiazzi, J.; Riffel, A.P.K.; Finamor, I.A.; de Oliveira Garcia, L.; Heldwein, C.G.; Heinzmann, B.M.; Baldisserotto, B.; Pavanato, M.A.; Llesuy, S.F. Effect of the Essential Oil of Lippia alba on Oxidative Stress Parameters in Silver Catfish (Rhamdia quelen) Subjected to Transport. Aquaculture 2011, 319, 156–161. [Google Scholar] [CrossRef]
- Salbego, J.; Toni, C.; Becker, A.G.; Zeppenfeld, C.C.; Menezes, C.C.; Loro, V.L.; Heinzmann, B.M.; Baldisserotto, B. Biochemical Parameters of Silver Catfish (Rhamdia quelen) after Transport with Eugenol or Essential Oil of Lippia alba Added to the Water. Braz. J. Biol. 2017, 77, 696–702. [Google Scholar] [CrossRef] [Green Version]
- Sönmez, A.Y.; Bilen, S.; Alak, G.; Hisar, O.; Yanık, T.; Biswas, G. Growth Performance and Antioxidant Enzyme Activities in Rainbow Trout (Oncorhynchus mykiss) Juveniles Fed Diets Supplemented with Sage, Mint and Thyme Oils. Fish Physiol. Biochem. 2015, 41, 165–175. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Silva, L.d.L.; Geihs, M.A.; Baldisserotto, B. Is Monoterpene Terpinen-4-Ol the Compound Responsible for the Anesthetic and Antioxidant Activity of Melaleuca alternifolia Essential Oil (Tea Tree Oil) in Silver Catfish? Aquaculture 2018, 486, 217–223. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Salbego, J.; Becker, A.G.; Gonçalves, J.F.; Menezes, C.C.; Heldwein, C.G.; Spanevello, R.M.; Loro, V.L.; Schetinger, M.R.C.; Morsch, V.M.; Heinzmann, B.M. The Essential Oil from Lippia alba Induces Biochemical Stress in the Silver Catfish (Rhamdia quelen) after Transportation. Neotrop. Ichthyol. 2014, 12, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Zeppenfeld, C.C.; Toni, C.; Becker, A.G.; dos Santos Miron, D.; Parodi, T.V.; Heinzmann, B.M.; Barcellos, L.J.G.; Koakoski, G.; da Rosa, J.G.S.; Loro, V.L. Physiological and Biochemical Responses of Silver Catfish, Rhamdia quelen, after Transport in Water with Essential Oil of Aloysia triphylla (L’Herit) Britton. Aquaculture 2014, 418, 101–107. [Google Scholar] [CrossRef]
- Morais, S.; Pratoomyot, J.; Taggart, J.B.; Bron, J.E.; Guy, D.R.; Bell, J.G.; Tocher, D.R. Genotype-Specific Responses in Atlantic Salmon (Salmo salar) Subject to Dietary Fish Oil Replacement by Vegetable Oil: A Liver Transcriptomic Analysis. BMC Genom. 2011, 12, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, M.; Vatnikov, Y.A.; Kulikov, E.V.; Ghelichpour, M. Change in Blood Stress and Antioxidant Markers and Hydromineral Balance of Common Carp (Cyprinus carpio) Anaesthetized with Citronellal and Linalool: Comparison with Eugenol. Aquac. Res. 2019, 50, 1313–1320. [Google Scholar] [CrossRef]
- Velíšek, J.; Stejskal, V.; Kouřil, J.; Svobodová, Z. Comparison of the Effects of Four Anaesthetics on Biochemical Blood Profiles of Perch. Aquac. Res. 2009, 40, 354–361. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Mahmoud, H.K.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Supplementation of Diets for Oreochromis niloticus with Essential Oil Extracts from Lemongrass (Cymbopogon citratus) and Geranium (Pelargonium graveolens) and Effects on Growth, Intestinal Microbiota, Antioxidant and Immune Activities. Aquac. Nutr. 2018, 24, 1006–1014. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, Anti-Inflammatory and Hepatoprotective Effects of Resveratrol on Oxidative Stress-Induced Liver Damage in Tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef]

| Total Polyphenol Content | 32.97 ± 1.63 | mmol GAE per 100 g of FW |
| Total Flavan-3-ol Content | 21.21 ± 1.04 | mmol A2-PACE per 100 g of FW |
| Radical Scavenging Activity | ||
| DPPH | 70.32 ± 1.39 | mmol TE per 100 g |
| ABTS | 29.92 ± 0.99 | mmol TE per 100 g |
| Reducing Activity | ||
| FRAP | 28.62 ± 2.05 | mmol TE per 100 g |
| Total Volatile Content | 73.18 ± 2.79 | mg per 100 g of FW | ||
| Formula | Compound | CAS ID | ||
| C10H18O | 1,8-Cineole | 470-82-6 | 9.33 ± 0.45 | % |
| C10H18O | Linalool | 78-70-6 | 25.29 ± 0.81 | % |
| C10H12O | Estragol | 140-67-0 | 18.79 ± 0.78 | % |
| C10H12O2 | Eugenol | 97-53-0 | 4.49 ± 0.12 | % |
| C10H10O2 | Methylcinnamylate | 103-26-4 | 8.71 ± 0.15 | % |
| C11H14O2 | Methyleugenol | 93-15-2 | 6.58 ± 0.08 | % |
| C15H24 | b-Caryophyllene | 87-44-5 | 7.47 ± 0.29 | % |
| C15H24 | α-Bergamotene | 17699-05-7 | 19.34 ± 1.09 | % |
| Total Fat Content | 9.97 ± 0.25 | g per 100g of FW | ||
| Formula | Compound | CAS ID | ||
| C14:0 | Myristic acid | 544-63-8 | 3.05 ± 0.12 | % |
| C16:1 trans | Palmitoleic acid | 373-49-9 | 1.89 ± 0.05 | % |
| C16:1 cis | Palmitovaccenic acid | 373-49-9 | 2.46 ± 0.08 | % |
| C16:0 | Palmitic acid | 57-10-3 | 37.28 ± 1.52 | % |
| C18:2 | Linoleic acid | 60-33-3 | 10.82 ± 0.41 | % |
| C18:1 cis | Oleic acid | 112-80-1 | 28.95 ± 1.32 | % |
| C18:1 trans | Elaidic acid | 112-79-8 | 1.06 ± 0.04 | % |
| C18:0 | Stearic acid | 57-11-4 | 6.53 ± 0.12 | % |
| C20:0 | Arachidic acid | 506-30-9 | 5.16 ± 0.15 | % |
| C22:0 | Behenic acid | 112-85-6 | 2.75 ± 0.06 | % |
| F Time (dfn, dfd) | Time (F-Value) | F Treatment-Interaction (dfn, dfd) | Treatment (F-Value) | Interaction (F-Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | Liver | Kidney | Liver | Kidney | Liver | Kidney | |
| SOD | (1; 70) | (1; 70) | 16.34 *** | 6.02 * | (4; 70) | (4; 70) | 1.00 | 0.55 | 0.98 | 3.72 ** |
| CAT | (1; 70) | (1; 70) | 0.002 | 41.36 *** | (4; 70) | (4; 70) | 2.12 | 3.36 * | 1.54 | 9.62 *** |
| GPx | (1; 70) | (1; 70) | 12.92 *** | 2.94 | (4; 70) | (4; 70) | 6.58 *** | 0.67 | 1.21 | 8.80 *** |
| GST | (1; 70) | (1; 70) | 65.07 *** | 1.91 | (4; 70) | (4; 70) | 23.81 *** | 34.10 *** | 4.88 ** | 3.92 ** |
| GR | (1; 70) | (1; 70) | 156.9 *** | 0.41 | (4; 70) | (4; 70) | 21.34 *** | 4.05 ** | 14.69 *** | 1.55 |
| GI | (1; 70) | (1; 70) | 19.99 *** | 1.63 * | (4; 70) | (4; 70) | 10.82 *** | 113.3 *** | 4.98 ** | 16.08 *** |
| GII | (1; 70) | (1; 70) | 8.01 ** | 29.48 *** | (4; 70) | (4; 70) | 2.52 * | 4.46 ** | 1.31 | 6.82 *** |
| LDH | (1; 70) | (1; 70) | 27.49 *** | 8.98 ** | (4; 70) | (4; 70) | 24.93 *** | 7.27 *** | 25.52 *** | 1.13 |
| Glut | (1; 70) | (1; 70) | 0.04 ** | 0.16 | (4; 70) | (4; 70) | 18.71 *** | 34.78 *** | 13.24 *** | 10.48 *** |
| MDA | (1; 70) | (1; 70) | 0.05 | 6.83 * | (4; 70) | (4; 70) | 3.88 ** | 16.11 *** | 2.69 * | 7.69 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magara, G.; Prearo, M.; Vercelli, C.; Barbero, R.; Micera, M.; Botto, A.; Caimi, C.; Caldaroni, B.; Bertea, C.M.; Mannino, G.; et al. Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum). Antioxidants 2022, 11, 415. https://doi.org/10.3390/antiox11020415
Magara G, Prearo M, Vercelli C, Barbero R, Micera M, Botto A, Caimi C, Caldaroni B, Bertea CM, Mannino G, et al. Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum). Antioxidants. 2022; 11(2):415. https://doi.org/10.3390/antiox11020415
Chicago/Turabian StyleMagara, Gabriele, Marino Prearo, Cristina Vercelli, Raffaella Barbero, Marco Micera, Alfonso Botto, Christian Caimi, Barbara Caldaroni, Cinzia Margherita Bertea, Giuseppe Mannino, and et al. 2022. "Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum)" Antioxidants 11, no. 2: 415. https://doi.org/10.3390/antiox11020415
APA StyleMagara, G., Prearo, M., Vercelli, C., Barbero, R., Micera, M., Botto, A., Caimi, C., Caldaroni, B., Bertea, C. M., Mannino, G., Barceló, D., Renzi, M., Gasco, L., Re, G., Dondo, A., Elia, A. C., & Pastorino, P. (2022). Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum). Antioxidants, 11(2), 415. https://doi.org/10.3390/antiox11020415














