Olanzapine-Induced Therapeutic Hypothermia Attenuates Renal Injury in Rats after Asphyxial Cardiac Arrest and Resuscitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
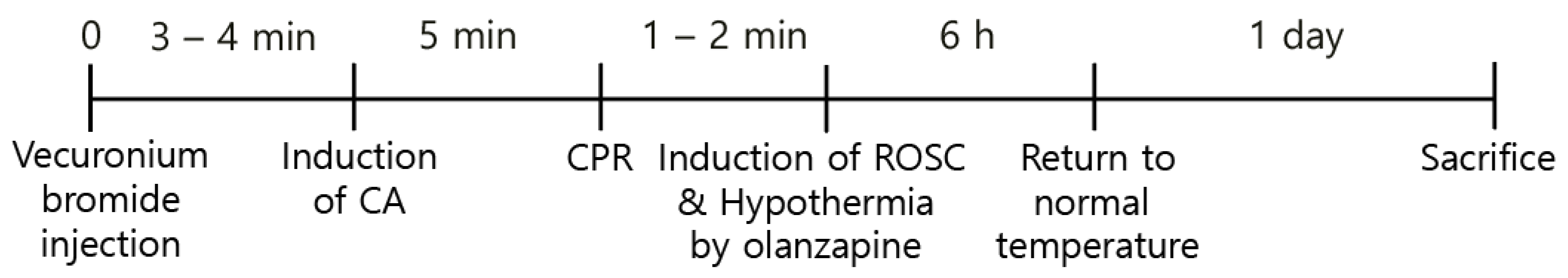
2.2. CA and CPR Procedures
2.3. Induction of Hypothermia
2.4. Hematoxylin and Eosin (H&E) and Periodic Acid–Schiff (PAS) Staining
2.5. Measurement of Serum Urea Nitrogen (BUN) and Creatinine
2.6. Immunostaining for Terminal Deoxynucleotidyl Transferase dUTP End Labeling (TUNEL); Intracellular Reactive Oxygen Species (ROS) Production; Mitochondrial Membrane Potential (MMP); Sirt3 and FOXO3a Proteins
2.7. Western Blot Analyses
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analyses
3. Results
3.1. Olanzapine-Induced TH Improves Overall Survival in CA-Induced Rats
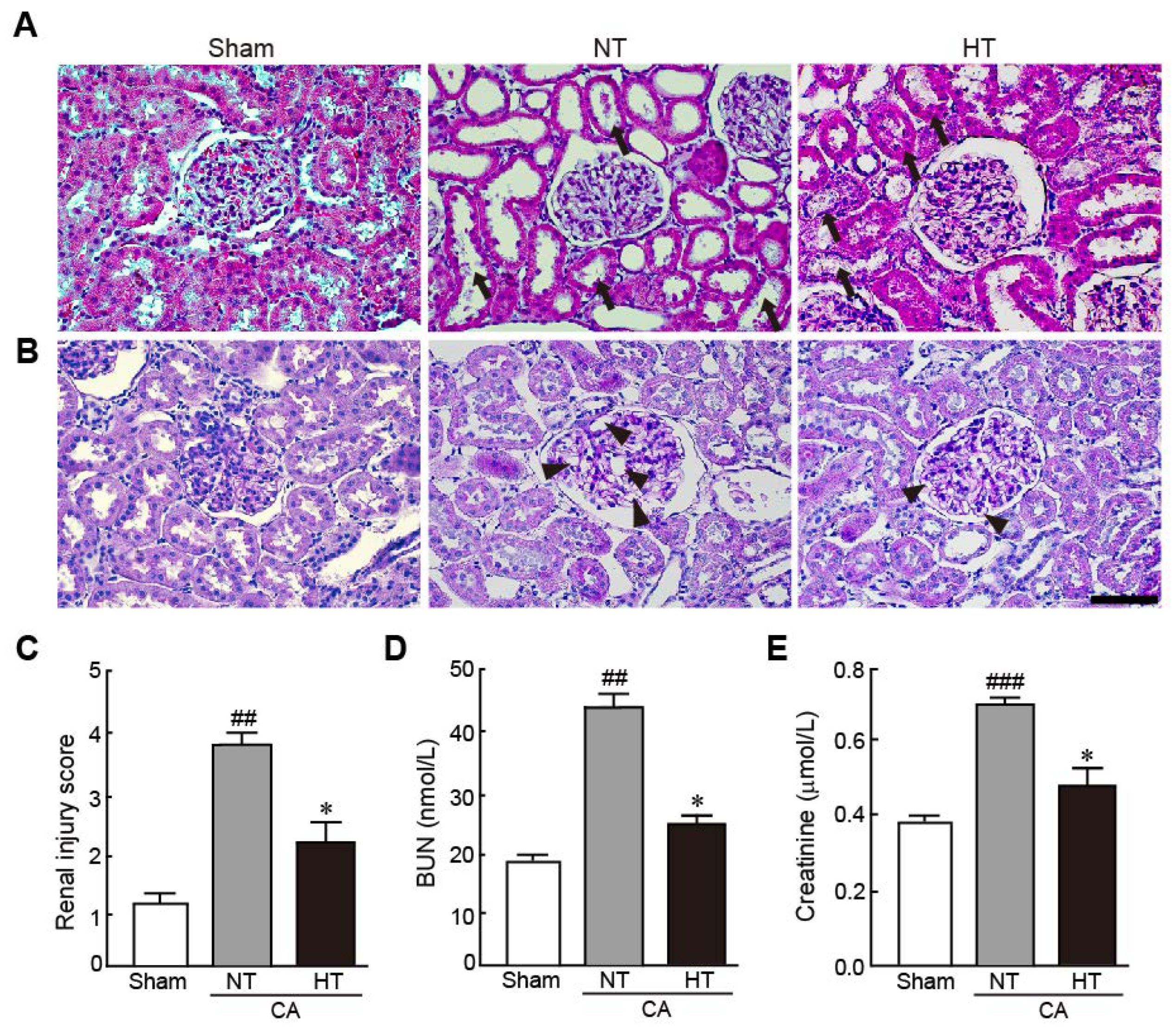
3.2. Olanzapine-Induced TH Attenuates Renal Injury in CA-Induced Rats
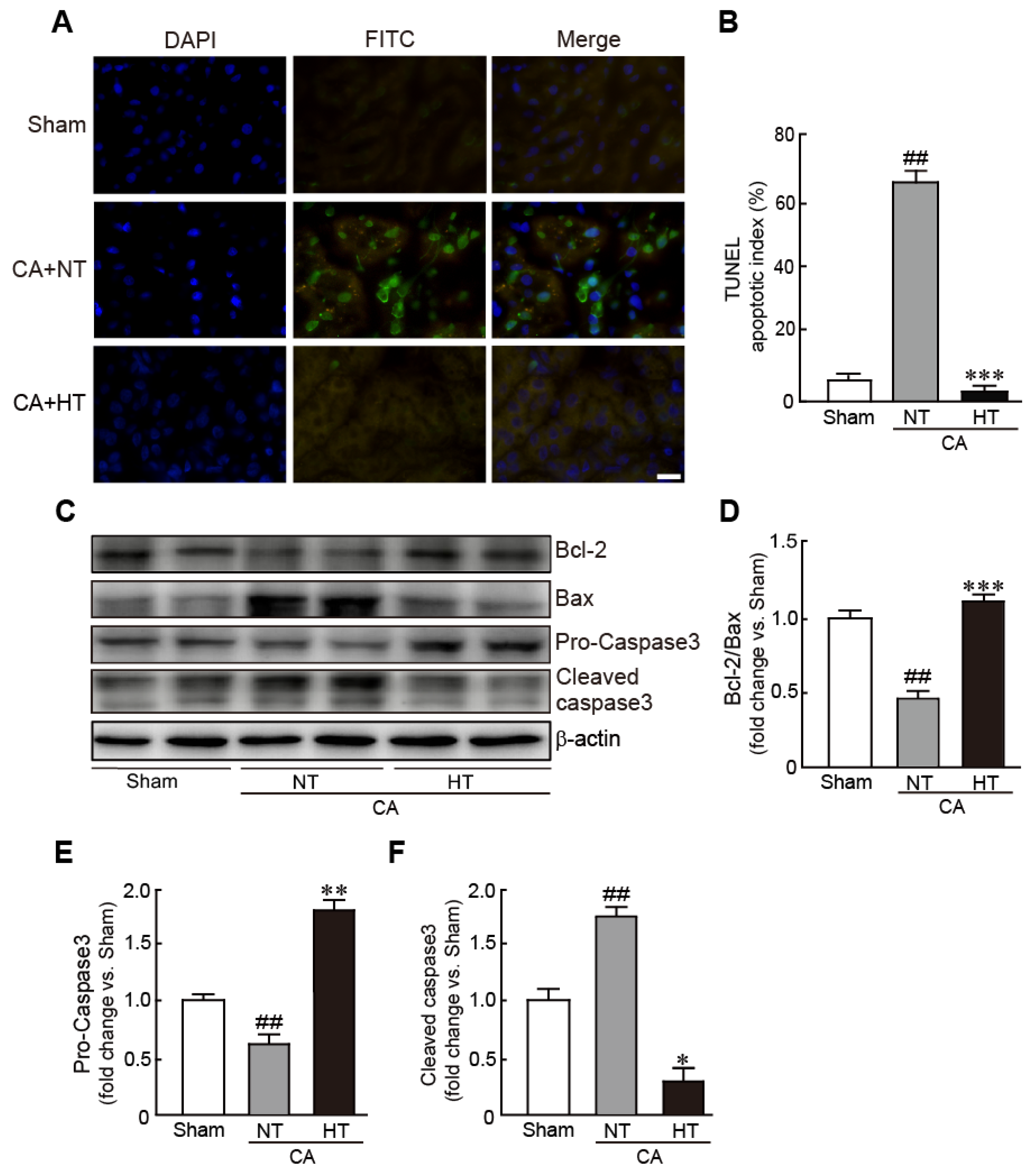
3.3. Olanzapine-Induced TH Inhibits CA-Induced Apoptotic Cell Death in Renal Tissues
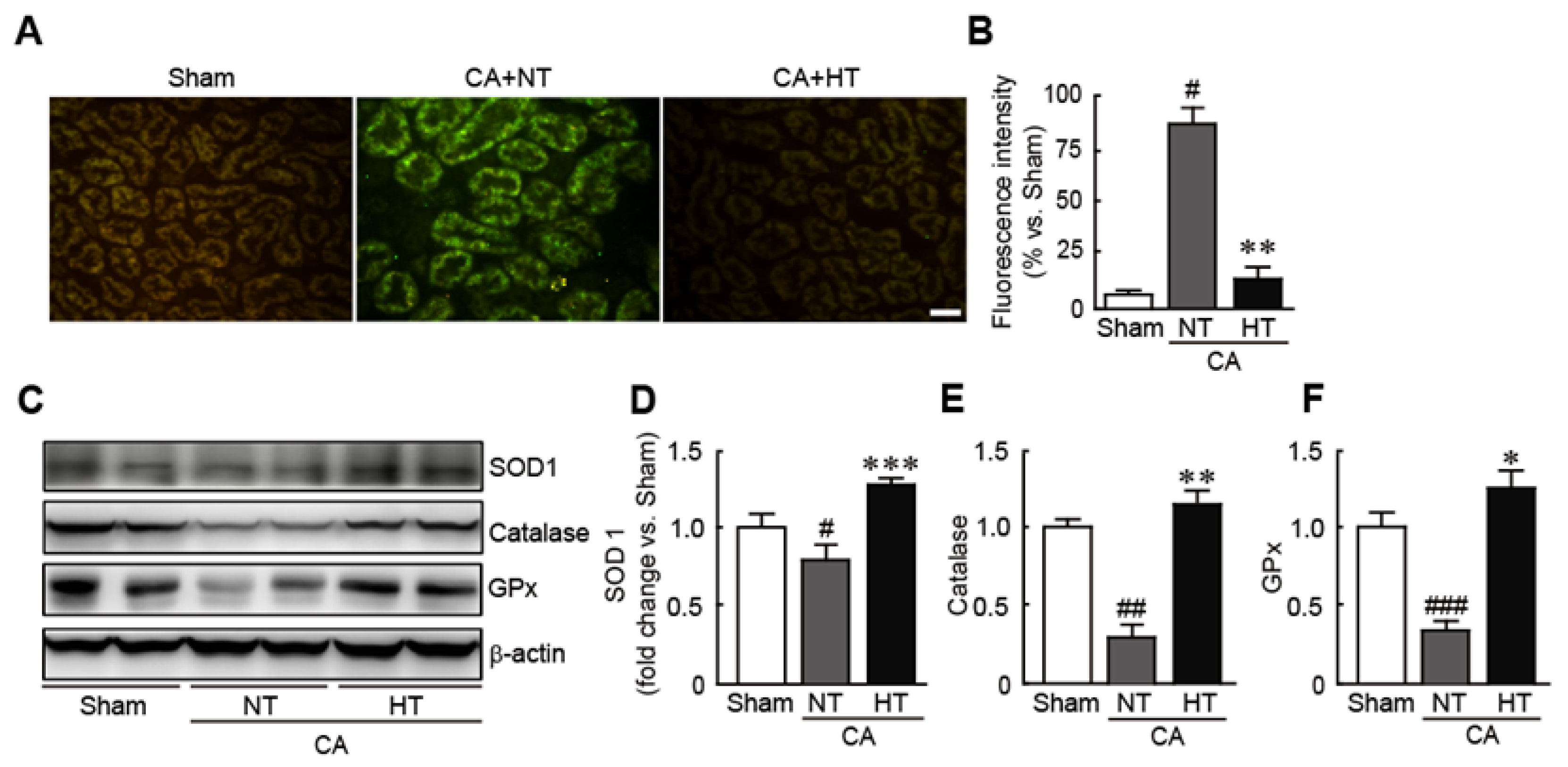
3.4. Olanzapine-Induced TH Inhibits CA-Induced Oxidative Stress in Renal Tissues
3.5. Olanzapine-Induced TH Preserves Mitochondrial Integrity Affected by CA in Renal Tissues
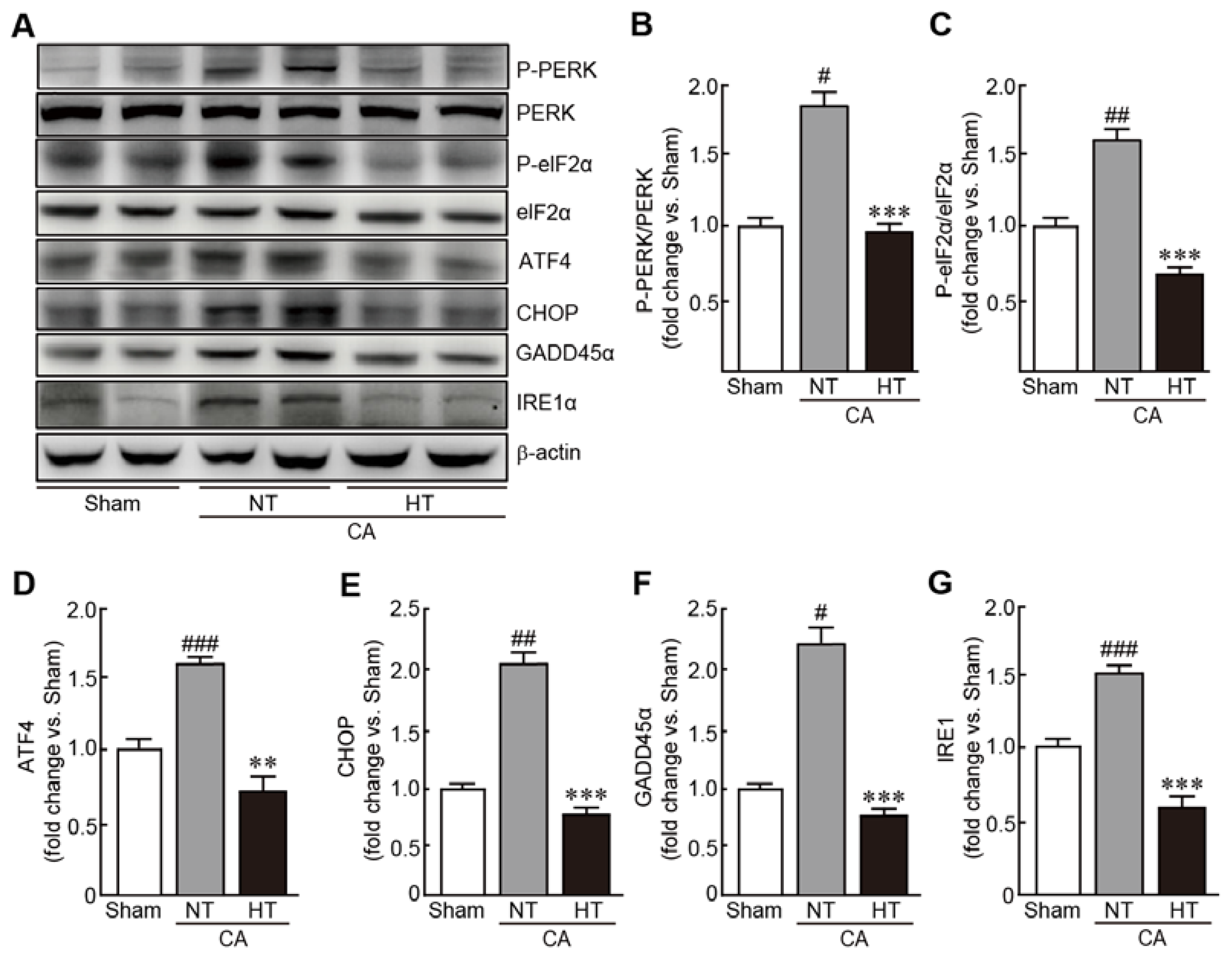
3.6. Olanzapine-Induced TH Inhibits CA-Induced ER Stress in Renal Tissues
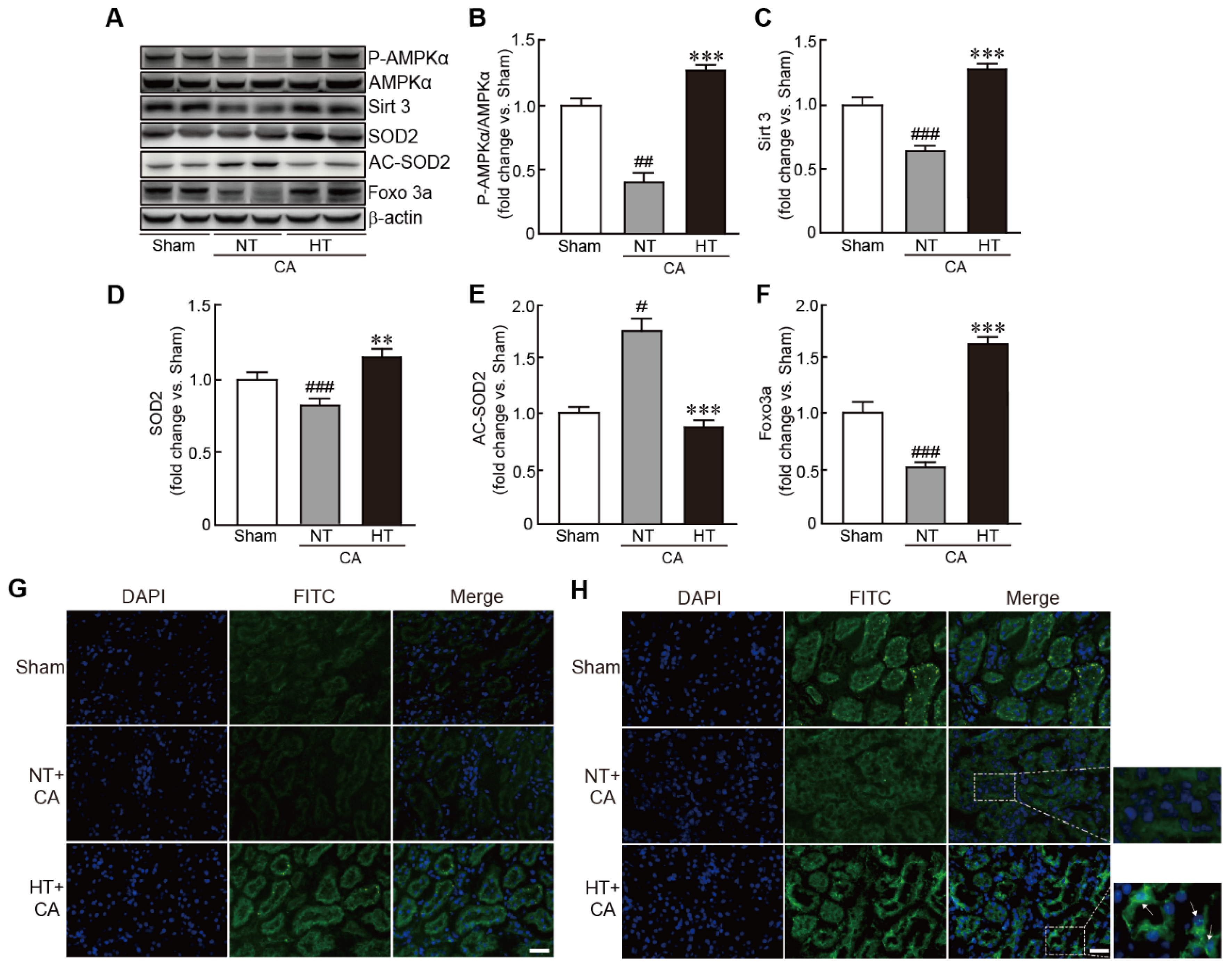
3.7. Olanzapine-Induced TH Activates the Sirt3-Related Signaling Pathway in CA-Induced Renal Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girotra, S.; Chan, P.S.; Bradley, S.M. Post-resuscitation care following out-of-hospital and in-hospital cardiac arrest. Heart 2015, 101, 1943–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Chan, P.S.; McNally, B.; Tang, F.; Kellermann, A. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation 2014, 130, 1876–1882. [Google Scholar] [CrossRef] [Green Version]
- Girotra, S.; Nallamothu, B.K.; Spertus, J.A.; Li, Y.; Krumholz, H.M.; Chan, P.S. Trends in survival after in-hospital cardiac arrest. N. Engl. J. Med. 2012, 367, 1912–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, B.W.; Kilgannon, J.H.; Chansky, M.E.; Mittal, N.; Wooden, J.; Parrillo, J.E.; Trzeciak, S. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit. Care Med. 2013, 41, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication: A Scientific Statement from the International Liaison Committee on Resuscitation; The American Heart Association Emergency Cardiovascular Care Committee; The Council on Cardiovascular Surgery and Anesthesia; The Council on Cardiopulmonary, Perioperative, and Critical Care; The Council on Clinical Cardiology; The Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar]
- Madl, C.; Holzer, M. Brain function after resuscitation from cardiac arrest. Curr. Opin. Crit. Care 2004, 10, 213–217. [Google Scholar] [CrossRef]
- Mongardon, N.; Dumas, F.; Ricome, S.; Grimaldi, D.; Hissem, T.; Pene, F.; Cariou, A. Postcardiac arrest syndrome: From immediate resuscitation to long-term outcome. Ann. Intensive Care 2011, 1, 45. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Hari, K.J.; Azizian, J.; Masmoudi, Y.; Khalid, F.; Kowal, J.L.; Ahmad, M.I.; Majeed, M.; Macdonald, L.; Sunkara, P.; et al. Incidence, Predictors, and Prognosis of Acute Kidney Injury Among Cardiac Arrest Survivors. J. Intensive Care Med. 2021, 36, 550–556. [Google Scholar] [CrossRef]
- Tujjar, O.; Mineo, G.; Dell’Anna, A.; Poyatos-Robles, B.; Donadello, K.; Scolletta, S.; Vincent, J.L.; Taccone, F.S. Acute kidney injury after cardiac arrest. Crit. Care 2015, 19, 169. [Google Scholar] [CrossRef] [Green Version]
- Nath, K.A.; Norby, S.M. Reactive oxygen species and acute renal failure. Am. J. Med. 2000, 109, 665–678. [Google Scholar] [CrossRef]
- Fay, T. Observations on generalized refrigeration in cases of severe cerebral trauma. Assoc. Res. Nerv. Ment. Dis. Proc. 1943, 24, 611–619. [Google Scholar]
- Omairi, A.M.; Pandey, S. Targeted Hypothermia Temperature Management; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, A.; Holzer, M.; Sterz, F.; Behringer, W.; Schorkhuber, W.; Mullner, M.; Frass, M.; Siostrzonek, P.; Ratheiser, K.; Kaff, A.; et al. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Stroke 2000, 31, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.M.; Lundbye, J.B. Managing the Complications of Mild Therapeutic Hypothermia in the Cardiac Arrest Patient. J. Intensive Care Med. 2015, 30, 259–269. [Google Scholar] [CrossRef]
- Black, P.R.; van Devanter, S.; Cohn, L.H. Effects of hypothermia on systemic and organ system metabolism and function. J. Surg. Res. 1976, 20, 49–63. [Google Scholar] [CrossRef]
- Fries, M.; Stoppe, C.; Brucken, D.; Rossaint, R.; Kuhlen, R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J. Crit. Care 2009, 24, 453–457. [Google Scholar] [CrossRef]
- Hackenhaar, F.S.; Medeiros, T.M.; Heemann, F.M.; Behling, C.S.; Putti, J.S.; Mahl, C.D.; Verona, C.; da Silva, A.C.A.; Guerra, M.C.; Goncalves, C.A.S.; et al. Therapeutic Hypothermia Reduces Oxidative Damage and Alters Antioxidant Defenses after Cardiac Arrest. Oxidative Med. Cell. Longev. 2017, 2017, 8704352. [Google Scholar] [CrossRef]
- Suh, G.J.; Kwon, W.Y.; Kim, K.S.; Lee, H.J.; Jeong, K.Y.; Jung, Y.S.; Lee, J.H. Prolonged therapeutic hypothermia is more effective in attenuating brain apoptosis in a Swine cardiac arrest model*. Crit. Care Med. 2014, 42, e132–e142. [Google Scholar] [CrossRef]
- Moler, F.W.; Silverstein, F.S.; Holubkov, R.; Slomine, B.S.; Christensen, J.R.; Nadkarni, V.M.; Meert, K.L.; Browning, B.; Pemberton, V.L.; Page, K.; et al. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N. Engl. J. Med. 2017, 376, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Legriel, S.; Lemiale, V.; Schenck, M.; Chelly, J.; Laurent, V.; Daviaud, F.; Srairi, M.; Hamdi, A.; Geri, G.; Rossignol, T.; et al. Hypothermia for Neuroprotection in Convulsive Status Epilepticus. N. Engl. J. Med. 2016, 375, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drabek, T.; Foley, L.M.; Janata, A.; Stezoski, J.; Hitchens, T.K.; Manole, M.D.; Kochanek, P.M. Global and regional differences in cerebral blood flow after asphyxial versus ventricular fibrillation cardiac arrest in rats using ASL-MRI. Resuscitation 2014, 85, 964–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, R.; Miao, W.; Evans, C.; Lu, L.; Lyu, J.; Li, X.; Warner, D.S.; Zhong, X.; Hoffmann, U.; et al. Development and Evaluation of a Novel Mouse Model of Asphyxial Cardiac Arrest Revealed Severely Impaired Lymphopoiesis After Resuscitation. J. Am. Heart Assoc. 2021, 10, e019142. [Google Scholar] [CrossRef]
- Lu, J.; Qian, H.Y.; Liu, L.J.; Zhou, B.C.; Xiao, Y.; Mao, J.N.; An, G.Y.; Rui, M.Z.; Wang, T.; Zhu, C.L. Mild hypothermia alleviates excessive autophagy and mitophagy in a rat model of asphyxial cardiac arrest. Neurol. Sci. 2014, 35, 1691–1699. [Google Scholar] [CrossRef]
- Klopfleisch, R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology—A systematic review. BMC Vet. Res. 2013, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Daneshgar, N.; Baguley, A.W.; Liang, P.I.; Wu, F.; Chu, Y.; Kinter, M.T.; Benavides, G.A.; Johnson, M.S.; Darley-Usmar, V.; Zhang, J.; et al. Metabolic derangement in polycystic kidney disease mouse models is ameliorated by mitochondrial-targeted antioxidants. Commun. Biol. 2021, 4, 1200. [Google Scholar] [CrossRef]
- Bhana, N.; Perry, C.M. Olanzapine: A review of its use in the treatment of bipolar I disorder. CNS Drugs 2001, 15, 871–904. [Google Scholar] [CrossRef]
- Bhana, N.; Foster, R.H.; Olney, R.; Plosker, G.L. Olanzapine: An updated review of its use in the management of schizophrenia. Drugs 2001, 61, 111–161. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Calligaro, D.O.; Falcone, J.F.; Marsh, R.D.; Moore, N.A.; Tye, N.C.; Seeman, P.; Wong, D.T. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996, 14, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Zonnenberg, C.; Bueno-de-Mesquita, J.M.; Ramlal, D.; Blom, J.D. Hypothermia due to Antipsychotic Medication: A Systematic Review. Front. Psychiatry 2017, 8, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blass, D.M.; Chuen, M. Olanzapine-associated hypothermia. Psychosomatics 2004, 45, 135–139. [Google Scholar] [CrossRef]
- Evers, S.S.; Calcagnoli, F.; van Dijk, G.; Scheurink, A.J. Olanzapine causes hypothermia, inactivity, a deranged feeding pattern and weight gain in female Wistar rats. Pharmacol. Biochem. Behav. 2010, 97, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oerther, S.; Ahlenius, S. Atypical antipsychotics and dopamine D(1) receptor agonism: An in vivo experimental study using core temperature measurements in the rat. J. Pharmacol. Exp. Ther. 2000, 292, 731–736. [Google Scholar]
- Sandroni, C.; Dell’anna, A.M.; Tujjar, O.; Geri, G.; Cariou, A.; Taccone, F.S. Acute kidney injury (AKI) after cardiac arrest: A systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016, 82, 989–999. [Google Scholar] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovcanin, B.; Medic, B.; Kocic, G.; Cebovic, T.; Ristic, M.; Prostran, M. Molecular Dissection of Renal Ischemia-Reperfusion: Oxidative Stress and Cellular Events. Curr. Med. Chem. 2016, 23, 1965–1980. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.J. The mitochondrial permeability transition pore: A molecular target for amyotrophic lateral sclerosis therapy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 186–197. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends. Cell. Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Pihan, P.; Carreras-Sureda, A.; Hetz, C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017, 24, 1478–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.A.; Kim, J.E.; Jo, M.; Ko, G.J. The Role of Sirtuins in Kidney Diseases. Int. J. Mol. Sci. 2020, 21, 6686. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Q.; Tu, S.; Qin, W. SIRT3 mediates mitofusin 2 ubiquitination and degradation to suppress ischemia reperfusion-induced acute kidney injury. Exp. Cell Res. 2021, 408, 112861. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef] [Green Version]
- Golson, M.L.; Kaestner, K.H. Fox transcription factors: From development to disease. Development 2016, 143, 4558–4570. [Google Scholar] [CrossRef] [Green Version]
- Fasano, C.; Disciglio, V.; Bertora, S.; Signorile, M.L.; Simone, C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef] [Green Version]


| Genes | Accesion No. | Primers | |
|---|---|---|---|
| PGC-1α | NM_031347.1 | forward | 5′-ACCGTAAATCTGCGGGATGA-3′ |
| reverse | 5′-AGTTTCATTCGACCTGCGTAAAGTA-3′ | ||
| NRF-1 | NM_001100708.1 | forward | 5′-CACTCTGGCTGAAGCCACCTTAC-3′ |
| reverse | 5′-TCACGGCTTTGCTGATGGTC-3′ | ||
| TFAM | NM_031326.1 | forward | 5′-TGAAGCTTGTAAATCAGGCTTGGA-3′ |
| reverse | 5′-GAGATCACTTCGCCCAACTTCAG-3′ | ||
| PPARα | NM_013196.1 | forward | 5′-GGCAATGCACTGAACATCGAG-3′ |
| reverse | 5′-GCCGAATAGTTCGCCGAAAG-3′ | ||
| ERRα | NM_001008511.2 | forward | 5′-GCTGAAAGCTCTGGCCCTTG-3′ |
| reverse | 5′-TGCTCCACAGCCTCAGCAT-3′ | ||
| ATP8 | NC_012920.1 | forward | 5′-TGCCACAACTAGACACATCCA-3′ |
| reverse | 5′-TGTGGGGGTAATGAAAGAGG-3′ | ||
| 18S rRNA (internal control) | NM_213557.1 | forward | 5′-AAGTTTCAGCACATCCTGCGAGTA-3′ |
| reverse | 5′-TTGGTGAGGTCAATGTCTGCTTTC-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungalag, T.; Yoo, Y.-J.; Tae, H.-J.; Yang, D.K. Olanzapine-Induced Therapeutic Hypothermia Attenuates Renal Injury in Rats after Asphyxial Cardiac Arrest and Resuscitation. Antioxidants 2022, 11, 443. https://doi.org/10.3390/antiox11030443
Tungalag T, Yoo Y-J, Tae H-J, Yang DK. Olanzapine-Induced Therapeutic Hypothermia Attenuates Renal Injury in Rats after Asphyxial Cardiac Arrest and Resuscitation. Antioxidants. 2022; 11(3):443. https://doi.org/10.3390/antiox11030443
Chicago/Turabian StyleTungalag, Tsendsuren, Yeo-Jin Yoo, Hyun-Jin Tae, and Dong Kwon Yang. 2022. "Olanzapine-Induced Therapeutic Hypothermia Attenuates Renal Injury in Rats after Asphyxial Cardiac Arrest and Resuscitation" Antioxidants 11, no. 3: 443. https://doi.org/10.3390/antiox11030443






