Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression
Abstract
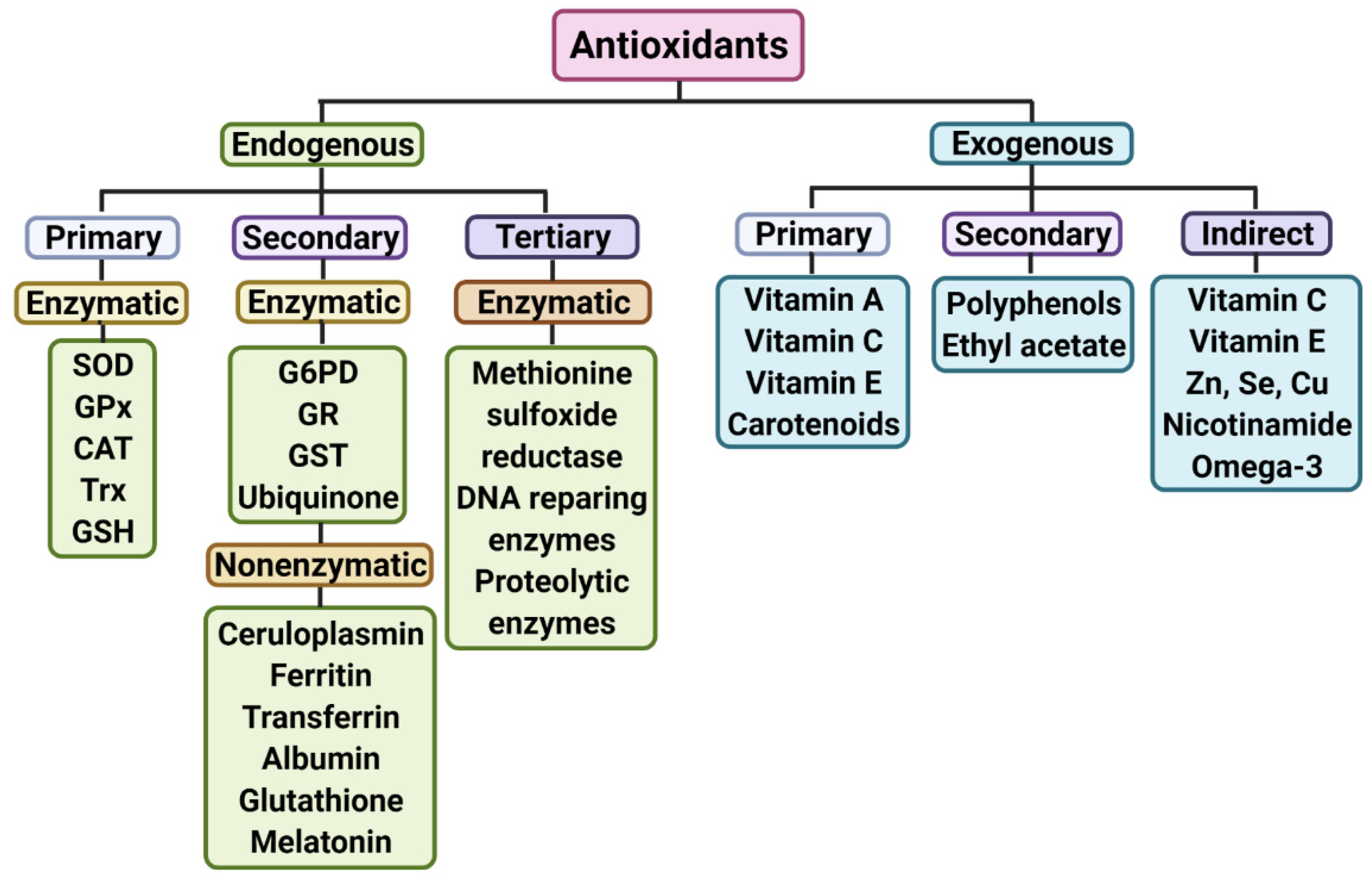
:1. Introduction
2. Current Treatments for MDD and Treatment Challenges
Proposed Treatments for Difficult-to-Treat Depression (DTD)
3. Oxidative Damage in the Brain and Its Association with MDD
4. Oxidative Stress and Inflammation Crosstalk in the Brain of Depressive Patients
5. Plant-Derived Antioxidant Molecules in MDD Treatment
6. Antioxidant and Anti-Inflammatory Potential of Mesenchymal Stem Cells in the Treatment of MDD
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, 2011–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain. Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, G.; Santaguida, P.; Keshavarz, H.; Jaworska, N.; Levine, M.; Beyene, J.; Raina, P. Systematic Review of Clinical Practice Guidelines for Failed Antidepressant Treatment Response in Major Depressive Disorder, Dysthymia, and Subthreshold Depression in Adults. Can. J. Psychiatry 2017, 62, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J. Persistent adverse effects of antidepressants. Epidemiol. Psychiatr. Sci. 2020, 29, E56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawam, E.A.; Laurencic, G.; Malone, D.A. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351–361. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; Da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid. Med. Cell. Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Kinlein, S.A.; Phillips, D.J.; Keller, C.R.; Karatsoreos, I.N. Role of corticosterone in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 2019, 102, 248–255. [Google Scholar] [CrossRef]
- McHugh, R.K.; Whitton, S.W.; Peckham, A.D.; Welge, J.A.; Otto, M.W. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: A meta-analytic review. J. Clin. Psychiatry 2013, 74, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Magnani, M.; Sasdelli, A.; Bellino, S.; Bellomo, A.; Carpiniello, B.; Politi, P.; Menchetti, M.; Berardi, D. Treating Depression: What Patients Want; Findings From a Randomized Controlled Trial in Primary Care. Psychosomatics 2016, 57, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Behr, G.A.; Moreira, J.C.F.; Frey, B.N. Preclinical and Clinical Evidence of Antioxidant Effects of Antidepressant Agents: Implications for the Pathophysiology of Major Depressive Disorder. Oxid. Med. Cell. Longev. 2012, 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, K.; Uher, R.; Crawford, A.A.; Lewis, G.; O’Donovan, M.C.; Keers, R.; Dernovšek, M.Z.; Mors, O.; Hauser, J.; Souery, D.; et al. Genetic predictors of antidepressant side effects: A grouped candidate gene approach in the Genome-Based Therapeutic Drugs for Depression (GENDEP) study. J. Psychopharmacol. 2014, 28, 142–150. [Google Scholar] [CrossRef]
- Ho, S.C.; Chong, H.Y.; Chaiyakunapruk, N.; Tangiisuran, B.; Jacob, S.A. Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: A systematic review. J. Affect. Disord. 2016, 193, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Munder, T.; Gerger, H.; Nüesch, E.; Trelle, S.; Znoj, H.; Jüni, P.; Cuijpers, P. Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis. PLoS Med. 2013, 10, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Loerinc, A.G.; Meuret, A.E.; Twohig, M.P.; Rosenfield, D.; Bluett, E.J.; Craske, M.G. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clin. Psychol. Rev. 2015, 42, 72–82. [Google Scholar] [CrossRef]
- Marwood, L.; Wise, T.; Perkins, A.M.; Cleare, A.J. Meta-analyses of the neural mechanisms and predictors of response to psychotherapy in depression and anxiety. Neurosci. Biobehav. Rev. 2018, 95, 61. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Gazal, M.; Mondin, T.C.; Cardoso, T.A.; Quevedo, L.T.; Souza, L.D.M.; Jansen, K.; Braganhol, E.; Oses, J.P.; Pinheiro, R.T.; et al. Cognitive psychotherapy treatment decreases peripheral oxidative stress parameters associated with major depression disorder. Biol. Psychol. 2015, 110, 175–181. [Google Scholar] [CrossRef]
- Dimidjian, S.; Hollon, S.D.; Dobson, K.S.; Schmaling, K.B.; Kohlenberg, R.J.; Addis, M.E.; Gallop, R.; McGlinchey, J.B.; Markley, D.K.; Gollan, J.K.; et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J. Consult. Clin. Psychol. 2006, 74, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Otto, M.W. Assessing BDNF as a mediator of the effects of exercise on depression. J. Psychiatr. Res. 2020, 123, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Bridges, L.; Sharma, M. The Efficacy of Yoga as a Form of Treatment for Depression. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Naveen, G.H.; Varambally, S.; Thirthalli, J.; Rao, M.; Christopher, R.; Gangadhar, B.N. Serum cortisol and BDNF in patients with major depression—Effect of yoga. Int. Rev. Psychiatry 2016, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M.; Sagar, R.; Dada, R. Impact of Yoga and Meditation on Cellular Aging in Apparently Healthy Individuals: A Prospective, Open-Label Single-Arm Exploratory Study. Oxid. Med. Cell. Longev. 2017, 2017, 7928981. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M.R.; Sagar, R.; Faiq, M.; Dada, R. Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 423–442. [Google Scholar] [CrossRef]
- Gautam, S.; Tolahunase, M.; Kumar, U.; Dada, R. Impact of yoga based mind-body intervention on systemic inflammatory markers and co-morbid depression in active Rheumatoid arthritis patients: A randomized controlled trial. Restor. Neurol. Neurosci. 2019, 37, 41–59. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Langhorst, J.; Dobos, G. Yoga for depression: A systematic review and meta-analysis. Depress. Anxiety 2013, 30, 1068–1083. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Z.; Wang, Y.; Ma, X.; Wang, X.; Wang, X.; Liu, J.; Huang, Y.; Zhang, J.; Li, L.; et al. Manual or electroacupuncture as an add-on therapy to SSRIs for depression: A randomized controlled trial. J. Psychiatr. Res. 2019, 114, 24–33. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Z.; Wang, Y.; Ma, X.; Wang, X.; Wang, X.; Liang, Y.; Yang, X.; Sun, Y.; Song, M.; et al. Can acupuncture combined with SSRIs improve clinical symptoms and quality of life in patients with depression? Secondary outcomes of a pragmatic randomized controlled trial. Complement. Ther. Med. 2019, 45, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, M.; Yin, X.; Lao, L.; Kuang, Z.; Xu, S. The effect of acupuncture on depression and its correlation with metabolic alterations: A randomized controlled trial. Medicine 2020, 99, e22752. [Google Scholar] [CrossRef]
- Li, M.; Niu, J.; Yan, P.; Yao, L.; He, W.; Wang, M.; Li, H.; Cao, L.; Li, X.; Shi, X.; et al. The effectiveness and safety of acupuncture for depression: An overview of meta-analyses. Complement. Ther. Med. 2020, 50, 102202. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gao, Y.; Yin, X.; Zhang, Z.; Lao, L.; Chen, Q.; Xu, S. The mechanism of electroacupuncture for depression on basic research: A systematic review. Chin. Med. 2021, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Kang, S.Y.; Kwon, O.S.; Bang, S.K.; Kim, S.P.; Choi, K.-H.; Moon, J.Y.; Ryu, Y. A mechanical acupuncture instrument mitigates the endoplasmic reticulum stress and oxidative stress of ovariectomized rats. Integr. Med. Res. 2019, 8, 187–194. [Google Scholar] [CrossRef]
- Yang, J.W.; Wang, X.R.; Ma, S.M.; Yang, N.N.; Li, Q.Q.; Liu, C.Z. Acupuncture attenuates cognitive impairment, oxidative stress and NF-κB activation in cerebral multi-infarct rats. Acupunct. Med. 2019, 37, 283–291. [Google Scholar] [CrossRef]
- Cai, W.; Ma, W.; Wang, G.T.; Li, Y.J.; Shen, W.D. Antidepressant, anti-inflammatory, and antioxidant effects of electroacupuncture through sonic hedgehog-signaling pathway in a rat model of poststroke depression. Neuropsychiatr. Dis. Treat. 2019, 15, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [Green Version]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav. Immun. 2013, 28, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAllister-Williams, R.H.; Arango, C.; Blier, P.; Demyttenaere, K.; Falkai, P.; Gorwood, P.; Hopwood, M.; Javed, A.; Kasper, S.; Malhi, G.S.; et al. The identification, assessment and management of difficult-to-treat depression: An international consensus statement. J. Affect. Disord. 2020, 267, 264–282. [Google Scholar] [CrossRef]
- Edwards, S.J.; Hamilton, V.; Nherera, L.; Trevor, N. Lithium or an atypical antipsychotic drug in the management of treatment resistant depression: A systematic review and economic evaluation. Health Technol. Assess. 2013, 17, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijaz, S.; Davies, P.; Williams, C.J.; Kessler, D.; Lewis, G.; Wiles, N. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst. Rev. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Zhang, X.; Zhao, W.; Ma, T.; Liu, Y.; Ma, P.; Zhao, Y.; Zhang, H. Ketamine relieves depression-like behaviors induced by chronic postsurgical pain in rats through anti-inflammatory, anti-oxidant effects and regulating BDNF expression. Psychopharmacology 2020, 237, 1657–1669. [Google Scholar] [CrossRef]
- Zhang, K.; Hashimoto, K. An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev. Neurother. 2019, 19, 83–92. [Google Scholar] [CrossRef]
- Leal, G.C.; Bandeira, I.D.; Correia-Melo, F.S.; Telles, M.; Mello, R.P.; Vieira, F.; Lima, C.S.; Jesus-Nunes, A.P.; Guerreiro-Costa, L.N.F.; Marback, R.F.; et al. Intravenous arketamine for treatment-resistant depression: Open-label pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 577–582. [Google Scholar] [CrossRef]
- Strumila, R.; Nobile, B.; Korsakova, L.; Lengvenyte, A.; Olie, E.; Lopez-Castroman, J.; Guillaume, S.; Courtet, P. Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors. Pharmaceuticals 2021, 14, 1213. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S.B.; Pace, B.T.; Nicholas, C.R.; Raison, C.L.; Hutson, P.R. The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry Res. 2020, 284, 112749. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.L.; Eloff, J.N. Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom. Plants 2020, 9, 1127. [Google Scholar] [CrossRef]
- Napierala, M.; Bodnar, A.; Chlopocka-Wozniak, M.; Permoda-Osip, A.; Rybakowski, J. Electroconvulsive therapy and autobiographical memory in patients with treatment-resistant depression. Psychiatr. Pol. 2019, 53, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Tsourtos, G.; Spong, J.; Stough, C. The effects of electro-convulsive therapy on the speed of information processing in major depression. J. Affect. Disord. 2007, 103, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Vasavada, M.M.; Leaver, A.M.; Njau, S.; Joshi, S.H.; Ercoli, L.; Hellemann, G.; Narr, K.L.; Espinoza, R. Short- and Long-term Cognitive Outcomes in Patients with Major Depression Treated with Electroconvulsive Therapy. J. ECT 2017, 33, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Geduldig, E.T.; Kellner, C.H. Electroconvulsive Therapy in the Elderly: New Findings in Geriatric Depression. Curr. Psychiatry Rep. 2016, 18, 1–6. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, Q.; Zhang, W.; Huang, X.; Zhu, M.; Geng, R.; Cheng, X.; Bao, C.; Wang, Y.; Zhang, C.; et al. Disturbance of Oxidative Stress Parameters in Treatment-Resistant Bipolar Disorder and Their Association with Electroconvulsive Therapy Response. Int. J. Neuropsychopharmacol. 2020, 23, 207. [Google Scholar] [CrossRef]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef]
- Wadhwa, R.; Gupta, R.; Maurya, P.K. Oxidative Stress and Accelerated Aging in Neurodegenerative and Neuropsychiatric Disorder. Curr. Pharm. Des. 2018, 24, 4711–4725. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef] [Green Version]
- Ekoue, D.N.; He, C.; Diamond, A.M.; Bonini, M.G. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E.; Maes, M. Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis/chronic fatigue syndrome: Another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuroendocrinol. Lett. 2011, 32, 133–140. [Google Scholar] [PubMed]
- Bal, N.; Acar, S.T.; Yazici, A.; Yazici, K.; Tamer, L. Altered Levels of Malondialdehyde and Vitamin E in Major Depressive Disorder and Generalized Anxiety Disorder. Dusunen Adam J. Psychiatry Neurol. Sci. 2012, 25, 206. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000, 58, 241–246. [Google Scholar] [CrossRef]
- Rybka, J.; Kedziora-Kornatowska, K.; Banaś-Lezańska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kedziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef]
- Szebeni, A.; Szebeni, K.; DiPeri, T.; Chandley, M.J.; Crawford, J.D.; Stockmeier, C.A.; Ordway, G.A. Shortened telomere length in white matter oligodendrocytes in major depression: Potential role of oxidative stress. Int. J. Neuropsychopharmacol. 2014, 17, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Wigner, P.; Synowiec, E.; Czarny, P.; Bijak, M.; Jóźwiak, P.; Szemraj, J.; Gruca, P.; Papp, M.; Śliwiński, T. Effects of venlafaxine on the expression level and methylation status of genes involved in oxidative stress in rats exposed to a chronic mild stress. J. Cell. Mol. Med. 2020, 24, 5675. [Google Scholar] [CrossRef] [Green Version]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, W.; Zhan, L.; Sui, H.; Zhang, L.; Zhao, C.; Lu, X. The ZiBuPiYin recipe regulates proteomic alterations in brain mitochondria-associated ER membranes caused by chronic psychological stress exposure: Implications for cognitive decline in Zucker diabetic fatty rats. Aging 2020, 12, 23698. [Google Scholar] [CrossRef] [PubMed]
- Colodro-Conde, L.; Couvy-Duchesne, B.; Zhu, G.; Coventry, W.L.; Byrne, E.M.; Gordon, S.; Wright, M.J.; Montgomery, G.W.; Madden, P.A.F.; Ripke, S.; et al. A direct test of the diathesis–stress model for depression. Mol. Psychiatry 2018, 23, 1590. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dai, J. Advance in Stress for Depressive Disorder. Adv. Exp. Med. Biol. 2019, 1180, 147–178. [Google Scholar] [CrossRef] [PubMed]
- Blugeot, A.; Rivat, C.; Bouvier, E.; Molet, J.; Mouchard, A.; Zeau, B.; Bernard, C.; Benoliel, J.J.; Becker, C. Vulnerability to depression: From brain neuroplasticity to identification of biomarkers. J. Neurosci. 2011, 31, 12889–12899. [Google Scholar] [CrossRef]
- Bouvier, E.; Brouillard, F.; Molet, J.; Claverie, D.; Cabungcal, J.H.; Cresto, N.; Doligez, N.; Rivat, C.; Do, K.Q.; Bernard, C.; et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry 2017, 22, 1701–1713. [Google Scholar] [CrossRef] [Green Version]
- Eren, I.; Naziroǧlu, M.; Demirdaş, A.; Çelik, Ö.; Uǧuz, A.C.; Altunbaşak, A.; Özmen, I.; Uz, E. Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem. Res. 2007, 32, 497–505. [Google Scholar] [CrossRef]
- Celikbilek, A.; Yesim Gocmen, A.; Tanik, N.; Yaras, N.; Yargicoglu, P.; Gumuslu, S. Serum lipid peroxidation markers are correlated with those in brain samples in different stress models. Acta Neuropsychiatr. 2014, 26, 51–57. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Steenkamp, L.R.; Hough, C.M.; Reus, V.I.; Jain, F.A.; Epel, E.S.; James, S.J.; Morford, A.E.; Mellon, S.H.; Wolkowitz, O.M.; Lindqvist, D. Severity of anxiety- but not depression- is associated with oxidative stress in Major Depressive Disorder. J. Affect. Disord. 2017, 219, 193–200. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain. Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Sutcigil, L.; Oktenli, C.; Musabak, U.; Bozkurt, A.; Cansever, A.; Uzun, O.; Sanisoglu, S.Y.; Yesilova, Z.; Ozmenler, N.; Ozsahin, A.; et al. Pro- and anti-inflammatory cytokine balance in major depression: Effect of sertraline therapy. Clin. Dev. Immunol. 2007, 2007, 76396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Bailey, M.L.; Andridge, R.; Peng, J.; Jaremka, L.M.; Fagundes, C.P.; Malarkey, W.B.; Laskowski, B.; Belury, M.A. Marital Distress, Depression, and a Leaky Gut: Translocation of Bacterial Endotoxin as a Pathway to Inflammation. Psychoneuroendocrinology 2018, 98, 52. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Herane Vives, A.; Cleare, A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Fagundes, C.P.; Andridge, R.; Peng, J.; Malarkey, W.B.; Habash, D.; Belury, M.A. Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol. Psychiatry 2017, 22, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Maes, M. Targeting cyclooxygenase-2 in depression is not a viable therapeutic approach and may even aggravate the pathophysiology underpinning depression. Metab. Brain Dis. 2012, 27, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Borisov, A.S.; Broadwell, S.D.; Capuron, L.; Woolwine, B.J.; Jacobson, I.M.; Nemeroff, C.B.; Miller, A.H. Depression during pegylated interferon-alpha plus ribavirin therapy: Prevalence and prediction. J. Clin. Psychiatry 2005, 66, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Nicholson, G.C.; Williams, L.J.; Jacka, F.N.; Henry, M.J.; Kotowicz, M.A.; Schneider, H.G.; Leonard, B.E.; Berk, M. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry 2010, 197, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeck, C.; Koenig, A.M.; Schury, K.; Geiger, M.L.; Karabatsiakis, A.; Wilker, S.; Waller, C.; Gündel, H.; Fegert, J.M.; Calzia, E.; et al. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion 2016, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2013, 76, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Fernandez, S.; Gurpegui, M.; Diaz-Atienza, F.; Perez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: Results from a meta-analysis. J. Clin. Psychiatry 2015, 76, 1658–1667. [Google Scholar] [CrossRef]
- Chung, C.P.; Schmidt, D.; Stein, C.M.; Morrow, J.D.; Salomon, R.M. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013, 206, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Szebeni, A.; Szebeni, K.; DiPeri, T.P.; Johnson, L.A.; Stockmeier, C.A.; Crawford, J.D.; Chandley, M.J.; Hernandez, L.J.; Burgess, K.C.; Brown, R.W.; et al. Elevated DNA Oxidation and DNA Repair Enzyme Expression in Brain White Matter in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2017, 20, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflam. 2021, 18, 1–23. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- O’neill Rothenberg, D.; Zhang, L. Mechanisms Underlying the Anti-Depressive Effects of Regular Tea Consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Z.; Fan, W.; Zhong, X.; Peng, M.; Liu, Z. Nano-Strategies for Enhancing the Bioavailability of Tea Polyphenols: Preparation, Applications, and Challenges. Foods 2022, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef]
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 9268083. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Agah, S.; Dulce Estêvão, M.; Hosseini, A.S.; Heydari, H.; Toupchian, O.; Abdollahi, S.; Persad, E.; Abu-Zaid, A.; Rezamand, G.; et al. Effect of saffron supplementation on oxidative stress parameters: A systematic review and meta-analysis of randomized placebo-controlled trials. Food Sci. Nutr. 2021, 9, 5809–5819. [Google Scholar] [CrossRef]
- Dai, L.; Chen, L.; Wang, W. Safety and Efficacy of Saffron (Crocus sativus L.) for Treating Mild to Moderate Depression: A Systematic Review and Meta-analysis. J. Nerv. Ment. Dis. 2020, 208, 269–276. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z.; et al. The Efficacy of Saffron in the Treatment of Mild to Moderate Depression: A Meta-analysis. Planta Med. 2019, 85, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum. Psychopharmacol. 2014, 29, 517–527. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2017, 207, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.; Huang, H.; Liu, Z. The efficacy and acceptability of curcumin for the treatment of depression or depressive symptoms: A systematic review and meta-analysis. J. Affect. Disord. 2021, 282, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gistau, V.; Colom, F.; Mané, A.; Romero, S.; Sugranyes, G.; Vieta, E. Atypical depression is associated with suicide attempt in bipolar disorder. Acta Psychiatr. Scand. 2009, 120, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kućma, M.; Styczeń, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: Effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancers. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Cas, M.D.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [Green Version]
- Al-Karawi, D.; Al Mamoori, D.A.; Tayyar, Y. The Role of Curcumin Administration in Patients with Major Depressive Disorder: Mini Meta-Analysis of Clinical Trials. Phytother. Res. 2016, 30, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liang, C.; Zheng, L.; Liu, L.; Li, Z.; Yang, G.; Li, Y. Anti-fatigue effect of hypericin in a chronic forced exercise mouse model. J. Ethnopharmacol. 2022, 284, 114767. [Google Scholar] [CrossRef]
- Huang, N.; Rizshsky, L.; Hauck, C.; Nikolau, B.J.; Murphy, P.A.; Birt, D.F. Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 2011, 72, 2015–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.V.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Alzoubi, K.H.; Abdel-Hafiz, L.; Khabour, O.F.; El-Elimat, T.; Alzubi, M.A.; Alali, F.Q. Evaluation of the Effect of Hypericum triquetrifolium Turra on Memory Impairment Induced by Chronic Psychosocial Stress in Rats: Role of BDNF. Drug Des. Devel. Ther. 2020, 14, 5299–5314. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Scicchitano, F.; Whalley, B.J.; Mazzitello, C.; Ciriaco, M.; Esposito, S.; Patanè, M.; Upton, R.; Pugliese, M.; Chimirri, S.; et al. Hypericum perforatum: Pharmacokinetic, mechanism of action, tolerability, and clinical drug-drug interactions. Phyther. Res. 2014, 28, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; do Céu Costa, M. Medicinal plants and their preparations in the European market: Why has the harmonization failed? The cases of St. John’s wort, valerian, ginkgo, ginseng, and green tea. Phytomedicine 2021, 81, 153421. [Google Scholar] [CrossRef] [PubMed]
- Furhad, S.; Bokhari, A.A. Herbal Supplements; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Moretti, M.; Rodrigues, A.L.S. Functional role of ascorbic acid in the central nervous system: A focus on neurogenic and synaptogenic processes. Nutr. Neurosci. 2021, 1–11. [Google Scholar] [CrossRef]
- Shivavedi, N.; Charan Tej, G.N.V.; Neogi, K.; Nayak, P.K. Ascorbic acid therapy: A potential strategy against comorbid depression-like behavior in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2019, 109, 351–359. [Google Scholar] [CrossRef]
- Maratha, S.; Sharma, V.; Walia, V. Antidepressant Like Effect of Ascorbic Acid in Mice: Possible Involvement of NO-sGC-cGMP Signaling. Neurochem. Res. 2021, 47, 967–978. [Google Scholar] [CrossRef]
- Fraga, D.B.; Camargo, A.; Olescowicz, G.; Azevedo Padilha, D.; Mina, F.; Budni, J.; Brocardo, P.S.; Rodrigues, A.L.S. A single administration of ascorbic acid rapidly reverses depressive-like behavior and hippocampal synaptic dysfunction induced by corticosterone in mice. Chem. Biol. Interact. 2021, 342, 109476. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, W.; Wu, Q.; Zheng, H.; Li, Y. Ascorbic acid intake is inversely associated with prevalence of depressive symptoms in US midlife women: A cross-sectional study. J. Affect. Disord. 2021, 299, 498–503. [Google Scholar] [CrossRef]
- Yosaee, S.; Keshtkaran, Z.; Abdollahi, S.; Shidfar, F.; Sarris, J.; Soltani, S. The effect of vitamin C supplementation on mood status in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Gen. Hosp. Psychiatry 2021, 71, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [Green Version]
- Bonsack, B.; Corey, S.; Shear, A.; Heyck, M.; Cozene, B.; Sadanandan, N.; Zhang, H.; Gonzales-Portillo, B.; Sheyner, M.; Borlongan, C.V. Mesenchymal stem cell therapy alleviates the neuroinflammation associated with acquired brain injury. CNS Neurosci. Ther. 2020, 26, 603–615. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflamm. 2019, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ezquer, F.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Lespay-Rebolledo, C.; Herrera-Marschitz, M.; Israel, Y. Activated mesenchymal stem cell administration inhibits chronic alcohol drinking and suppresses relapse-like drinking in high-alcohol drinker rats. Addict. Biol. 2019, 24, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Francos, S.; Eiro, N.; Costa, L.A.; Escudero-Cernuda, S.; Fernández-Sánchez, M.L.; Vizoso, F.J. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Santapau, D.; Berríos-Cárcamo, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y. Intranasal mesenchymal stem cell secretome administration markedly inhibits alcohol and nicotine self-administration and blocks relapse-intake: Mechanism and translational options. Stem Cell Res. Ther. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Fei, G.Q.; Liu, W.J.; Ding, J.; Wang, Y.; Wang, H.; Jin, J.; Wang, X. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-κB signaling pathways. Acta Pharmacol. Sin. 2020, 41, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Chen, C.-N.; Zhan, J.-Q.; Liu, Q.-S.; Liu, Y.; Jiang, S.-Z.; Wei, B. Decreased Plasma Hydrogen Sulfide Level Is Associated with the Severity of Depression in Patients with Depressive Disorder. Front. Psychiatry 2021, 12, 5664. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Shefa, U.; Kim, M.S.; Jeong, N.Y.; Jung, J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxid. Med. Cell. Longev. 2018, 2018, 1873962. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Shi, S. Hydrogen Sulfide Regulates Homeostasis of Mesenchymal Stem Cells and Regulatory T Cells. J. Dent. Res. 2016, 95, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.; Jiang, L.; Lan, F.; Tang, Y.Y.; Zhang, P.; Zou, W.; Chen, Y.J.; Tang, X.Q. Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res. Bull. 2021, 177, 194–202. [Google Scholar] [CrossRef]
- Tseng, N.; Lambie, S.C.; Huynh, C.Q.; Sanford, B.; Patel, M.; Herson, P.S.; Ormond, D.R. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow Metab. 2021, 41, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dai, Z.; Jin, Y.; Chen, P. Emerging Antioxidant Paradigm of Mesenchymal Stem Cell-Derived Exosome Therapy. Front. Endocrinol. 2021, 12, 727272. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riveros, M.E.; Ávila, A.; Schruers, K.; Ezquer, F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants 2022, 11, 540. https://doi.org/10.3390/antiox11030540
Riveros ME, Ávila A, Schruers K, Ezquer F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants. 2022; 11(3):540. https://doi.org/10.3390/antiox11030540
Chicago/Turabian StyleRiveros, María Eugenia, Alba Ávila, Koen Schruers, and Fernando Ezquer. 2022. "Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression" Antioxidants 11, no. 3: 540. https://doi.org/10.3390/antiox11030540
APA StyleRiveros, M. E., Ávila, A., Schruers, K., & Ezquer, F. (2022). Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants, 11(3), 540. https://doi.org/10.3390/antiox11030540





