Extra Virgin Olive Oil Extracts of Indigenous Southern Tuscany Cultivar Act as Anti-Inflammatory and Vasorelaxant Nutraceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Chemical Considerations
2.2. EVOO Samples
2.3. Determination of Free Fatty Acids
2.4. Determination of Peroxide Value
2.5. Determination of Biophenols by HPLC
2.6. Determination of Tocopherols by HPLC
2.7. Determination of Methyl Esters of Fatty Acids by GC
2.8. Determination of Total Polyphenol Content by Folin–Ciocalteu Method
2.9. Preparation of Polar Fraction Extracts
2.10. 1H-NMR Analysis
2.11. UHPLC-HRMS
2.12. Stable Isotope Ratio Analysis
| δi E = (i RSA − i RREF) |
| i RREF |
2.13. Antioxidant–Anti-Inflammatory Activity of EVOO Extracts
2.13.1. Cell Culture and Treatments
2.13.2. Cytotoxicity Study (MTT Assay)
2.13.3. AmplexRed Assay
2.13.4. Immunofluorescence Staining
2.13.5. RNA Extraction and Quantitative Real Time PCR (qRT-PCR)
2.14. Vasoactivity Assessments of EVOO Extracts
2.14.1. Animals
2.14.2. Preparation of Rat Aortic Rings
2.14.3. Effect of EVOO Extracts on Phenylephrine-Induced Contraction
2.15. Statistical Analysis
3. Results and Discussion
3.1. Acidity and Peroxides Analysis
3.2. Determination of Methyl Esters of Fatty Acids by GC
3.3. Tocopherols
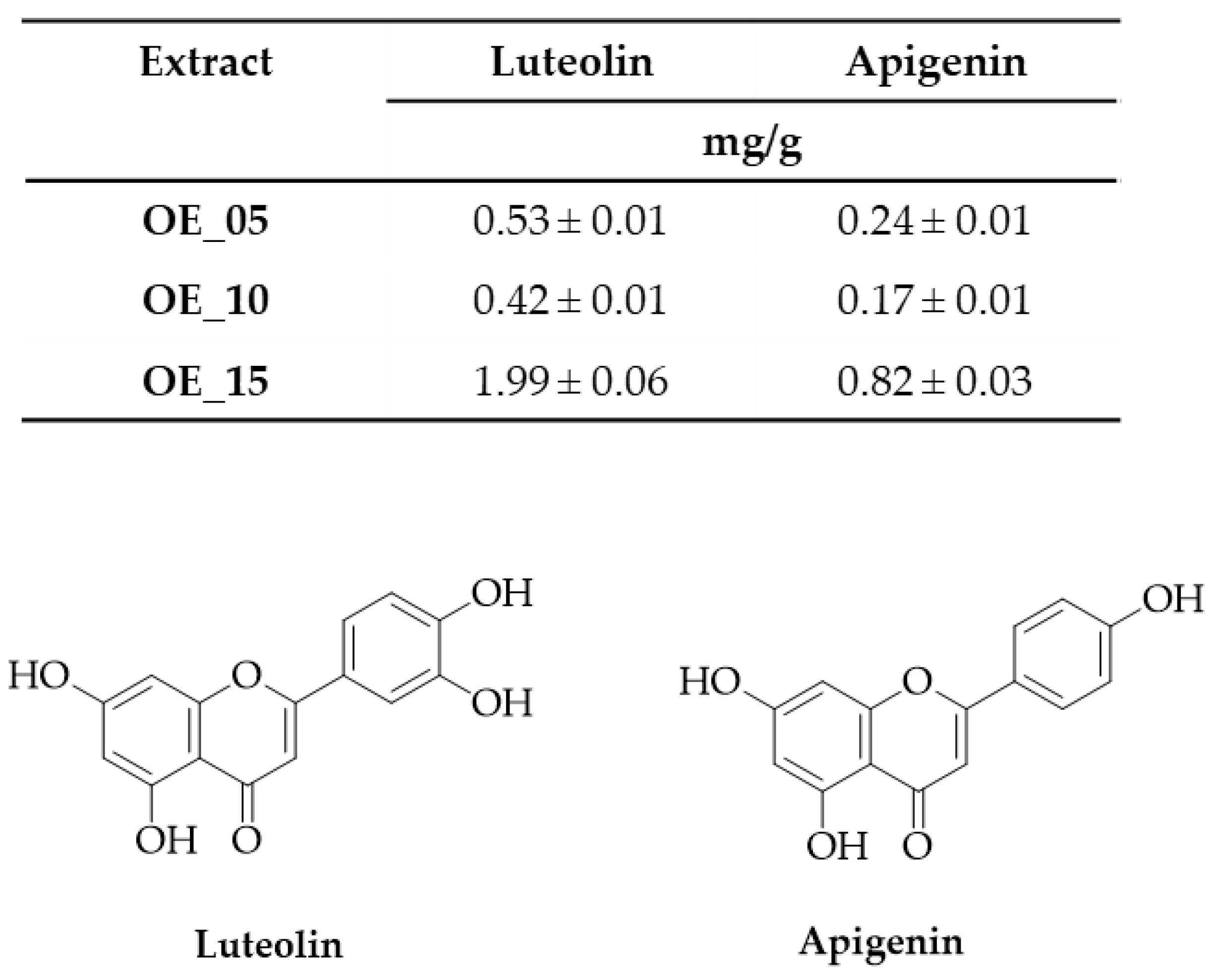
3.4. Total Phenolic Content and Biophenols
3.5. Isotopic Ratio Mass Spectrometry
3.6. Extraction and Chemical Characterization via 1H NMR and HRMS
3.7. Evaluation of Protective Effects of OE_05, OE_10, and OE_15 on CaCo-2 Cells
3.7.1. MTT Assay
3.7.2. Antioxidant Properties of EVOO Extracts
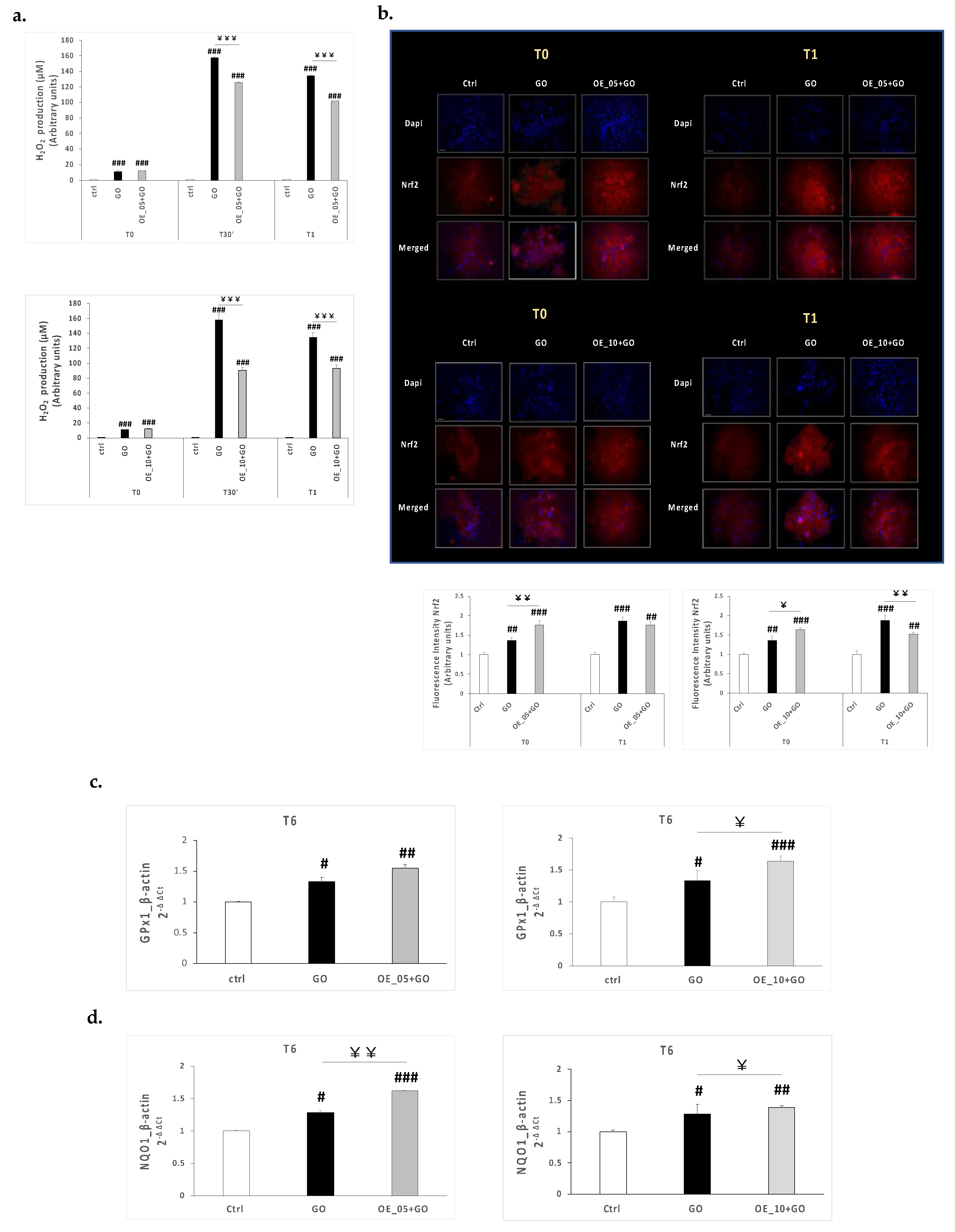
3.7.3. EVOO Extracts Showed Anti-Inflammatory Properties against Glucose Oxidase Insult
3.8. Effects of EVOO Extracts on Phenylephrine-Induced Contraction in Rat Thoracic Aorta Rings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, O.M.; Ashor, A.W.; Scialo, F.; Saretzki, G.; Martin-Ruiz, C.; Lara, J.; Matu, J.; Griffiths, A.; Robinson, N.; Lillà, L.; et al. Mediterranean diet and the hallmarks of ageing. Eur. J. Clin. Nutr. 2021, 75, 1176–1192. [Google Scholar] [CrossRef]
- D’Alessandro, A.; De Pergola, G. Mediterranean Diet and Cardiovascular Disease: A Critical Evaluation of a Priori Dietary Indexes. Nutrients 2015, 7, 7863–7888. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Shahbaz, M.; Sacanella, E.; Tahiri, I.; Casas, R. Chapter 17—Mediterranean diet and role of olive oil. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 205–214. ISBN 978-0-12-819528-4. [Google Scholar]
- Carullo, G.; Mazzotta, S.; Koch, A.; Hartmann, K.M.; Friedrich, O.; Gilbert, D.F.; Vega-Holm, M.; Schneider-Stock, R.; Aiello, F. New oleoyl hybrids of natural antioxidants: Synthesis and in vitro evaluation as inducers of apoptosis in colorectal cancer cells. Antioxidants 2020, 9, 1077. [Google Scholar] [CrossRef]
- Celano, R.; Piccinelli, A.L.; Pugliese, A.; Carabetta, S.; Di Sanzo, R.; Rastrelli, L.; Russo, M. Insights into the Analysis of Phenolic Secoiridoids in Extra Virgin Olive Oil. J. Agric. Food Chem. 2018, 66, 6053–6063. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Olmo-Cunillera, A.; Jáuregui, O.; Pérez, M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Total Analysis of the Major Secoiridoids in Extra Virgin Olive Oil: Validation of an UHPLC-ESI-MS/MS Method. Antioxidants 2021, 10, 540. [Google Scholar] [CrossRef]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Zallocco, L.; Beghelli, D.; Ronci, M.; Scortichini, S.; Digiacomo, M.; Macchia, M.; Mazzoni, M.R.; Fiorini, D.; Lucacchini, A.; et al. Antioxidant and Neuroprotective Activity of Extra Virgin Olive Oil Extracts Obtained from Quercetano Cultivar Trees Grown in Different Areas of the Tuscany Region (Italy). Antioxidants 2021, 10, 421. [Google Scholar] [CrossRef]
- Otrante, A.; Trigui, A.; Walha, R.; Berrougui, H.; Fulop, T.; Khalil, A. Extra Virgin Olive Oil Prevents the Age-Related Shifts of the Distribution of HDL Subclasses and Improves Their Functionality. Nutrients 2021, 13, 2235. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef]
- Longhi, R.; Santos, A.S.e.A.d.C.; López-Yerena, A.; Rodrigues, A.P.S.; de Oliveira, C.; Silveira, E.A. The Effectiveness of Extra Virgin Olive Oil and the Traditional Brazilian Diet in Reducing the Inflammatory Profile of Individuals with Severe Obesity: A Randomized Clinical Trial. Nutrients 2021, 13, 4139. [Google Scholar] [CrossRef]
- Njike, V.Y.; Ayettey, R.; Treu, J.A.; Doughty, K.N.; Katz, D.L. Post-prandial effects of high-polyphenolic extra virgin olive oil on endothelial function in adults at risk for type 2 diabetes: A randomized controlled crossover trial. Int. J. Cardiol. 2021, 330, 171–176. [Google Scholar] [CrossRef]
- Carullo, G.; Governa, P.; Spizzirri, U.G.; Biagi, M.; Sciubba, F.; Giorgi, G.; Loizzo, M.R.; Di Cocco, M.E.; Aiello, F.; Restuccia, D. Sangiovese cv pomace seeds extract-fortified kefir exerts anti-inflammatory activity in an in vitro model of intestinal epithelium using caco-2 cells. Antioxidants 2020, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Dini, I.; Graziani, G.; Gaspari, A.; Fedele, F.L.; Sicari, A.; Vinale, F.; Cavallo, P.; Lorito, M.; Ritieni, A. New strategies in the cultivation of olive trees and repercussions on the nutritional value of the extra virgin olive oil. Molecules 2020, 25, 2345. [Google Scholar] [CrossRef]
- Carullo, G.; Sciubba, F.; Governa, P.; Mazzotta, S.; Frattaruolo, L.; Grillo, G.; Cappello, A.R.; Cravotto, G.; Di Cocco, M.E.; Aiello, F. Mantonico and pecorello grape seed extracts: Chemical characterization and evaluation of in vitro wound-healing and anti-inflammatory activities. Pharmaceuticals 2020, 13, 97. [Google Scholar] [CrossRef]
- Lammi, C.; Bellumori, M.; Cecchi, L.; Bartolomei, M.; Bollati, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A.; Mulinacci, N. Extra virgin olive oil phenol extracts exert hypocholesterolemic effects through the modulation of the LDLR pathway: In vitro and cellular mechanism of action elucidation. Nutrients 2020, 12, 1723. [Google Scholar] [CrossRef]
