Role of the Antioxidant Activity of Melatonin in Myocardial Ischemia-Reperfusion Injury
Abstract
1. Introduction
2. Melatonin and Physiology
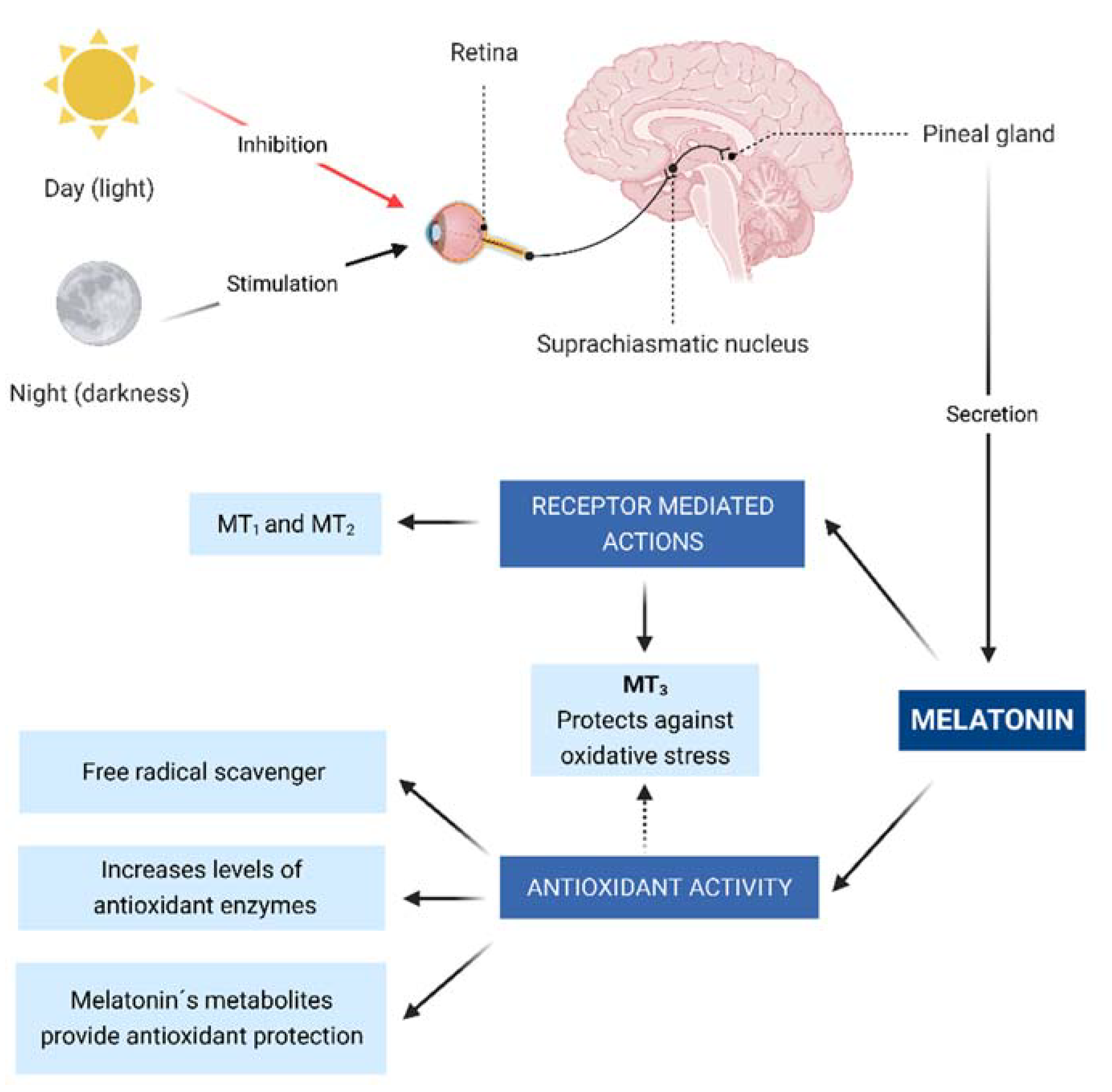
2.1. Byosynthesis and Secretion
2.2. Receptor-Mediated Actions
2.3. Antioxidant Activity and Anti-Inflammatory Effect
3. Overview of Ischemia-Reperfusion Injury
3.1. Oxidative Stress
3.2. Ion Accumulation
3.3. Nitric Oxide Metabolism
3.4. Mitochondrial Dysfunction
3.5. Inflammation
3.6. Cellular Death Pathways
4. Experimental Studies
5. Clinical Studies
6. Melatonin Analogs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neumann, F.-J.; Uva, M.S.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2019, 14, 1435–1534. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Gragnano, F.; Branca, M.; Franzone, A.; Baber, U.; Jang, Y.; Kimura, T.; Hahn, J.Y.; Zhao, Q.; Windecker, S.; et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: Individual patient level meta-analysis of randomised controlled trials. BMJ 2021, 373, 332. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Neural control of the pineal gland. Behav. Brain Res. 1995, 73, 125–130. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Haslmayer, P.; Philipp, C.; Mehrabi, M.R.; Glogar, H.D.; Grimm, M.; Leibetseder, V.J.; Thalhammer, T.; Marktl, W. Expression of the mt1melatonin receptor subtype in human coronary arteries. J. Recept. Signal Transduct. 2001, 21, 85–91. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Thalhammer, T.; Humpeler, S.; Mehrabi, M.R.; Glogar, H.D.; Hölzenbein, T.; Markovic, O.; Leibetseder, V.J.; Strauss-Blasche, G.; Marktl, W. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J. Pineal Res. 2003, 35, 40–44. [Google Scholar] [CrossRef]
- Baker, J.; Kimpinski, K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018, 45, 755–766. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.-M.; Lefoulon, F.; Fauchère, J.-L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the Melatonin-binding SiteMT 3 as the Quinone Reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Lin-Du, C.; Burkhard, P.; Lucien, C.; Russel, R. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.-X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc. Res. 2003, 58, 10–19. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores-Alvarado, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2006, 42, 28–42. [Google Scholar] [CrossRef]
- Dobsak, P.; Siegelova, J.; Eicher, J.C.; Jancik, J.; Svacinova, H.; Vasku, J.; Kuchtickova, S.; Horky, M.; Wolf, J.E. Melatonin protects against ischemia-reperfusion injury and inhibits apoptosis in isolated working rat heart. Pathophysiology 2003, 9, 179–187. [Google Scholar] [CrossRef]
- Fu, Z.; Jiao, Y.; Wang, J.; Zhang, Y.; Shen, M.; Reiter, R.J.; Xi, Q.; Chen, Y. Cardioprotective Role of Melatonin in Acute Myocardial Infarction. Front. Physiol. 2020, 11, 366. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Chen, W.; Chen, L.; Liu, D.; Wang, X.; Wang, X. Melatonin attenuates white matter damage after focal brain ischemia in rats by regulating the TLR4/NF-κB pathway. Brain Res. Bull. 2019, 150, 168–178. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Karimi, G.; Hosseinzadeh, H. Effects of melatonin on cardiovascular risk factors and metabolic syndrome: A comprehensive review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 521–536. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Y.; Xu, Y.; Zhou, Q.; Wang, Y.; Zhu, H. Melatonin alleviates myosin light chain kinase expression and activity via the mitogen-activated protein kinase pathway during atherosclerosis in rabbits. Mol. Med. Rep. 2014, 11, 99–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jennings, R.B.; Sommers, H.M.; A Smyth, G.; A Flack, H.; Linn, H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960, 70, 68–78. [Google Scholar] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Gunata, M.; Parlakpinar, H. A review of myocardial ischaemia/reperfusion injury: Pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem. Funct. 2020, 39, 190–217. [Google Scholar] [CrossRef]
- Yang, C.-F. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Tzu Chi Med J. 2018, 30, 209–215. [Google Scholar] [CrossRef]
- Caccioppo, A.; Franchin, L.; Grosso, A.; Angelini, F.; D’Ascenzo, F.; Brizzi, M.F. Ischemia Reperfusion Injury: Mechanisms of Damage/Protection and Novel Strategies for Cardiac Recovery/Regeneration. Int J. Mol. Sci. 2019, 20, 5024. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Kim, S.J.; El-Sokkary, G.H. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: Prevention by melatonin. J. Pineal Res. 1998, 25, 184–191. [Google Scholar] [CrossRef]
- Lagneux, C.; Joyeux, M.; Demenge, P.; Ribuot, C.; Godin-Ribuot, D. Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 2000, 66, 503–509. [Google Scholar] [CrossRef]
- Kaneko, S.; Okumura, K.; Numaguchi, Y.; Matsui, H.; Murase, K.; Mokuno, S.; Morishima, I.; Hira, K.; Toki, Y.; Ito, T.; et al. Melatonin scavenges hydroxyl radical and protects isolated rat hearts from ischemic reperfusion injury. Life Sci. 2000, 67, 101–112. [Google Scholar] [CrossRef]
- Szárszoi, O.; Asemu, G.; Vanecek, J.; Ost’ádal, B.; Kolar, F. Effects of melatonin on ischemia and reperfusion injury of the rat heart. Cardiovasc. Drugs Ther. 2001, 15, 251–257. [Google Scholar] [CrossRef]
- Sahna, E.; Parlakpinar, H.; Turkoz, Y.; Acet, A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol. Res. 2005, 54, 491–495. [Google Scholar] [PubMed]
- