Role of a Novel Silver Fir (Abies alba) Extract, Abigenol®/AlbiPhenol®, in Modulating Cardiovascular Disorders: Key Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
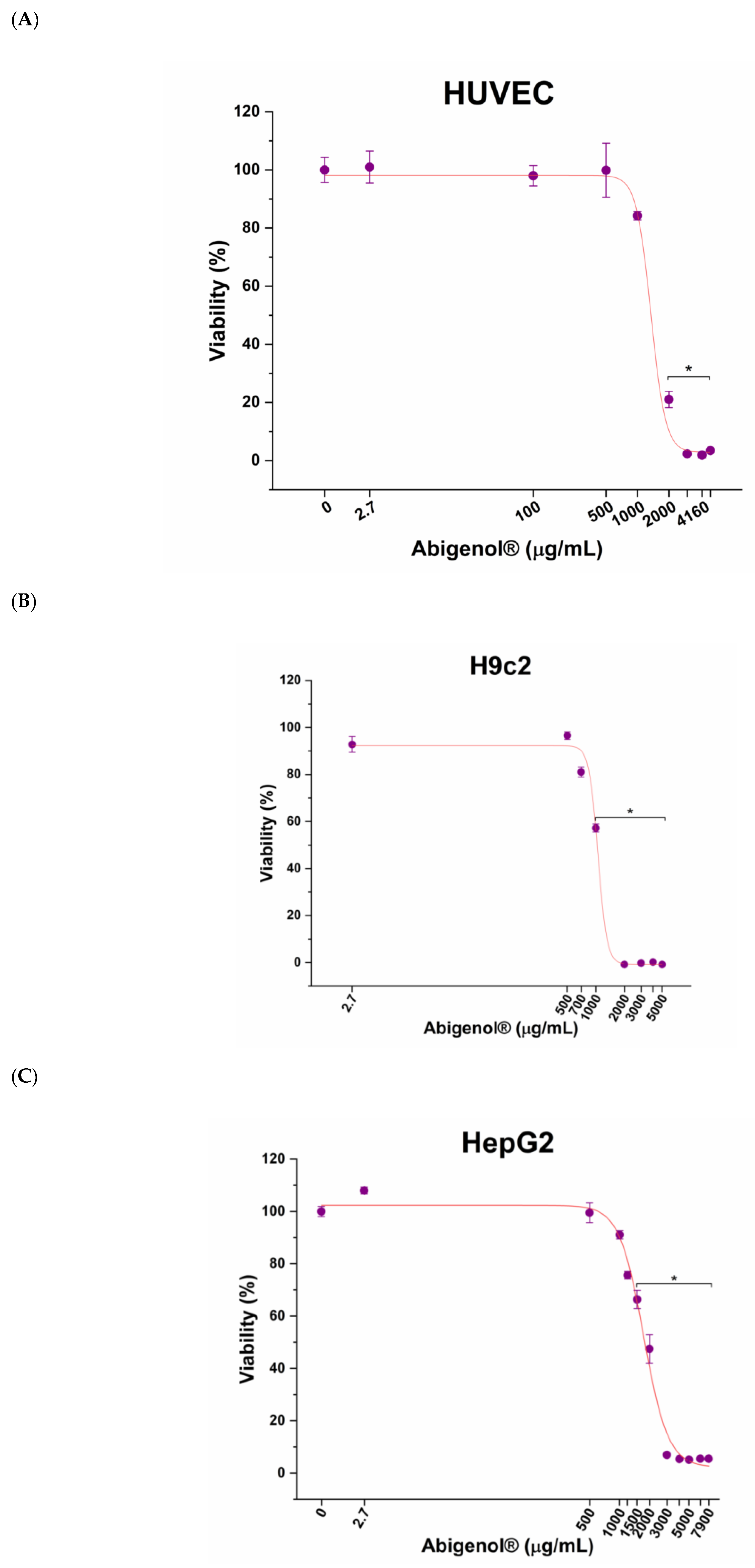
2.2. Cell Cultures
2.2.1. Caco-2 Cell Culture
2.2.2. HepG2 Cell Culture
2.2.3. H9c2 Cell Culture
2.2.4. HUVEC Cell Culture
2.3. Determination of Catechin Content within Abigenol®/AlbiPhenol®
2.4. Determination of Abigenol®/AlbiPhenol® Bioaccessibility
2.5. In Vitro Model of Human Intestinal Epithelium
2.6. Evaluation of Abigenol®/AlbiPhenol® Bioavailability
2.7. Evaluation of the Impact of Digested Formulations on the Intestinal Epithelium Viability
2.8. Barrier Integrity and Cell Viability
2.9. Evaluation of Abigenol®/AlbiPhenol® Cytotoxicity
2.10. Determination of Antioxidant Activity of Abigenol®/AlbiPhenol® on Cultured Cells
2.11. Glutathione System (GSH/GSSG) Assay
2.12. Diclorofluorescein (DCFDA) Assay
2.13. Superoxide Dismutase (SOD) Assay Kit
2.14. Determination of Antioxidant Activity on HDL and LDL
2.15. Determination of Antioxidant Activity by ORAC Test
2.16. Evaluation of Anti-Steatotic Activity of Abigenol®/AlbiPhenol® on Hepatocytes
2.17. Determination of Intracellular Lipids
2.18. Determination of Triglycerides
2.19. Determination of Bile Acids
2.20. Determination of Cholesterol
2.21. Evaluation of Abigenol®/AlbiPhenol® Effect on ACE Activity
2.22. Statistical Analysis
3. Results
3.1. Determination of Abigenol®/AlbiPhenol® Concentration
3.2. Abigenol®/AlbiPhenol® Intestinal Absorption
3.3. Impact of Digested Formulations on Intestinal Mucosa Viability and Integrity
3.4. Evaluation of Abigenol®/AlbiPhenol® Cytotoxicity
3.5. Determination of Antioxidant Activity of Abigenol®/AlbiPhenol®
3.6. Evaluation of Anti-Steatotic Activity of Abigenol®/AlbiPhenol®
3.7. Evaluation of Abigenol®/AlbiPhenol® Effect on ACE Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Bajaj, S.; Virk, H.; Bikkina, M.; Shamoon, F. Rapid progression of coronary atherosclerosis: A review. Thrombosis 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.H.; Park, W.J. Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 956, pp. 511–540. [Google Scholar]
- Castellon, X.; Bogdanova, V. Chronic inflammatory diseases and endothelial dysfunction. Aging Dis. 2016, 7, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Brown, L. Cholesterol versus inflammation as cause of chronic diseases. Nutrients 2019, 11, 2332. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, A.O.; Jacobs, D.R.; Sanchez, O.A.; Goff, D.C.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Gaudio, E.; Nobili, V.; Franchitto, A.; Onori, P.; Carpino, G. Nonalcoholic fatty liver disease and atherosclerosis. Intern. Emerg. Med. 2012, 7, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin Resistance in Nonalcoholic Fatty Liver Disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Cusi, K. Role of Insulin Resistance and Lipotoxicity in Non-Alcoholic Steatohepatitis. Clin. Liver Dis. 2009, 13, 545–563. [Google Scholar] [CrossRef]
- Hurjui, D.M.; Niţă, O.; Graur, L.I.; Mihalache, L.; Popescu, D.S.; Graur, M. The central role of the non alcoholic fatty liver disease in metabolic syndrome. Rev. Med.-Chir. Soc. Med. Nat. Iasi 2012, 116, 425–431. [Google Scholar] [PubMed]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.V.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. J. Heart Fail. 2008, 10, 933–989. [Google Scholar] [CrossRef]
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Executive summary: Heart disease and stroke statistics-2016 update: A Report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Chair, F.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement From the American Heart Association Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Stroke Council. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Coronel, R.; De Groot, J.R.; Van Lieshout, J.J. Defining heart failure. Cardiovasc. Res. 2001, 50, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Williams, S.G.; Tan, D.K.H.; Cohen-solal, A.; Williams, S.G.; Tan, D.K.H.; Cohen-solal, A.; Tan, L.; Simon, G.; Tan, D.K.H.; et al. So many definitions of heart failure: Are they all So many definitions of heart failure: Are they all universally valid? A critical appraisal. Expert Rev. Cardiovasc. Ther. 2010, 8, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2016, 14, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- Behradmanesh, S.; Nasri, P. Serum cholesterol and LDL-C in association with level of diastolic blood pressure in type 2 diabetic patients. J. Ren. Inj. Prev. 2012, 1, 23–236. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Limberg, J.K. Blood pressure regulation: Every adaptation is an integration? Eur. J. Appl. Physiol. 2014, 114, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matchar, D.B.; McCrory, D.C.; Orlando, L.A.; Patel, M.R.; Patel, U.D.; Patwardhan, M.B.; Powers, B.; Samsa, G.P.; Gray, R.N. Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 2008, 148, 16–29. [Google Scholar] [CrossRef]
- Montezano, A.C.; Touyz, R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012, 44 (Suppl. S1), S2–S16. [Google Scholar] [CrossRef]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Redón, J.; Oliva, M.R.; Tormos, C.; Giner, V.; Chaves, J.; Iradi, A.; Sáez, G.T. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 2003, 41, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Bocalandro, C.; Sanhueza, V.; Gómez-Caravaca, A.M.; González-álvarez, J.; Fernández, K.; Roeckel, M.; Rodríguez-Estrada, M.T. Comparison of the composition of Pinus radiata bark extracts obtained at bench- and pilot-scales. Ind. Crops Prod. 2012, 38, 21–26. [Google Scholar] [CrossRef]
- Fradinho, D.M.; Neto, C.P.; Evtuguin, D.; Jorge, F.C.; Irle, M.A.; Gil, M.H.; Pedrosa de Jesus, J. Chemical characterisation of bark and of alkaline bark extracts from maritime pine grown in Portugal. Ind. Crops Prod. 2002, 16, 23–32. [Google Scholar] [CrossRef]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Štrukelj, B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crops Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Walczak, A.P.; Fokkink, R.; Peters, R.; Tromp, P.; Herrera Rivera, Z.E.; Rietjens, I.M.C.M.; Hendriksen, P.J.M.; Bouwmeester, H. Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology 2013, 7, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Covas, M.I.; Martín, S.; Rubiés-Prat, J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J. Hum. Hypertens. 2000, 14, 343–345. [Google Scholar] [CrossRef] [Green Version]
- Pickering, T.G. Diagnosis and evaluation of renovascular hypertension. Indications for therapy. Proc. Circ. 1991, 83 (Suppl. S2), I147-54. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Bhagwat, S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2; United States Department of Agriculture: Beltsville, MD, USA, 2010; pp. 1–46.
- Baudin, B.; Berard, M.; Carrier, J.L.; Legrand, Y.; Drouet, L. Vascular Origin Determines Angiotensin I-Converting Enzyme Expression in Endothelial Cells. Endothelium J. Endothel. Cell Res. 1997, 5, 73–84. [Google Scholar] [CrossRef]
- Wan, W.; Jiang, X.; Li, X.; Zhang, C.; Yi, X. Silencing of angiotensin-converting enzyme by RNA interference prevents H9c2 cardiomyocytes from apoptosis induced by anoxia/reoxygenation through regulation of the intracellular renin-angiotensin system. Int. J. Mol. Med. 2013, 32, 1380–1386. [Google Scholar] [CrossRef]














| Complete | Supernatant | Pellet | ||||
|---|---|---|---|---|---|---|
| (CTCN) (µg/mL) | CTCN (%) | (CTCN) (µg/mL) | CTCN (%) | (CTCN) (µg/mL) | CTCN (%) | |
| Abigenol®/AlbiPhenol® | 493.4 ± 18.7 | 102.4 ± 3.9 | 255.0 ± 14.5 | 53.0 ± 3.0 | 206.9 ± 9.7 | 43.0 ± 2.0 |
| Inhibition of Oxidation (%) | ||
|---|---|---|
| Concentration of Abigenol®/AlbiPhenol® | HDL | LDL |
| 2.7 µg/mL | 51.8 | 43.9 |
| 15.5 µg/mL (HDL) or 8.1 µg/mL (LDL) | 98.5 | 85.8 |
| Amount of Abigenol®/AlbiPhenol®/mL | Mean ORAC Value (µMol TE/100 g) |
|---|---|
| H-ORAC | 221,359 |
| L-ORAC | 10,538 |
| Total-ORAC | 231,897 |
| Food | Mean ORAC Value (µMol TE/100 g) |
|---|---|
| Sumac, bran, raw | 312,400 * |
| Spices, cloves, ground | 290,283 * |
| Sorghum, bran, hi-tannin | 240,000 * |
| Abigenol®/AlbiPhenol® | 231,897 |
| Total Cholesterol Decrease (%) | ||
|---|---|---|
| Concentration of Abigenol®/AlbiPhenol® | Physiological Conditions | Steatosis-Inducing Conditions |
| 2.7 µg/mL | 12.0 | 2.5 |
| 1200 µg/mL | 68.8 | 63.0 |
| Bile Acid Increase (%) | ||
|---|---|---|
| Concentration of Abigenol®/AlbiPhenol® | Normal Conditions | Steatosis |
| 2.7 µg/mL | 54.7 | −7.2 |
| 1200 µg/mL | 11.4 | 63.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, K.; Micheletto, M.; Di Maira, G.; Tedesco, E.; Benetti, F.; Zaloker, U. Role of a Novel Silver Fir (Abies alba) Extract, Abigenol®/AlbiPhenol®, in Modulating Cardiovascular Disorders: Key Factors. Antioxidants 2022, 11, 618. https://doi.org/10.3390/antiox11040618
Leone K, Micheletto M, Di Maira G, Tedesco E, Benetti F, Zaloker U. Role of a Novel Silver Fir (Abies alba) Extract, Abigenol®/AlbiPhenol®, in Modulating Cardiovascular Disorders: Key Factors. Antioxidants. 2022; 11(4):618. https://doi.org/10.3390/antiox11040618
Chicago/Turabian StyleLeone, Kevin, Marta Micheletto, Giovanni Di Maira, Erik Tedesco, Federico Benetti, and Urška Zaloker. 2022. "Role of a Novel Silver Fir (Abies alba) Extract, Abigenol®/AlbiPhenol®, in Modulating Cardiovascular Disorders: Key Factors" Antioxidants 11, no. 4: 618. https://doi.org/10.3390/antiox11040618