- Lammi, C.; Mulinacci, N.; Cecchi, L.; Bellumori, M.; Bollati, C.; Bartolomei, M.; Franchini, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A. Virgin Olive Oil Extracts Reduce Oxidative Stress and Modulate Cholesterol Metabolism: Comparison between Oils Obtained with Traditional and Innovative Processes. Antioxidants 2020, 9, 798. [Google Scholar] [CrossRef]
- D’agostino, R.; Barberio, L.; Gatto, M.; Tropea, T.; De Luca, M.; Mandalà, M. Extra virgin olive oil phenols vasodilate rat mesenteric resistance artery via phospholipase c (Plc)-calcium microdomains-potassium channels (bkca) signals. Biomolecules 2021, 11, 137. [Google Scholar] [CrossRef]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra virgin olive oil extracts modulate the inflammatory ability of murine dendritic cells based on their polyphenols pattern: Correlation between chemical composition and biological function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- Rizzo, R.; Caleca, V. Resistance to the attack of Bactrocera oleae (Gmelin) of some Sicilian olive cultivars. In Proceedings of the Olivebioteq 2006, Second International Seminar “Biotechnology and quality of olive tree products around the Mediterranean Basin”, Mazara del Vallo, Marsala, Italy, 5–10 November 2006; Volume II, pp. 35–42. [Google Scholar]
- EU Commission. Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. L 1991, 248, 1. [Google Scholar]
- EU Commission. Commission Implementing Regulation (EU) No 1348/2013 of 16 December 2013 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. OJ L 338, 17.12.2013, p. 31–67. Off. J. Eur. Union 2014, 57, 1–28. [Google Scholar]
- International Olive Council Method of Analysis Determination of Biophenols in Olive Oils by Hplc. 2017, pp. 1–8. Available online: https://www.oelea.de/downloads/COI-T20-DOC-29-2009-DETERMINATION-OF-BIOPHENOLS-IN-OLIVE-OILS-BY-HPLC.pdf (accessed on 24 January 2022).
- UNI EN 12822:2014. Foodstuffs—Determination of Vitamin E by High Performance Liquid Chromatography—Measurement of α-, β-, γ- and δ-Tocopherols. Available online: https://infostore.saiglobal.com/preview/98701590166.pdf?sku=858459_SAIG_NSAI_NSAI_2042196 (accessed on 24 January 2022).
- EU Commission. Commission Implementing Regulation (EU) 2015/1833 of 12 October 2015 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. OJ L 266, 13.10.2015, p. 29–52. Off. J. Eur. Union 2015, 2015, 29–52. [Google Scholar]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Tsimidou, M.; Papadopoulos, G.; Boskou, D. Determination of phenolic compounds in virgin olive oil by reversed-phase HPLC with emphasis on UV detection. Food Chem. 1992, 44, 53–60. [Google Scholar] [CrossRef]
- Christophoridou, S.; Dais, P. Detection and quantification of phenolic compounds in olive oil by high resolution 1H nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2009, 633, 283–292. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of olive oil pomace extracts for a functional pear beverage formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- Castejón, D.; Fricke, P.; Cambero, M.I.; Herrera, A. Automatic 1H-NMR Screening of Fatty Acid Composition in Edible Oils. Nutrients 2016, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- Camin, F.; Bertoldi, D.; Santato, A.; Bontempo, L.; Perini, M.; Ziller, L.; Stroppa, A.; Larcher, R. Validation of methods for H, C, N and S stable isotopes and elemental analysis of cheese: Results of an international collaborative study. Rapid Commun. Mass Spectrom. 2015, 29, 415–423. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Hobson, K.A. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ. Health Stud. 2003, 39, 211–217. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Pambianchi, E.; Pecorelli, A.; Woodby, B.; Messano, N.; Therrien, J.P.; Lila, M.A.; Valacchi, G. Redox regulation of cutaneous inflammasome by ozone exposure. Free Radic. Biol. Med. 2019, 152, 561–570. [Google Scholar] [CrossRef]
- Romani, A.; Cervellati, C.; Muresan, X.M.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Benedusi, M.; Evelson, P. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, F.; Woodby, B.; Benedusi, M.; Ferrara, F.; Guiotto, A.; Valacchi, G. Evaluation of oxidative damage and Nrf2 activation by combined pollution exposure in lung epithelial cells. Environ. Sci. Pollut. Res. 2020, 27, 31841–31853. [Google Scholar] [CrossRef]
- Saponara, S.; Durante, M.; Spiga, O.; Mugnai, P.; Sgaragli, G.; Huong, T.; Khanh, P.; Son, N.; Cuong, N.; Fusi, F. Functional, electrophysiological and molecular docking analysis of the modulation of Cav1.2 channels in rat vascular myocytes by murrayafoline A. Br. J. Pharmacol. 2016, 173, 292–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carullo, G.; Ahmed, A.; Trezza, A.; Spiga, O.; Brizzi, A.; Saponara, S.; Fusi, F.; Aiello, F. A multitarget semi-synthetic derivative of the flavonoid morin with improved in vitro vasorelaxant activity: Role of CaV1.2 and KCa1.1 channels. Biochem. Pharmacol. 2021, 185, 114429. [Google Scholar] [CrossRef]
- Mazzotta, S.; Governa, P.; Borgonetti, V.; Marcolongo, P.; Nanni, C.; Gamberucci, A.; Manetti, F.; Pessina, F.; Carullo, G.; Brizzi, A.; et al. Pinocembrin and its linolenoyl ester derivative induce wound healing activity in HaCaT cell line potentially involving a GPR120/FFA4 mediated pathway. Bioorg. Chem. 2021, 108, 104657. [Google Scholar] [CrossRef]
- Farías, J.G.; Carrasco-Pozo, C.; Carrasco Loza, R.; Sepúlveda, N.; Álvarez, P.; Quezada, M.; Quiñones, J.; Molina, V.; Castillo, R.L. Polyunsaturated fatty acid induces cardioprotection against ischemia-reperfusion through the inhibition of NF-kappaB and induction of Nrf2. Exp. Biol. Med. 2017, 242, 1104–1114. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.G.; León, L.; Pascual, M.; de la Rosa, R.; Belaj, A.; Sanz, C. Analysis of Olive (Olea Europaea L.) Genetic Resources in Relation to the Content of Vitamin E in Virgin Olive Oil. Antioxidants 2019, 8, 242. [Google Scholar] [CrossRef] [Green Version]
