Vitamin D Deficiency, Excessive Gestational Weight Gain, and Oxidative Stress Predict Small for Gestational Age Newborns Using an Artificial Neural Network Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval Statement
2.2. Study Design
2.3. Samples, Biochemical Analysis, and Oxidative Stress Markers
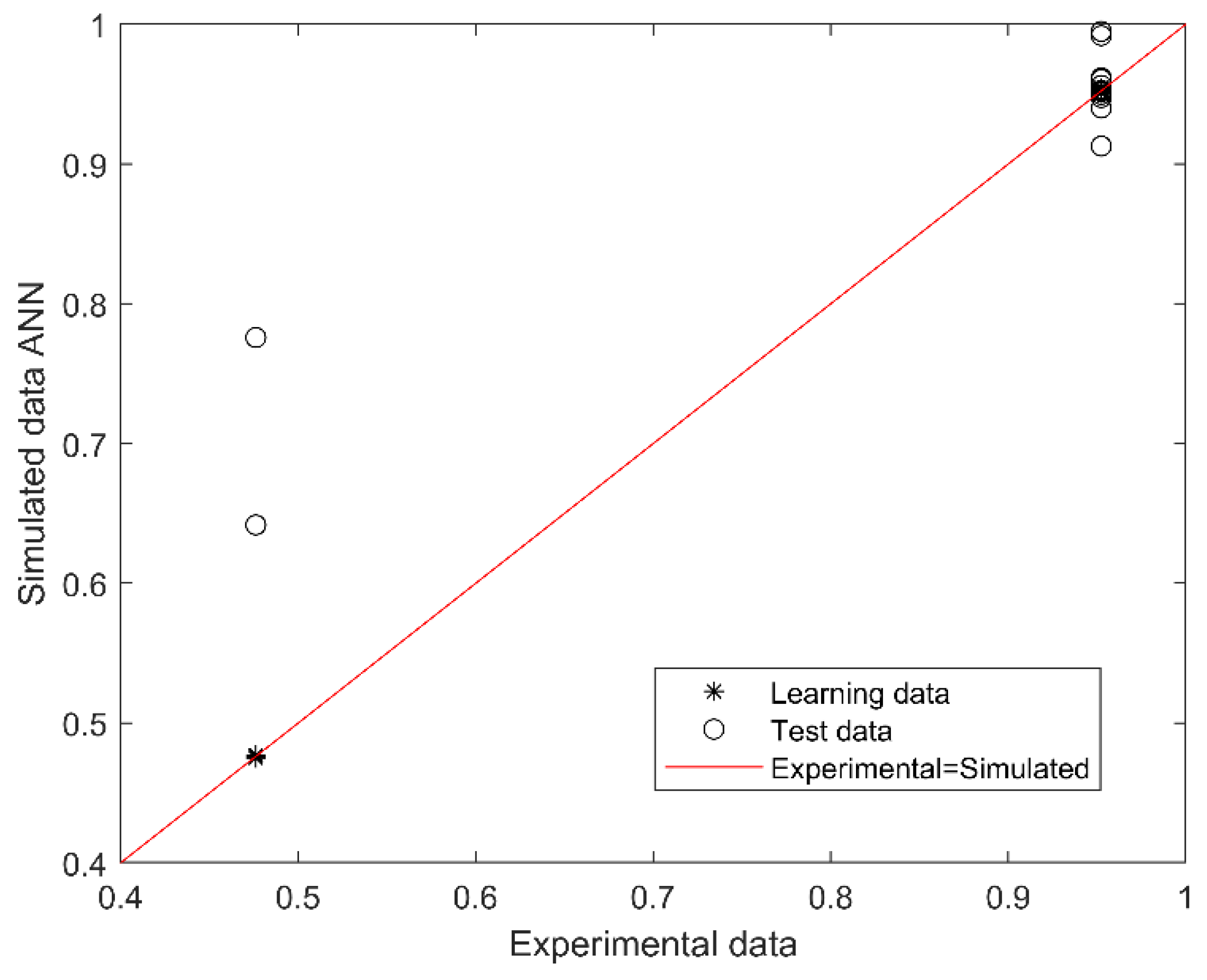
2.4. Artificial Neural Network Model Development and Validation
2.5. Predictive Performance of the Model
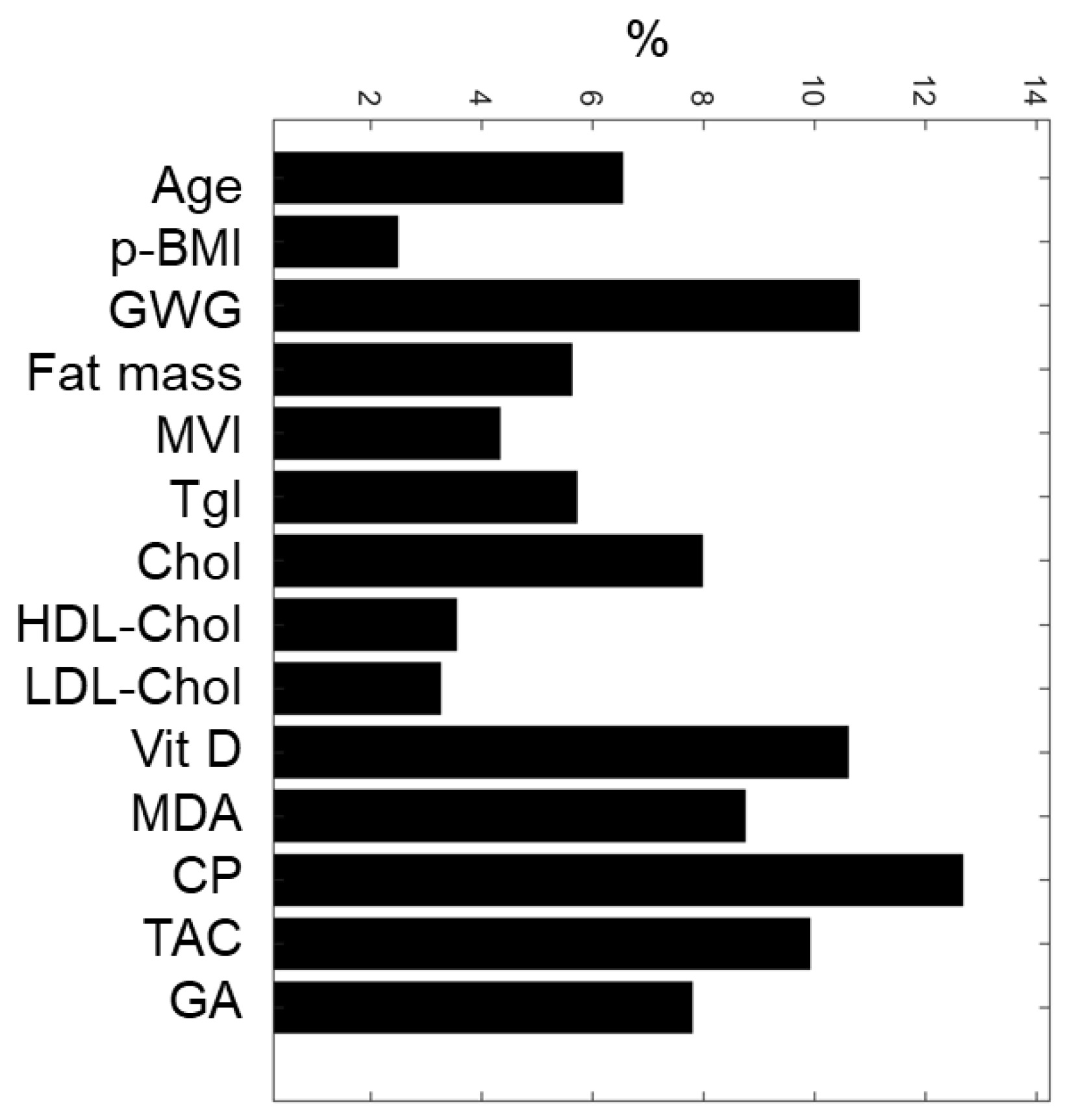
2.6. The Relative Importance of the Maternal Variables in the Prediction
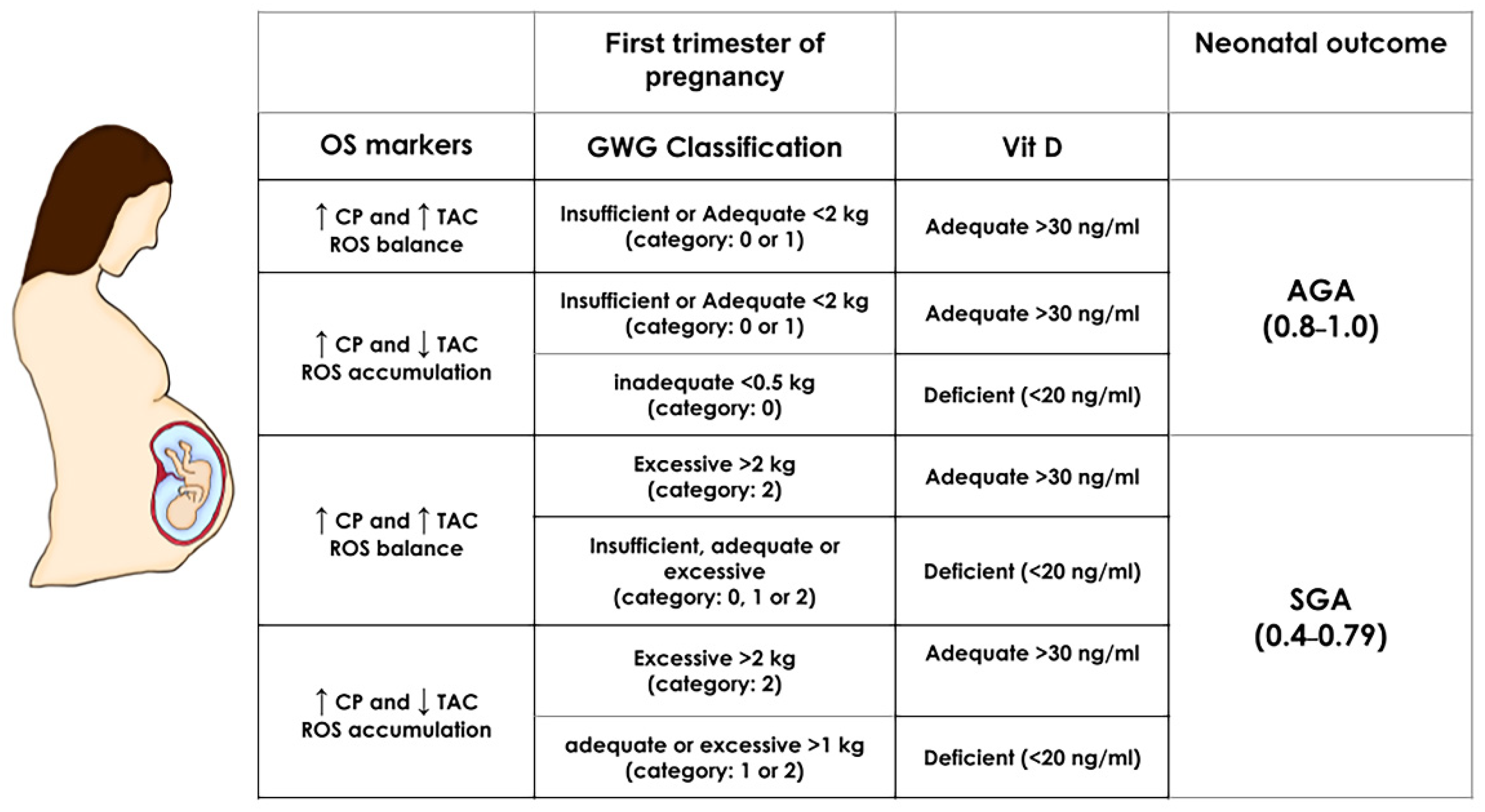
2.7. The Simulator of SGA
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Population
3.2. Development and Validation of SGA Predictive Model
3.3. Performance-Based on Confusion Matrix
3.4. The Relative Importance of Maternal Variables
3.5. Simulator for SGA
4. Discussion
4.1. Strengths and Limitations of the Study
4.2. Challenge and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Drake, A.J.; Reynolds, R.M. Impact of Maternal Obesity on Offspring Obesity and Cardiometabolic Disease Risk. J. Reprod. Fertil. 2010, 140, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Myatt, L. The Human Placenta in Health and Disease, an Issue of Obstetrics and Gynecology Clinics E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; ISBN 9780323710985. [Google Scholar]
