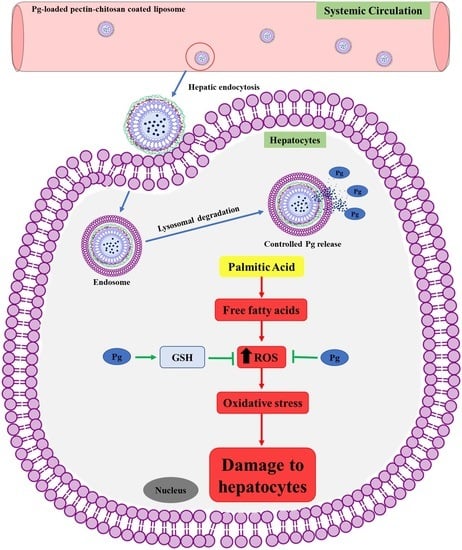
Pelargonidin-3-O-Glucoside Encapsulated Pectin-Chitosan-Nanoliposomes Recovers Palmitic Acid-Induced Hepatocytes Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Nanoliposomes Formation
2.3. Modification of Nanoliposomes Surface Using Chitosan and Pectin Coating
2.4. Characterization of Different Liposomal Particles
2.4.1. Dynamic Light Scattering (DLS)
2.4.2. Encapsulation Efficiency (EE)
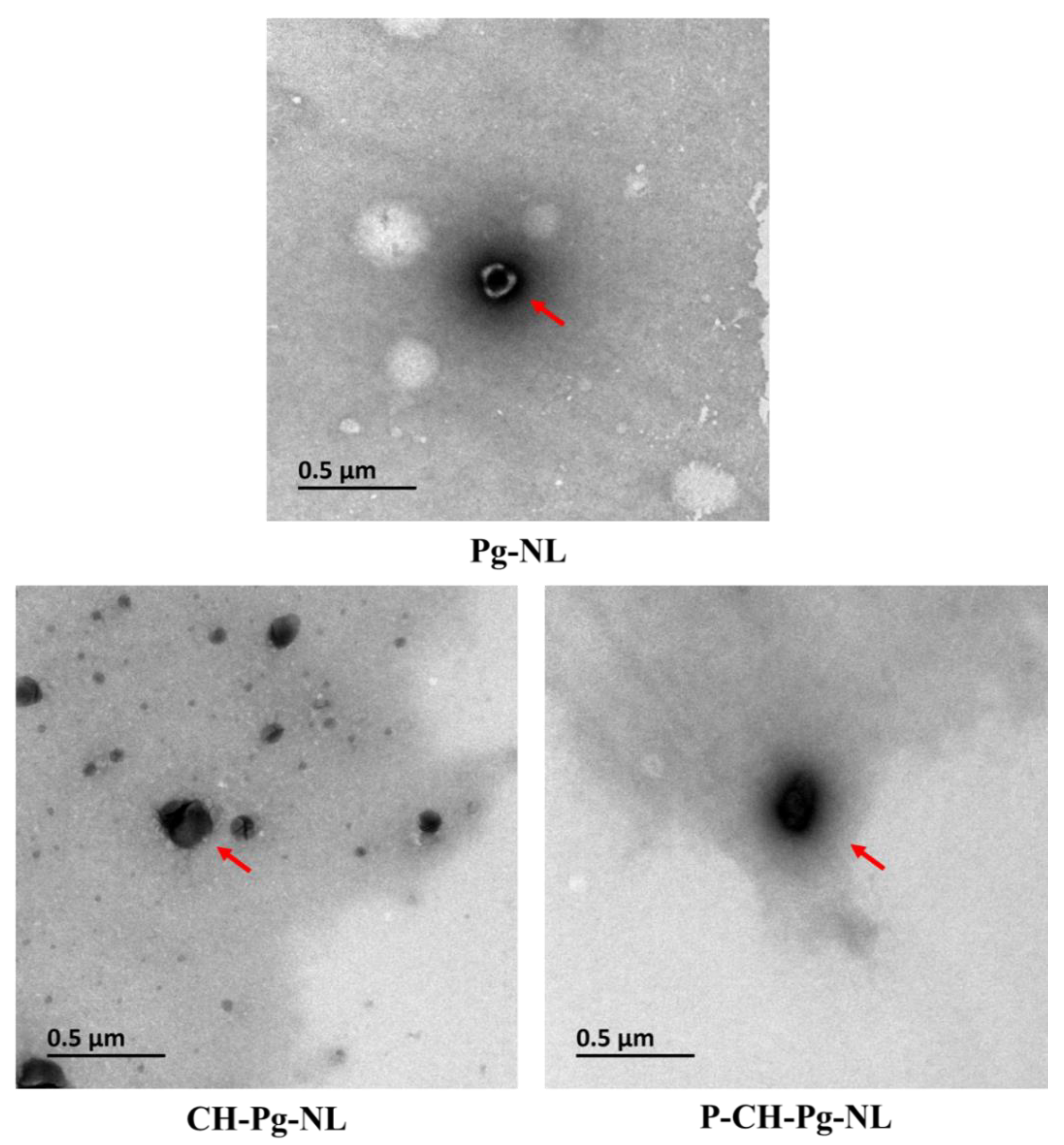
2.4.3. Transmission Electron Microscope (TEM)
2.5. Cell Culture
2.5.1. Cytotoxicity Study
2.5.2. Cytoprotective Study
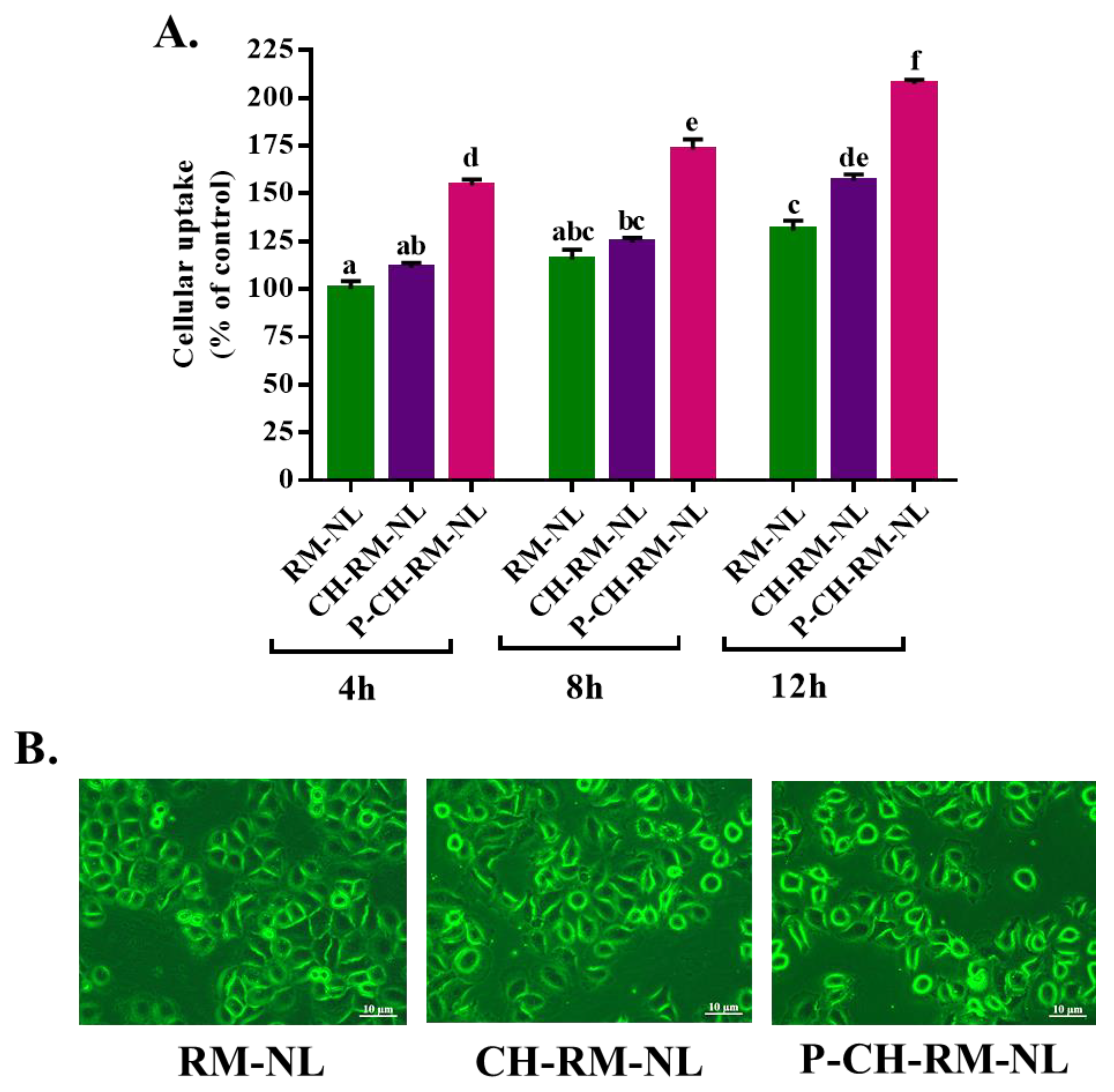
2.5.3. Evaluation of Cellular Uptake of Liposomal Particles to L02 Cells
2.5.4. Determination of Intracellular Reactive Oxygen Species (ROS) Generation
2.5.5. Determination of Superoxide Anion Radical (O2•−) Generation
2.5.6. Evaluation of Cellular Mitochondrial Membrane Potential (MMP)
2.5.7. Evaluation of Intracellular Glutathione (GSH) Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Pg-Loaded Nanoliposomal Carriers
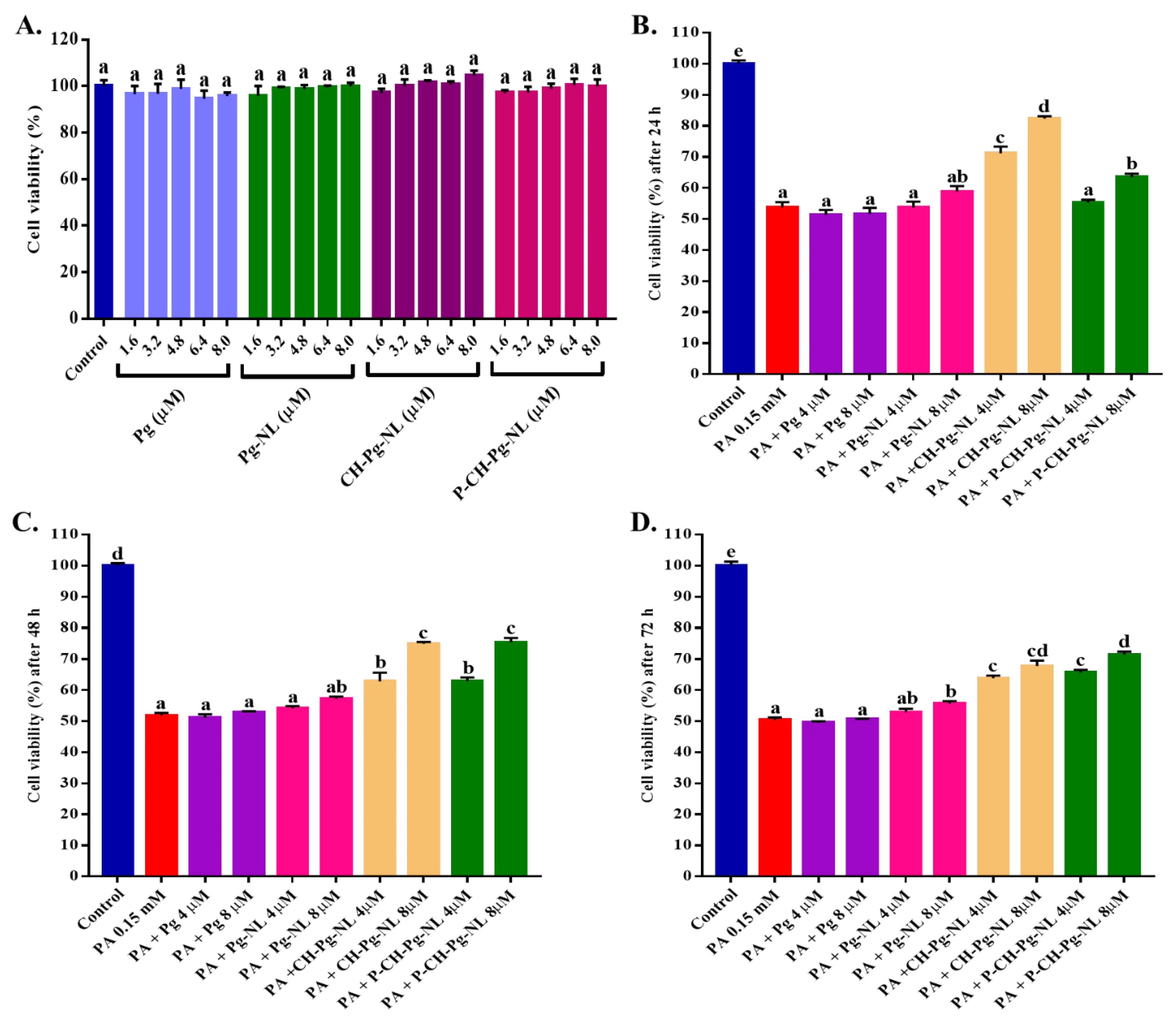
3.2. Effect of Free-Pg and Pg-Loaded Liposomal Systems on Cell Viability
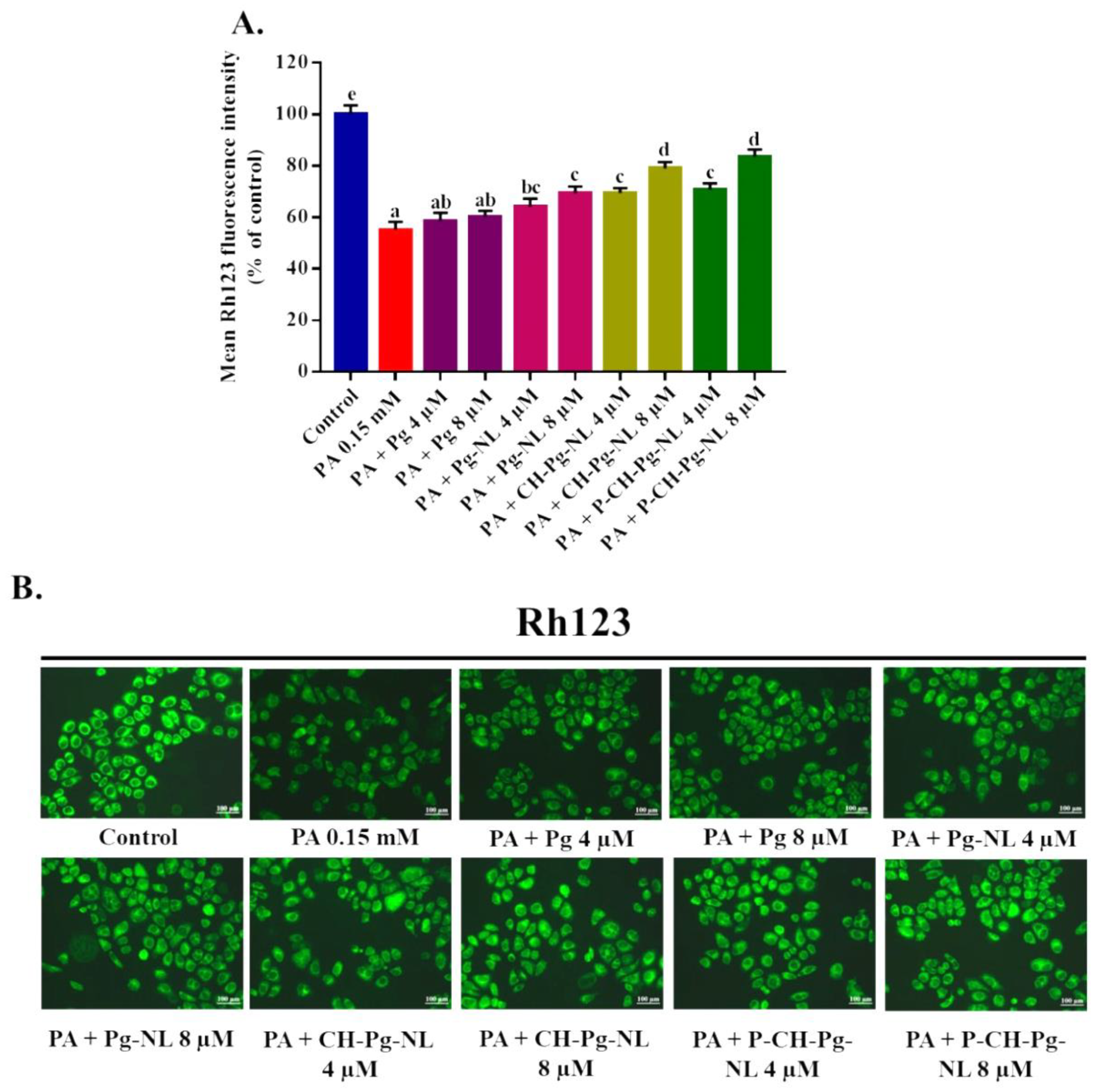
3.3. Cellular Uptake Study of Rhodamine 123 (RM)-Loaded Liposomal Systems
3.4. Effect of Free-Pg and Pg-Loaded Liposomal Systems against PA-Induced Lipotoxicity
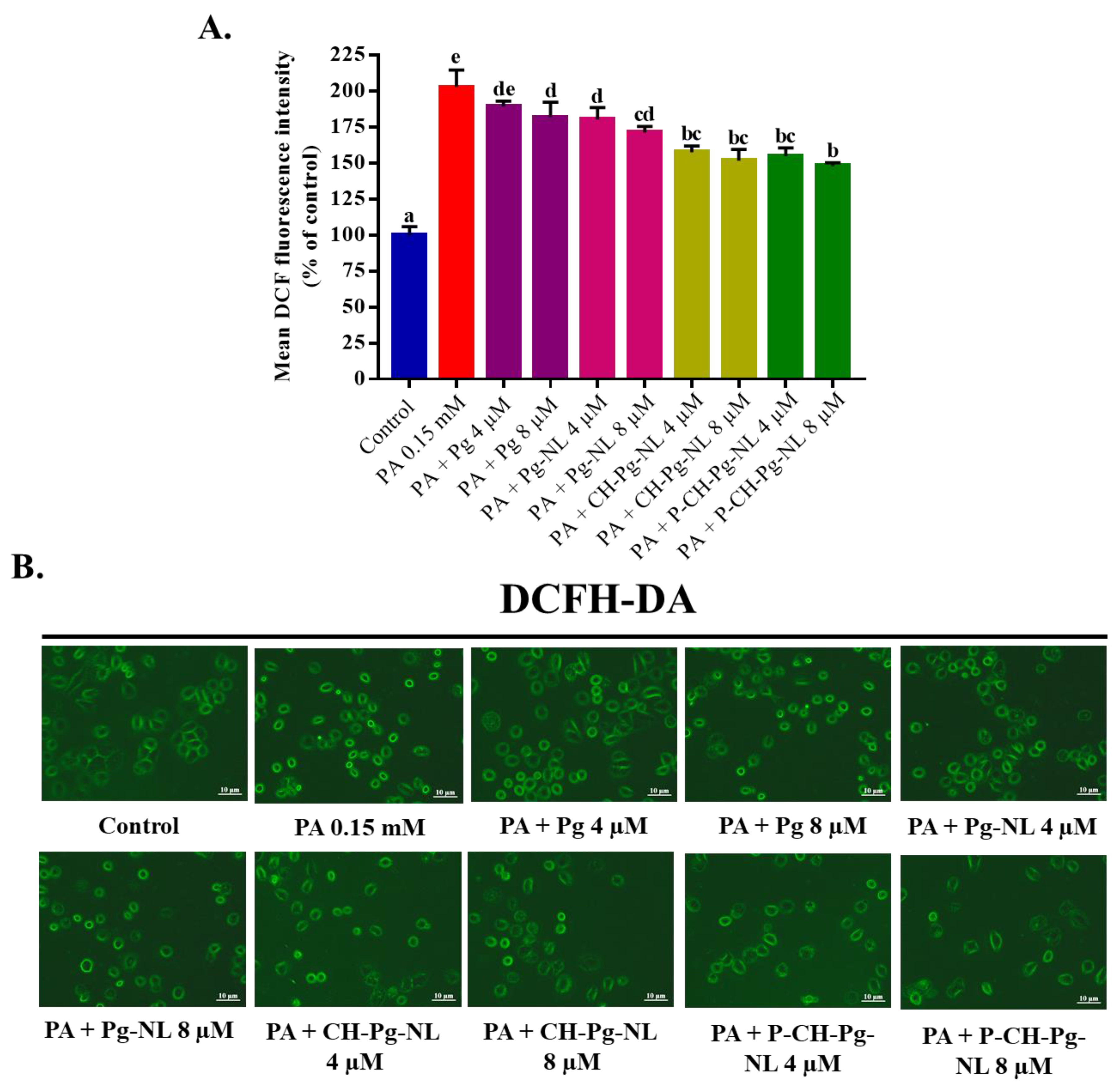
3.5. Effect of Free-Pg and Pg-Loaded Liposomal Systems against PA-Induced Intracellular ROS Generation
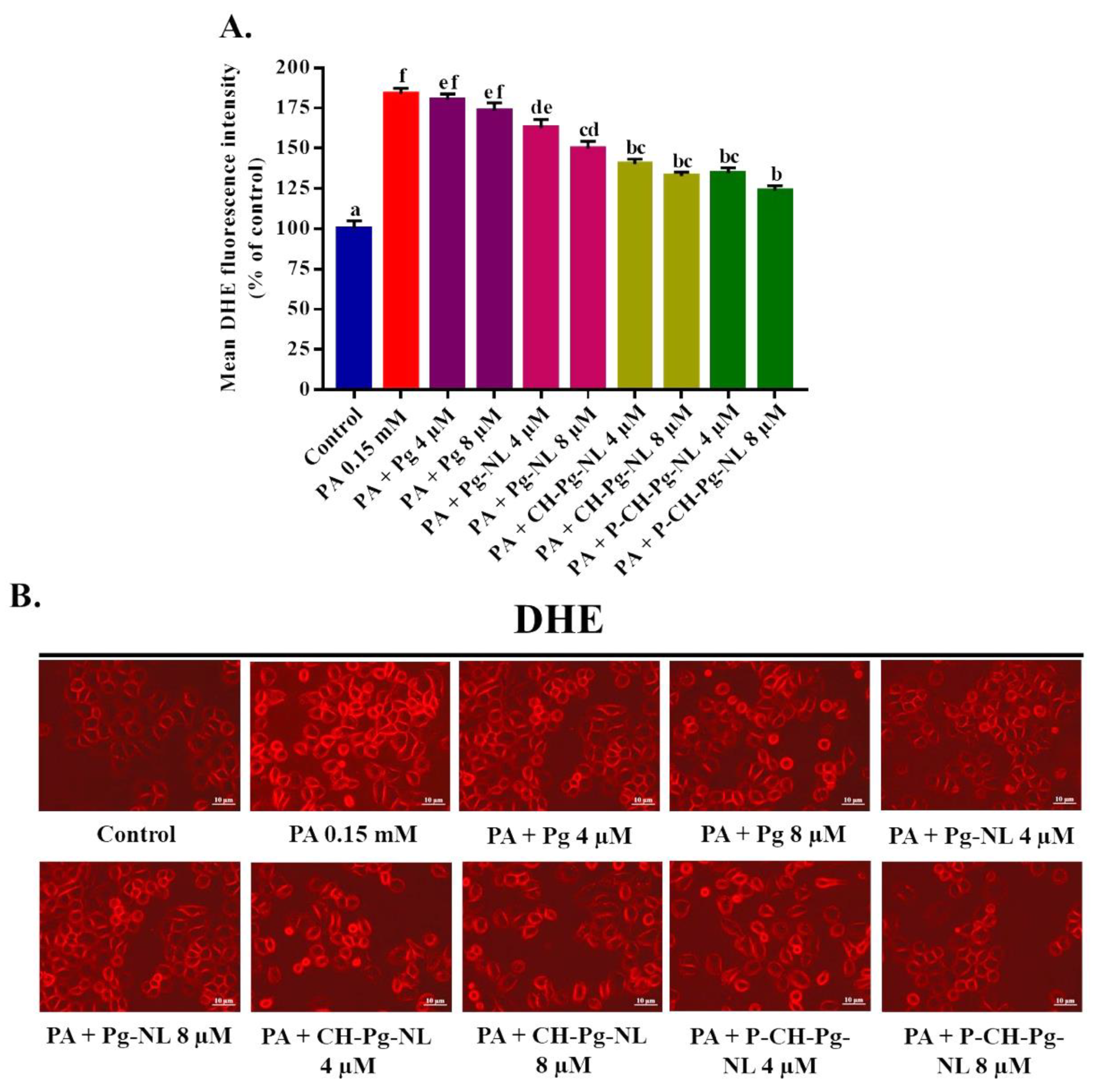
3.6. Effect of Free-Pg and Pg-Loaded Liposomal Systems against PA-Induced Intracellular O2•− Generation
3.7. Effect of Free-Pg and Pg-Loaded Liposomal Systems against PA-Induced Mitochondrial Dysfunction
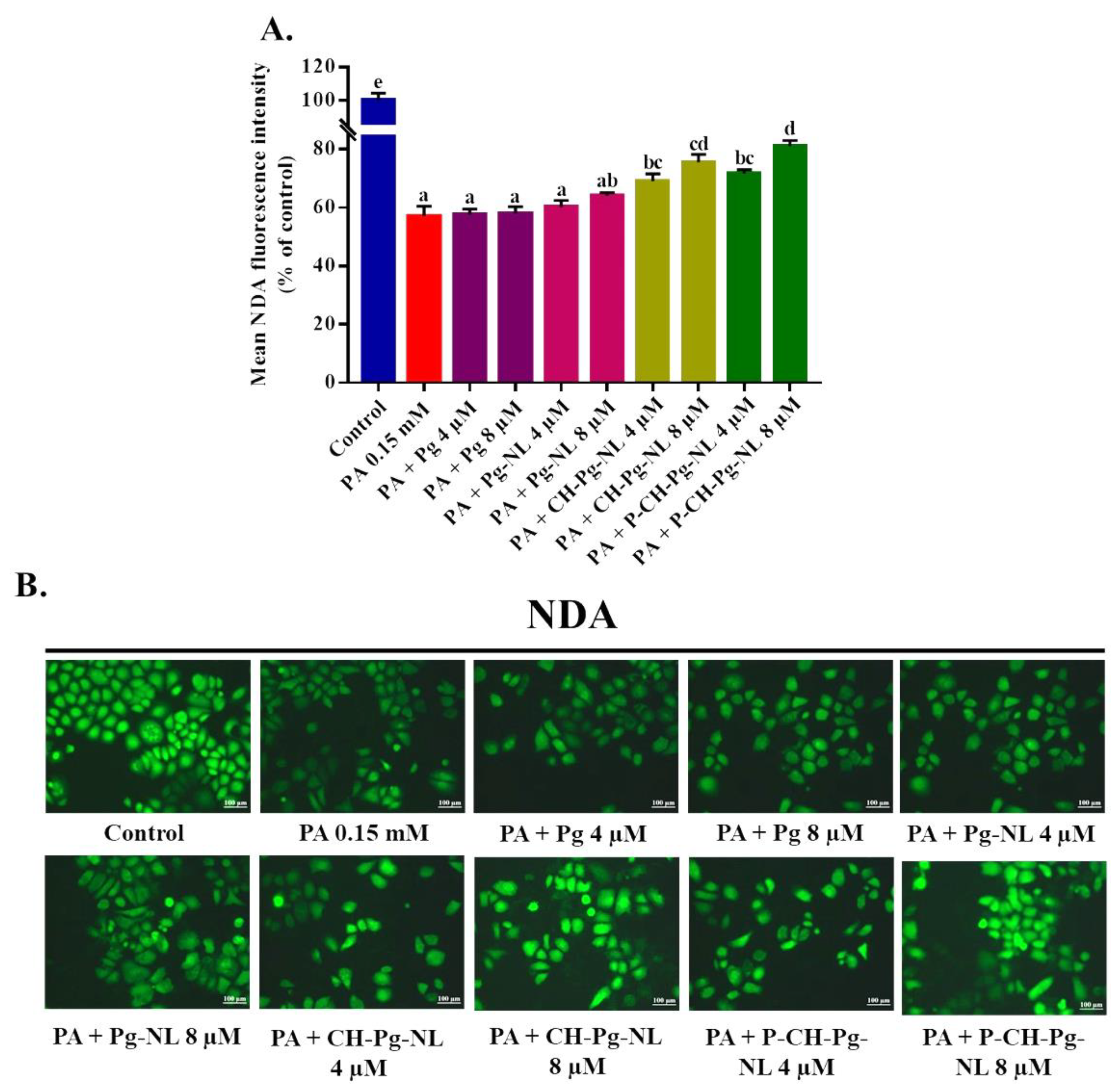
3.8. Effect of Free-Pg and Pg-Loaded Liposomal Systems against PA-Induced Cellular Glutathione (GSH) Depletion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Hu, D.; Li, Y.; Sun, C.; Chen, W. An effective method for preparation of high-purity pelargonidin-3-O-glucoside from strawberry and its protective effect on cellular oxidative stress. J. Chromatogr. B 2018, 1072, 211–220. [Google Scholar]
- Duarte, L.J.; Chaves, V.C.; Nascimento, M.V.P.d.S.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simões, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65. [Google Scholar] [PubMed]
- Park, M.J.; Ryu, D.H.; Cho, J.Y.; Lee, D.G.; Lee, J.N.; Kang, Y.-H. Potential for antioxidant and antihyperglycemic activities of four everbearing strawberry cultivars. Hortic. Environ. Biote. 2020, 61, 615–623. [Google Scholar]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [PubMed]
- Mirshekar, M.; Roghani, M.; Khalili, M.; Baluchnejadmojarad, T. Chronic oral pelargonidin alleviates learning and memory disturbances in streptozotocin diabetic rats. Iran. J. Pharm. Res. 2011, 10, 569–575. [Google Scholar]
- Sohanaki, H.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Pelargonidin improves memory deficit in amyloid β25–35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016, 83, 85–91. [Google Scholar] [PubMed]
- Karthi, N.; Kalaiyarasu, T.; Kandakumar, S.; Mariyappan, P.; Manju, V. Pelargonidin induces apoptosis and cell cycle arrest via a mitochondria mediated intrinsic apoptotic pathway in HT29 cells. RSC Adv. 2016, 6, 45064–45076. [Google Scholar]
- Shishir, M.R.I.; Karim, N.; Xu, Y.; Xie, J.; Chen, W. Improving the physicochemical stability and functionality of nanoliposome using green polymer for the delivery of pelargonidin-3-O-glucoside. Food Chem. 2021, 337, 127654. [Google Scholar] [PubMed]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal delivery of natural product: A promising approach in health research. Trends Food Sci. Technol. 2019, 85, 177–200. [Google Scholar]
- Karim, N.; Shishir, M.R.I.; Chen, W. Surface decoration of neohesperidin-loaded nanoliposome using chitosan and pectin for improving stability and controlled release. Int. J. Biol. Macromol. 2020, 164, 2903–2914. [Google Scholar] [PubMed]
- Lee, E.-H.; Lim, S.-J.; Lee, M.-K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [PubMed]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloid Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [PubMed]
- Caddeo, C.; Pons, R.; Carbone, C.; Fernàndez-Busquets, X.; Cardia, M.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr. Polym. 2017, 157, 1853–1861. [Google Scholar] [PubMed]
- Li, Y.; Zhao, H.; Duan, L.-R.; Li, H.; Yang, Q.; Tu, H.-H.; Cao, W.; Wang, S.-W. Preparation, characterization and evaluation of bufalin liposomes coated with citrus pectin. Colloid Surf. A Physicochem. Eng. Asp. 2014, 444, 54–62. [Google Scholar]
- Gottesmann, M.; Goycoolea, F.M.; Steinbacher, T.; Menogni, T.; Hensel, A. Smart drug delivery against Helicobacter pylori: Pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo. Appl. Microbiol. Biotechnol. 2020, 104, 5943–5957. [Google Scholar]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [PubMed]
- Manca, M.L.; Manconi, M.; Valenti, D.; Lai, F.; Loy, G.; Matricardi, P.; Fadda, A.M. Liposomes coated with chitosan–xanthan gum (chitosomes) as potential carriers for pulmonary delivery of rifampicin. J. Pharm. Sci. 2012, 101, 566–575. [Google Scholar] [PubMed]
- Liu, W.; Tian, M.; Kong, Y.; Lu, J.; Li, N.; Han, J. Multilayered vitamin C nanoliposomes by self-assembly of alginate and chitosan: Long-term stability and feasibility application in mandarin juice. LWT Food Sci. Technol. 2017, 75, 608–615. [Google Scholar]
- Gomaa, A.I.; Martinent, C.; Hammami, R.; Fliss, I.; Subirade, M. Dual coating of liposomes as encapsulating matrix of antimicrobial peptides: Development and characterization. Front. Chem. 2017, 5, 1–12. [Google Scholar]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [PubMed]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [PubMed] [Green Version]
- Chen, Q.; Su, Y.; Ju, Y.; Ma, K.; Li, W.; Li, W. Astragalosides IV protected the renal tubular epithelial cells from free fatty acids-induced injury by reducing oxidative stress and apoptosis. Biomed. Pharmacother. 2018, 108, 679–686. [Google Scholar] [PubMed]
- Park, E.-J.; Lee, A.Y.; Park, S.; Kim, J.-H.; Cho, M.-H. Multiple pathways are involved in palmitic acid-induced toxicity. Food Chem. Toxicol. 2014, 67, 26–34. [Google Scholar] [PubMed]
- Srivastava, S.; Chan, C.; Srivastava, S.; Chan, C. Hydrogen peroxide and hydroxyl radicals mediate palmitate-induced cytotoxicity to hepatoma cells: Relation to mitochondrial permeability transition. Free Radic. Res. 2007, 41, 38–49. [Google Scholar] [PubMed]
- Yao, H.; Qiao, Y.-J.; Zhao, Y.-L.; Tao, X.-F.; Xu, L.-N.; Yin, L.-H.; Qi, Y.; Peng, J.-Y. Herbal medicines and nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 6890. [Google Scholar] [PubMed]
- Shishir, M.R.I.; Karim, N.; Xie, J.; Rashwan, A.K.; Chen, W. Colonic delivery of pelargonidin-3-O-glucoside using pectin-chitosan-nanoliposome: Transport mechanism and bioactivity retention. Int. J. Biol. Macromol. 2020, 159, 341–355. [Google Scholar]
- Yang, W.; Zhu, L.; Lai, S.; Ding, Q.; Xu, T.; Guo, R.; Dou, X.; Chai, H.; Yu, Z.; Li, S. Cimifugin ameliorates lipotoxicity-induced hepatocyte damage and steatosis through TLR4/p38 MAPK- and SIRT1-involved pathways. Oxidative Med. Cell. Longev. 2022, 2022, 4557532. [Google Scholar]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar]
- Mo, L.; Song, J.G.; Lee, H.; Zhao, M.; Kim, H.Y.; Lee, Y.J.; Ko, H.W.; Han, H.-K. PEGylated hyaluronic acid-coated liposome for enhanced in vivo efficacy of sorafenib via active tumor cell targeting and prolonged systemic exposure. Nanomed.-Nanotechnol. Biol. Med. 2018, 14, 557–567. [Google Scholar]
- Hu, D.; Bao, T.; Lu, Y.; Su, H.; Ke, H.; Chen, W. Polysaccharide from mulberry fruit (Morus alba L.) protects against palmitic-acid-induced hepatocyte lipotoxicity by activating the Nrf2/ARE signaling pathway. J. Agric. Food Chem. 2019, 68, 13016–13024. [Google Scholar]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloid Surf. B 2015, 129, 7–14. [Google Scholar]
- Alshraim, M.O.; Sangi, S.; Harisa, G.I.; Alomrani, A.H.; Yusuf, O.; Badran, M.M. Chitosan-coated flexible liposomes magnify the anticancer activity and bioavailability of docetaxel: Impact on composition. Molecules 2019, 24, 250. [Google Scholar]
- Hasan, M.; Ben Messaoud, G.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-coated liposomes encapsulating curcumin: Study of lipid–polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Pectin and polygalacturonic acid-coated liposomes as novel delivery system for nisin: Preparation, characterization and release behavior. Food Hydrocoll. 2017, 70, 1–7. [Google Scholar]
- Karim, N.; Shishir, M.R.I.; Rashwan, A.K.; Ke, H.; Chen, W. Suppression of palmitic acid-induced hepatic oxidative injury by neohesperidin-loaded pectin-chitosan decorated nanoliposomes. Int. J. Biol. Macromol. 2021, 183, 908–917. [Google Scholar]
- Yin Win, K.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar]
- Niu, Z.; Conejos-Sánchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar]
- Jiang, Y.; Du, J. Properties of high-methoxyl pectin extracted from “Fuji” apple pomace in China. J. Food Process Eng. 2017, 40, e12497. [Google Scholar]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar]
- Chen, B.-H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar]
- Pohland, M.; Pellowska, M.; Asseburg, H.; Hagl, S.; Reutzel, M.; Joppe, A.; Berressem, D.; Eckert, S.H.; Wurglics, M.; Schubert-Zsilavecz, M.; et al. MH84 improves mitochondrial dysfunction in a mouse model of early Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 18. [Google Scholar]
- Gui, T.; Li, Y.; Zhang, S.; Zhang, N.; Sun, Y.; Liu, F.; Chen, Q.; Gai, Z. Docosahexaenoic acid protects against palmitate-induced mitochondrial dysfunction in diabetic cardiomyopathy. Biomed. Pharmacother. 2020, 128, 110306. [Google Scholar]
- Yamada, Y.; Akita, H.; Kamiya, H.; Kogure, K.; Yamamoto, T.; Shinohara, Y.; Yamashita, K.; Kobayashi, H.; Kikuchi, H.; Harashima, H. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 423–432. [Google Scholar]
- Bollenbach, A.; Tsikas, D. Measurement of the tripeptides glutathione and ophthalmic acid by gas chromatography-mass spectrometry. Anal. Biochem. 2020, 113841. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Glutathione in cellular redox homeostasis: Association with the excitatory amino acid carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [PubMed] [Green Version]
- Oh, J.M.; Choi, J.M.; Lee, J.Y.; Oh, S.J.; Kim, H.C.; Kim, B.H.; Ma, J.Y.; Kim, S.K. Effects of palmitic acid on TNF-α-induced cytotoxicity in SK-Hep-1 cells. Toxicol. In Vitro 2012, 26, 783–790. [Google Scholar] [PubMed]
- Ugbaja, R.N.; James, A.S.; Ugwor, E.I.; Akamo, A.J.; Thomas, F.C.; Kosoko, A.M. Lycopene suppresses palmitic acid-induced brain oxidative stress, hyperactivity of some neuro-signalling enzymes, and inflammation in female Wistar rat. Sci. Rep. 2021, 11, 15038. [Google Scholar]
- Hu, D.; Xu, Y.; Xie, J.; Sun, C.; Zheng, X.; Chen, W. Systematic evaluation of phenolic compounds and protective capacity of a new mulberry cultivar J33 against palmitic acid-induced lipotoxicity using a simulated digestion method. Food Chem. 2018, 258, 43–50. [Google Scholar] [PubMed]
| DLS Analysis | HPLC Analysis | |||
|---|---|---|---|---|
| Sample | Particle Size (nm) | Zeta Potential (mV) | Polydispersity Index (PDI) | Encapsulation Efficiency (% of EE) |
| Pg-NL | 64.80 ± 0.36 | −30.37 ± 0.33 | 0.21 ± 0.01 | 28.54 ± 1.36 |
| CH-Pg-NL | 252.63 ± 1.92 | 21.93 ± 0.56 | 0.13 ± 0.00 | 53.04 ± 1.19 |
| P-CH-Pg-NL | 352.00 ± 4.03 | −20.00 ± 0.78 | 0.24 ± 0.02 | 61.17 ± 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, N.; Shishir, M.R.I.; Li, Y.; Zineb, O.Y.; Mo, J.; Tangpong, J.; Chen, W. Pelargonidin-3-O-Glucoside Encapsulated Pectin-Chitosan-Nanoliposomes Recovers Palmitic Acid-Induced Hepatocytes Injury. Antioxidants 2022, 11, 623. https://doi.org/10.3390/antiox11040623
Karim N, Shishir MRI, Li Y, Zineb OY, Mo J, Tangpong J, Chen W. Pelargonidin-3-O-Glucoside Encapsulated Pectin-Chitosan-Nanoliposomes Recovers Palmitic Acid-Induced Hepatocytes Injury. Antioxidants. 2022; 11(4):623. https://doi.org/10.3390/antiox11040623
Chicago/Turabian StyleKarim, Naymul, Mohammad Rezaul Islam Shishir, Yuting Li, Ould Yahia Zineb, Jianling Mo, Jitbanjong Tangpong, and Wei Chen. 2022. "Pelargonidin-3-O-Glucoside Encapsulated Pectin-Chitosan-Nanoliposomes Recovers Palmitic Acid-Induced Hepatocytes Injury" Antioxidants 11, no. 4: 623. https://doi.org/10.3390/antiox11040623