- Bontempo, L.; Paolini, M.; Franceschi, P.; Ziller, L.; García-González, D.L.; Camin, F. Characterisation and attempted differentiation of European and extra-European olive oils using stable isotope ratio analysis. Food Chem. 2019, 276, 782–789. [Google Scholar] [CrossRef]
- Bocci, V.; Valacchi, G. Nrf2 activation as target to implement therapeutic treatments. Front. Chem. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, F.; Woodby, B.; Pecorelli, A.; Schiavone, M.L.; Pambianchi, E.; Messano, N.; Therrien, J.P.; Choudhary, H.; Valacchi, G. Additive effect of combined pollutants to UV induced skin OxInflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. 2020, 34, 101481. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Cervellati, C.; Hayek, J.; Valacchi, G. OxInflammation in Rett syndrome. Int. J. Biochem. Cell Biol. 2016, 81, 246–253. [Google Scholar] [CrossRef]
- Pecorelli, A.; Cordone, V.; Messano, N.; Zhang, C.; Falone, S.; Amicarelli, F.; Hayek, J.; Valacchi, G. Altered inflammasome machinery as a key player in the perpetuation of Rett syndrome oxinflammation. Redox Biol. 2020, 28, 101334. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Camargo, A.; Rangel-Zuñiga, O.A.; Haro, C.; Meza-Miranda, E.R.; Peña-Orihuela, P.; Meneses, M.E.; Marin, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem. 2014, 162, 161–171. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Liu, Y.; Huang, C.; Xia, W.; Zhou, H.; Zhou, Z.; Zhou, X. Luteolin Protects Chondrocytes from H2O2-Induced Oxidative Injury and Attenuates Osteoarthritis Progression by Activating AMPK-Nrf2 Signaling. Oxid. Med. Cell. Longev. 2022, 2022, 5635797. [Google Scholar] [CrossRef]
- Cicek, M.; Unsal, V.; Doganer, A.; Demir, M. Investigation of oxidant/antioxidant and anti-inflammatory effects of apigenin on apoptosis in sepsis-induced rat lung. J. Biochem. Mol. Toxicol. 2021, 35, e22743. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Carullo, G.; Ahmed, A.; Fusi, F.; Sciubba, F.; Di Cocco, M.E.; Restuccia, D.; Spizzirri, U.G.; Saponara, S.; Aiello, F. Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv. Pharmaceuticals 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Durante, M.; Sciubba, F.; Restuccia, D.; Spizzirri, U.G.; Ahmed, A.; Di Cocco, M.E.; Saponara, S.; Aiello, F.; Fusi, F. Vasoactivity of Mantonico and Pecorello grape pomaces on rat aorta rings: An insight into nutraceutical development. J. Funct. Foods 2019, 57, 328–334. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saponara, S.; Carosati, E.; Mugnai, P.; Sgaragli, G.; Fusi, F. The flavonoid scaffold as a template for the design of modulators of the vascular Ca v1.2 channels. Br. J. Pharmacol. 2011, 164, 1684–1697. [Google Scholar] [CrossRef] [Green Version]
- Fusi, F.; Spiga, O.; Trezza, A.; Sgaragli, G.; Saponara, S. The surge of flavonoids as novel, fine regulators of cardiovascular Cavchannels. Eur. J. Pharmacol. 2017, 796, 158–174. [Google Scholar] [CrossRef]
- Ilic, S.; Stojiljkovic, N.; Stojanovic, N.; Stoiljkovic, M.; Mitic, K.; Salinger-Martinovic, S.; Randjelovic, P. Effects of oleuropein on rat’s atria and thoracic aorta: A study of antihypertensive mechanisms. Can. J. Physiol. Pharmacol. 2021, 99, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; Woodman, O.L. Tocotrienol Rich Palm Oil Extract Is More Effective Than Pure Tocotrienols at Improving Endothelium-Dependent Relaxation in the Presence of Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015, 150829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carullo, G.; Badolato, M.; Aiello, F. Chapter 26—Bioavailability and Biochemistry of Quercetin and Applications to Health and Diseases. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 361–371. ISBN 978-0-12-813006-3. [Google Scholar]
- Carullo, G.; Aiello, F. Quercetin-3-oleate. Molbank 2018, 2018, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Menendez, C.; Dueñas, M.; Galindo, P.; González-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodríguez-Gómez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular deconjugation of quercetin glucuronide: The flavonoid paradox revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef]



| EVOO Samples | Cultivars | Acidity (% Oleic Acid) | Oleic Acid (%) | Linoleic Acid (%) | TPC (mg/kg) | Biophenols (mg/kg) | Tocopherols (mg/kg) | Peroxides (meq O2/kg) |
|---|---|---|---|---|---|---|---|---|
| O_01 | Leccino (80%) Frantoio (10%) Moraiolo (10%) | 0.15 | 75.48 | 6.28 | 703 | 664 | 298 | 7.70 |
| O_02 | Leccino (10%) Frantoio (80%) Moraiolo (10%) | 0.11 | 76.68 | 5.86 | 517 | 405 | 164 | 8.00 |
| O_03 | Minuta (70%) Greggiolo (30%) | 0.12 | 75.72 | 6.01 | 632 | 451 | 260 | 7.20 |
| O_04 | Leccino (100%) | 0.11 | 76.92 | 5.75 | 467 | 321 | 228 | 6.80 |
| O_05 | Greggiolo (50%) Leccino (10%) Moraiolo (10%) Frantoio (10%) Morello (20%) | 0.11 | 77.04 | 5.95 | 406 | 314 | 221 | 6.50 |
| O_06 | Leccino (50%) Frantoio (50%) | 0.14 | 77.54 | 5.63 | 474 | 293 | 228 | 8.40 |
| O_07 | Leccino (60%) Frantoio (30%) Moraiolo (10%) | 0.10 | 76.18 | 5.99 | 547 | 345 | 223 | 9.30 |
| O_08 | Leccino (50%) Frantoio (50%) | 0.11 | 75.96 | 6.07 | 627 | 560 | 270 | 7.30 |
| O_09 | Frantoio (40%) Moraiolo (30%) Leccino (30%) | 0.13 | 77.34 | 5.78 | 465 | 372 | 167 | 8.50 |
| O_10 | Leccino (100%) | 0.11 | 76.45 | 5.73 | 713 | 477 | 446 | 5.30 |
| O_11 | Leccino (100 %) | 0.08 | 76.47 | 5.68 | 444 | 290 | 291 | 6.20 |
| O_12 | Leccino (60%) Frantoio (20%) Moraiolo (20%) | 0.09 | 77.20 | 5.75 | 586 | 405 | 207 | 5.70 |
| O_13 | Leccino (80%) Frantoio (10%) Moraiolo (10%) | 0.09 | 77.14 | 5.40 | 410 | 227 | 238 | 7.10 |
| O_14 | Leccino (10%) Frantoio (70%) Moraiolo (20%) | 0.09 | 75.75 | 5.98 | 602 | 434 | 254 | 6.30 |
| O_15 | Minuta (100%) | 0.13 | 75.53 | 6.34 | 681 | 553 | 239 | 4.90 |
| O_16 | Leccino (80%) Frantoio (10%) Moraiolo (10%) | 0.12 | 75.83 | 6.16 | 540 | 380 | 216 | 6.20 |
| O_17 | Leccino (30%) Frantoio (30%) Moraiolo (30%) Others (10%) | 0.10 | 77.09 | 5.66 | 440 | 243 | 222 | 7.90 |
| O_18 | Frantoio (80%) Leccino (10%) Moraiolo (10%) | 0.10 | 76.44 | 5.98 | 529 | 385 | 261 | 7.00 |
| EVOO Samples | Cultivars | Altitude (m asl) | 13C/12C (δ13C) (‰ vs. V-PDB) | 18O/16O (δ18O) (‰ vs. V-SMOW) | 2H/1H (δ2H) (‰ vs. V-SMOW) |
|---|---|---|---|---|---|
| O_05 | Greggiolo (50%) Leccino (10%) Moraiolo (10%) Frantoio (10%) Morello (20%) | 450 | −30.2‰ | 23.2‰ | −146‰ |
| O_08 | Leccino (50%) Frantoio (50%) | 350 | −30.9‰ | 22.8‰ | −149‰ |
| O_09 | Frantoio (40%) Moraiolo (30%) Leccino (30%) | 300 | −30.1‰ | 23.2‰ | −150‰ |
| O_10 | Leccino (100%) | 300 | −30.5‰ | 22.1‰ | −148‰ |
| O_15 | Minuta (100%) | 330 | −29.6‰ | 23.5‰ | −149‰ |
| O_16 | Leccino (80%) Frantoio (10%) Moraiolo (10%) | 600 | −30.5‰ | 23.2‰ | −150‰ |
| Compound | Assignment | 1H (ppm) | Multiplicity | Samples | ||
|---|---|---|---|---|---|---|
| OE_05 | OE_10 | OE_15 | ||||
| Oleuropein | CHOH | 9.49 | m | + | + | + |
| Oleocanthal | CHO | 9.23 | s | + | + | + |
| p-Coumaric acid | CH=CH | 7.54 | d | + | + | + |
| Tyrosol (total) | - | 7.08–6.96 | not assigned | + | + | + |
| Hydrotyrosol | - | 6.54–6.41 | not assigned | + | + | + |
| Carotenoids (total) | CH | 6.68 | m | + | + | + |
| Luteolin | C6-H | 6.18 | m | − | + | − |
| Ligstroside | - | 4.20–4.00 | not assigned | + | + | + |
| Pinoresinol | OCH3 | 3.75 | s | + | − | + |
| 1-Acetoxypinoresinol | OCH3 | 3.76 | s | − | + | + |
| Campesterol | CH3–18 | 0.70 | s | + | + | + |
| β-Sitosterol | CH3–18 | 0.68 | s | + | + | + |
| Peak | Retention Time (min) | [M-H]- (m/z) | MS/MS | Error (ppm) | Proposed Compound | Molecular Formula |
|---|---|---|---|---|---|---|
| 00 | 3.07 ± 0.01 | 153.0556 | 123.4023; 95.4011 | −0.65 | 3-Hydroxytyrosol | C8H10O3 |
| 0 | 4.46 ± 0.03 | 137.0602 | 112.0045 | −4.37 | Tyrosol | C8H10O2 |
| 1 | 6.47 ± 0.03 | 335.1093 | 199.0564; 155.0665 | −8.95 | Oleacinic acid | C17H20O7 |
| 2 | 8.77 ± 0.02 | 319.1168 | 181.0452; 199.0551 | −5.95 | Oleocanthalic acid | C17H20O6 |
| 3 | 9.41 ± 0.02 | 285.0410 | 175.0382; 199.0373 | 2.10 | Luteolin | C15H10O6 |
| 4 | 9.57 ± 0.11 | 357.1368 | 136.0393; 342.0891 | 7.00 | Pinoresinol | C20H21O6 |
| 5 | 10.14 ± 0.19 | 377.1205 | 275.0829; 149.0197; 139.0074 | 0.7 | Oleuropein aglycone | C19H21O8 |
| 6 | 10.32 ± 0.04 | 377.1205 | 275.0831; 149.0199; 139.0076 | 0.9 | Oleuropein aglycone Isomer II | C19H21O8 |
| 7 | 10.51 ± 0.02 | 269.0414 | 225.0481; 150.0228; 117.0289 | 5.7 | Apigenin | C15H10O5 |
| 8 | 10.67 ± 0.16 | 377.1205 | 275.0829; 149.0197; 139.0074 | −4.4 | Oleuropein aglycone Isomer III | C19H21O8 |
| 9 | 10.90 ± 0.10 | 377.1201 | 275.0842; 149.0174; 139.0010 | −5.4 | Oleuropein aglycone Isomer IV | C19H21O8 |
| 10 | 11.42 ± 0.19 | 361.1250 | 291.0780; 259.0926 | −4.1 | Ligstroside-aglycone | C19H22O7 |
| 11 | 12.21 ± 0.02 | 361.1242 | 291.0801; 259.0865 | −9.0 | Ligstroside-aglycone Isomer II | C19H22O7 |
| 12 | 12.63 ± 0.02 | 361.1271 | 291.0808; 259.0909 | −4.2 | Ligstroside-aglycone Isomer III | C19H22O7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzetti, L.; Ferrara, F.; Marotta, L.; Gemma, S.; Butini, S.; Benedusi, M.; Fusi, F.; Ahmed, A.; Pomponi, S.; Ferrari, S.; et al. Extra Virgin Olive Oil Extracts of Indigenous Southern Tuscany Cultivar Act as Anti-Inflammatory and Vasorelaxant Nutraceuticals. Antioxidants 2022, 11, 437. https://doi.org/10.3390/antiox11030437
Pozzetti L, Ferrara F, Marotta L, Gemma S, Butini S, Benedusi M, Fusi F, Ahmed A, Pomponi S, Ferrari S, et al. Extra Virgin Olive Oil Extracts of Indigenous Southern Tuscany Cultivar Act as Anti-Inflammatory and Vasorelaxant Nutraceuticals. Antioxidants. 2022; 11(3):437. https://doi.org/10.3390/antiox11030437
Chicago/Turabian StylePozzetti, Luca, Francesca Ferrara, Ludovica Marotta, Sandra Gemma, Stefania Butini, Mascia Benedusi, Fabio Fusi, Amer Ahmed, Serena Pomponi, Stefano Ferrari, and et al. 2022. "Extra Virgin Olive Oil Extracts of Indigenous Southern Tuscany Cultivar Act as Anti-Inflammatory and Vasorelaxant Nutraceuticals" Antioxidants 11, no. 3: 437. https://doi.org/10.3390/antiox11030437
APA StylePozzetti, L., Ferrara, F., Marotta, L., Gemma, S., Butini, S., Benedusi, M., Fusi, F., Ahmed, A., Pomponi, S., Ferrari, S., Perini, M., Ramunno, A., Pepe, G., Campiglia, P., Valacchi, G., Carullo, G., & Campiani, G. (2022). Extra Virgin Olive Oil Extracts of Indigenous Southern Tuscany Cultivar Act as Anti-Inflammatory and Vasorelaxant Nutraceuticals. Antioxidants, 11(3), 437. https://doi.org/10.3390/antiox11030437