- Lee, A.C.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and Regional Estimates of Term and Preterm Babies Born Small for Gestational Age in 138 Low-Income and Middle-Income Countries in 2010. Lancet Glob. Health 2013, 1, e26–e36. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.C.; Kozuki, N.; Cousens, S.; Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.; Schmiegelow, C.; Adair, L.S.; et al. Estimates of Burden and Consequences of Infants Born Small for Gestational Age in Low and Middle-Income Countries with INTERGROWTH-21 Standard: Analysis of CHERG Datasets. BMJ 2017, 358, j3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, E.; Fernandes, M.; Fazel, M.; Kennedy, S.H.; Villar, J.; Stein, A. Differential Effect of Intrauterine Growth Restriction on Childhood Neurodevelopment: A Systematic Review. BJOG 2015, 122, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.H. Handbook of Parenting: Volume 2: Biology and Ecology of Parenting, 3rd ed.; Routledge: London, UK, 2019; ISBN 9780429685880. [Google Scholar]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dede, H.; Takmaz, O.; Ozbasli, E.; Dede, S.; Gungor, M. Higher Level of Oxidative Stress Markers in Small for Gestational Age Newborns Delivered by Cesarean Section at Term. Fetal Pediatr. Pathol. 2017, 36, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Westermeier, F.; Sáez, P.J.; Villalobos-Labra, R.; Sobrevia, L.; Farías-Jofré, M. Programming of Fetal Insulin Resistance in Pregnancies with Maternal Obesity by ER Stress and Inflammation. BioMed Res. Int. 2014, 2014, 917672. [Google Scholar] [CrossRef]
- Perichart-Perera, O.; Muñoz-Manrique, C.; Reyes-López, A.; Tolentino-Dolores, M.; Espino, Y.; Sosa, S.; Ramírez-González, M.C. Metabolic Markers during Pregnancy and Their Association with Maternal and Newborn Weight Status. PLoS ONE 2017, 12, e0180874. [Google Scholar] [CrossRef]
- Feghali, M.N.; Catov, J.M.; Zantow, E.; Mission, J.; Caritis, S.N.; Scifres, C.M. Timing of Gestational Weight Gain and Adverse Perinatal Outcomes in Overweight and Obese Women. Obstet. Gynecol. 2019, 133, 962–970. [Google Scholar] [CrossRef]
- Prado, E.L.; Sebayang, S.K.; Apriatni, M.; Adawiyah, S.R.; Hidayati, N.; Islamiyah, A.; Siddiq, S.; Harefa, B.; Lum, J.; Alcock, K.J.; et al. Maternal Multiple Micronutrient Supplementation and Other Biomedical and Socioenvironmental Influences on Children’s Cognition at Age 9-12 Years in Indonesia: Follow-up of the SUMMIT Randomised Trial. Lancet Glob. Health 2017, 5, e217–e228. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Liu, Z.B.; Ma, L.; Zhang, Z.C.; Fu, L.; Yu, Z.; Chen, W.; Song, Y.P.; Wang, P.; Wang, H.; et al. Gestational Vitamin D Deficiency Causes Placental Insufficiency and Fetal Intrauterine Growth Restriction Partially through Inducing Placental Inflammation. J. Steroid Biochem. Mol. Biol. 2020, 203, 105733. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Blanco, I.; Agodi, A. Effects of Vitamin D Supplementation During Pregnancy on Birth Size: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgen, F.; Onal, E.; Varol, F.G. IUGR Detection by Ultrasonographic Examinations Using Neural Networks. IEEE Eng. Med. Biol. Mag. 1997, 16, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Wright, D.; Nicolaides, K.H. Competing-Risks Model for Prediction of Small-for-Gestational-Age Neonate from Maternal Characteristics and Medical History. Ultrasound Obstet. Gynecol. 2020, 56, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Wright, D.; Lolos, M.; Anampousi, K.; Mamalis, M.; Nicolaides, K.H. Competing-Risks Model for Prediction of Small-for-Gestational-Age Neonate from Maternal Characteristics, Serum Pregnancy-Associated Plasma Protein-A and Placental Growth Factor at 11-13 Weeks’ Gestation. Ultrasound Obstet. Gynecol. 2021, 57, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452.
- Xu, Z.; Wen, Z.; Zhou, Y.; Li, D.; Luo, Z. Inadequate Weight Gain in Obese Women and the Risk of Small for Gestational Age (SGA): A Systematic Review and Meta-Analysis. J. Matern. Fetal. Neonatal Med. 2017, 30, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM. Pregnancy Weight Guidelines Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press (US): Washington, DC, USA, 2010; ISBN 9780309131131. [Google Scholar]
- Pelletiet, D. Anthropometric Standardization Reference Manual: Abridged Edition. Edited by T.G. Lohman, A.F. Roche, and R. Martorell. vi 90 Pp. Champaign, IL: Human Kinetics Books. 1991. U.S. 15.00, Canada 18.50 (paper). Am. J. Hum. Biol. 1992, 4, 425. [Google Scholar] [CrossRef]
- Safety, N.A.F. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 2 November 2021).
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Altman, D.G.; Victora, C.; Noble, J.A.; et al. Postnatal Growth Standards for Preterm Infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21(st) Project. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef] [Green Version]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl Groups as Biomarkers of Oxidative Stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total Antioxidant Capacity Assay of Human Serum Using copper(II)-Neocuproine as Chromogenic Oxidant: The CUPRAC Method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Perichart-Perera, O.; González-Leyva, C.P.; González-Ludlow, I.; Tolentino-Dolores, M.; Solis-Paredes, M.; Reyes-Muñoz, E.; Borboa-Olivares, H.; Sánchez-Martínez, M.; Parra-Hernández, S.; Monterrubio-Flores, E.; et al. Vitamin D Deficiency in Mexican Pregnant Women: Is Supplementation with ≤400 IU/day Enough? Nutrients 2020, 12, 2517. [Google Scholar] [CrossRef] [PubMed]
- Helguera-Repetto, A.C.; Soto-Ramírez, M.D.; Villavicencio-Carrisoza, O.; Yong-Mendoza, S.; Yong-Mendoza, A.; León-Juárez, M.; González-Y.-Merchand, J.A.; Zaga-Clavellina, V.; Irles, C. Neonatal Sepsis Diagnosis Decision-Making Based on Artificial Neural Networks. Front. Pediatr. 2020, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.P.; Andaverde, J.; Santoyo, E. Application of the Error Propagation Theory in Estimates of Static Formation Temperatures in Geothermal and Petroleum Boreholes. Energy Convers. Manag. 2006, 47, 3659–3671. [Google Scholar] [CrossRef]
- Aleboyeh, A.; Kasiri, M.B.; Olya, M.E.; Aleboyeh, H. Prediction of Azo Dye Decolorization by UV/H2O2 Using Artificial Neural Networks. Dyes Pigments 2008, 77, 288–294. [Google Scholar] [CrossRef]
- Papastefanou, I.; Souka, A.P.; Pilalis, A.; Eleftheriades, M.; Michalitsi, V.; Kassanos, D. First Trimester Prediction of Small- and Large-for-Gestation Neonates by an Integrated Model Incorporating Ultrasound Parameters, Biochemical Indices and Maternal Characteristics. Acta Obstet. Gynecol. Scand. 2012, 91, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Triunfo, S.; Crispi, F.; Rodriguez-Sureda, V.; Dominguez, C.; Figueras, F.; Gratacos, E. Differential Performance of First-Trimester Screening in Predicting Small-for-Gestational-Age Neonate or Fetal Growth Restriction. Ultrasound Obstet. Gynecol. 2017, 49, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naimi, A.I.; Platt, R.W.; Larkin, J.C. Machine Learning for Fetal Growth Prediction. Epidemiology 2018, 29, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Fung, R.; Villar, J.; Dashti, A.; Ismail, L.C.; Staines-Urias, E.; Ohuma, E.O.; Salomon, L.J.; Victora, C.G.; Barros, F.C.; Lambert, A.; et al. Achieving Accurate Estimates of Fetal Gestational Age and Personalised Predictions of Fetal Growth Based on Data from an International Prospective Cohort Study: A Population-Based Machine Learning Study. Lancet Digit. Health 2020, 2, e368–e375. [Google Scholar] [CrossRef]
- Kuhle, S.; Maguire, B.; Zhang, H.; Hamilton, D.; Allen, A.C.; Joseph, K.S.; Allen, V.M. Comparison of Logistic Regression with Machine Learning Methods for the Prediction of Fetal Growth Abnormalities: A Retrospective Cohort Study. BMC Pregnancy Childbirth 2018, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Ochi, D.; Matsukawa, N.; Saigusa, D.; Ishikuro, M.; Obara, T.; Tsunemoto, Y.; Kumatani, S.; Yamashita, R.; Tanabe, O.; et al. Machine learning approaches to predict gestational age in normal and complicated pregnancies via urinary metabolomics analysis. Sci. Rep. 2021, 11, 17777. [Google Scholar] [CrossRef] [PubMed]
- Bertini, A.; Salas, R.; Chabert, S.; Sobrevia, L.; Pardo, F. Using Machine learning to predict complications in pregnancy: A systematic review. Front. Bioeng. Biotechnol. 2022, 9, 780389. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tam, C.H.-T.; Yuen, L.Y.; Catalano, P.M.; Ma, R.C.-W.; Tam, W.H. Optimal Gestational Weight Gain for Chinese Women—Analysis from a Longitudinal Cohort with Childhood Follow-Up. Lancet Reg. Health West Pac. 2021, 13, 100190. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Mannan, M.; Doi, S.A.R. Gestational Weight Gain in Relation to Offspring Obesity over the Life Course: A Systematic Review and Bias-Adjusted Meta-Analysis. Obes. Rev. 2014, 15, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.G.; White, J.; Murphy, P.; Burrage, L.; Hutchens, D. The Effect of Gestational Weight Gain by Body Mass Index on Maternal and Neonatal Outcomes. J. Obstet. Gynaecol. Can. 2009, 31, 28–35. [Google Scholar] [CrossRef]
- Gallo, L.A.; Barrett, H.L.; Dekker Nitert, M. Review: Placental Transport and Metabolism of Energy Substrates in Maternal Obesity and Diabetes. Placenta 2017, 54, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [Green Version]
- Lausten-Thomsen, U.; Olsen, M.; Greisen, G.; Schmiegelow, K. Inflammatory Markers in Umbilical Cord Blood from Small-for-Gestational-Age Newborns. Fetal Pediatr. Pathol. 2014, 33, 114–118. [Google Scholar] [CrossRef]
- Palacios, C.; Trak-Fellermeier, M.A.; Martinez, R.X.; Lopez-Perez, L.; Lips, P.; Salisi, J.A.; John, J.C.; Peña-Rosas, J.P. Regimens of Vitamin D Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2019, 10, CD013446. [Google Scholar] [CrossRef]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D Status during Pregnancy and Offspring Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Clin. Nutr. 2020, 74, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhang, Q.; Zhao, J.; Shen, Q.; Yao, D.; Wang, T.; Liu, Z. Relationship between Maternal Vitamin D Status in the First Trimester of Pregnancy and Maternal and Neonatal Outcomes: A Retrospective Single Center Study. BMC Pediatr. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between Maternal Serum 25-Hydroxyvitamin D Level and Pregnancy and Neonatal Outcomes: Systematic Review and Meta-Analysis of Observational Studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiao, Y.; Zhang, L.; Gao, Q. Maternal Early Pregnancy Vitamin D Status in Relation to Low Birth Weight and Small-for-Gestational-Age Offspring. J. Steroid Biochem. Mol. Biol. 2018, 175, 146–150. [Google Scholar] [CrossRef]
- Longini, M.; Perrone, S.; Kenanidis, A.; Vezzosi, P.; Marzocchi, B.; Petraglia, F.; Centini, G.; Buonocore, G. Isoprostanes in Amniotic Fluid: A Predictive Marker for Fetal Growth Restriction in Pregnancy. Free Radic. Biol. Med. 2005, 38, 1537–1541. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, İ. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Mert, I.; Oruc, A.S.; Yuksel, S.; Cakar, E.S.; Buyukkagnıcı, U.; Karaer, A.; Danısman, N. Role of Oxidative Stress in Preeclampsia and Intrauterine Growth Restriction. J. Obstet. Gynaecol. Res. 2012, 38, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Alcala, M.; Gutierrez-Vega, S.; Castro, E.; Guzman-Gutiérrez, E.; Ramos-Álvarez, M.P.; Viana, M. Antioxidants and Oxidative Stress: Focus in Obese Pregnancies. Front. Physiol. 2018, 9, 1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grobler, L.; Visser, M.; Siegfried, N. Healthy Life Trajectories Initiative: Summary of the Evidence Base for Pregnancy-Related Interventions to Prevent Overweight and Obesity in Children. Obes. Rev. 2019, 20, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Yuan, H.; Zhang, L.; Zhang, J. SGW-SGN: An integrated machine learning approach for workload forecasting in geo-distributed cloud data centers. Inf. Sci. 2019, 481, 57. [Google Scholar] [CrossRef]
| Variables | All Women (n = 77) Mean ± SD n (%) | SGA (n = 14) Mean ± SD n (%) | AGA (n = 63) Mean ± SD n (%) |
|---|---|---|---|
| Age (years) | 28 ± 5 | 29 ± 4 | 28 ± 5 |
| Parity: | |||
| Nulliparous | 35 (45.5) | 7 (50) | 28 (44.4) |
| Multiparous | 42 (54.5) | 7 (50) | 35 (55.6) |
| Socioeconomic Status: | |||
| Low/lower-middle-income | 49 (63.9) | 11 (78.6) | 28 (60.3) |
| Upper middle-/high-income | 28 (36.4) | 3 (21.4) | 25 (39.7) |
| p-BMI (kg/m2) | 26.9 ± 5.5 | 28.2 ± 8.0 | 26.6 ± 4.9 |
| p-BMI group: | |||
| Normal | 33 (42.9) | 5 (35.7) | 28 (44.4) |
| Overweight/obesity | 44 (57.1) | 9 (64.3) | 35 (55.6) |
| GWG (kg) | 1.5 ± 3.2 | 2 ± 3.1 | 1.4 ± 3.2 |
| Fat mass (%) | 38.8 ± 7.1 | 39.7 ± 8.9 | 38.6 ± 6.8 |
| MVI supplementation: | |||
| Yes | 28 (36.4) | 4 (28.6) | 24 (38.1) |
| No | 49 (63.6) | 10 (71.4) | 39 (61.9) |
| Medication: | |||
| Yes | 5 (6.5) | 1 (7.1) | 4 (6.3) |
| No | 72 (93.5) | 13 (92.9) | 59 (93.7) |
| Glucose (mg/dL) | 80.8 ± 9.6 | 80 ± 11.4 | 81 ± 9.3 |
| Triglycerides (mg/dL) | 136 ± 46.4 | 157 ± 63.4 | 132 ± 41.1 |
| Total Cholesterol (mg/dL) | 187 ± 38.5 | 201 ± 32.6 | 184 ± 39.3 |
| HDL-Cholesterol (mg/dL) | 60.5 ± 12.4 | 59.7 ± 11.1 | 60.7 ± 12.8 |
| LDL-Cholesterol (mg/dL) | 92.1 ± 25.6 | 89.9 ± 27.9 | 92.7 ± 25.3 |
| HbA1c (%) | 5.3 ± 0.4 | 5.2 ± 0.5 | 5.3 ± 0.4 |
| 25-OH-D (ng/mL) | 21.6 ± 6.8 | 19.9 ± 3.4 | 22 ± 7.2 |
| MDA (pmol MDA/mg dry weight) | 170 ± 174 | 153 ± 180 | 173 ± 173 |
| CP (pmol CP/mg protein) | 5397 ± 2617 | 5710 ± 2388 | 5327 ± 2679 |
| TAC (pmol of Trolox equivalent/mg protein) | 81.1 ± 28.4 | 78 ± 30.7 | 81.8 ± 28 |
| Term birth: | |||
| Yes | 68 (88.3) | 12 (85.7) | 56 (88.9) |
| No | 9 (11.7) | 2 (14.3) | 7 (11.1) |
| Newborn sex: | |||
| Female | 39 (50.6) | 5 (35.7) | 34 (54) |
| Male | 38 (49.4) | 9 (64.3) | 29 (46) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perichart-Perera, O.; Avila-Sosa, V.; Solis-Paredes, J.M.; Montoya-Estrada, A.; Reyes-Muñoz, E.; Rodríguez-Cano, A.M.; González-Leyva, C.P.; Sánchez-Martínez, M.; Estrada-Gutierrez, G.; Irles, C. Vitamin D Deficiency, Excessive Gestational Weight Gain, and Oxidative Stress Predict Small for Gestational Age Newborns Using an Artificial Neural Network Model. Antioxidants 2022, 11, 574. https://doi.org/10.3390/antiox11030574
Perichart-Perera O, Avila-Sosa V, Solis-Paredes JM, Montoya-Estrada A, Reyes-Muñoz E, Rodríguez-Cano AM, González-Leyva CP, Sánchez-Martínez M, Estrada-Gutierrez G, Irles C. Vitamin D Deficiency, Excessive Gestational Weight Gain, and Oxidative Stress Predict Small for Gestational Age Newborns Using an Artificial Neural Network Model. Antioxidants. 2022; 11(3):574. https://doi.org/10.3390/antiox11030574
Chicago/Turabian StylePerichart-Perera, Otilia, Valeria Avila-Sosa, Juan Mario Solis-Paredes, Araceli Montoya-Estrada, Enrique Reyes-Muñoz, Ameyalli M. Rodríguez-Cano, Carla P. González-Leyva, Maribel Sánchez-Martínez, Guadalupe Estrada-Gutierrez, and Claudine Irles. 2022. "Vitamin D Deficiency, Excessive Gestational Weight Gain, and Oxidative Stress Predict Small for Gestational Age Newborns Using an Artificial Neural Network Model" Antioxidants 11, no. 3: 574. https://doi.org/10.3390/antiox11030574
APA StylePerichart-Perera, O., Avila-Sosa, V., Solis-Paredes, J. M., Montoya-Estrada, A., Reyes-Muñoz, E., Rodríguez-Cano, A. M., González-Leyva, C. P., Sánchez-Martínez, M., Estrada-Gutierrez, G., & Irles, C. (2022). Vitamin D Deficiency, Excessive Gestational Weight Gain, and Oxidative Stress Predict Small for Gestational Age Newborns Using an Artificial Neural Network Model. Antioxidants, 11(3), 574. https://doi.org/10.3390/antiox11030574







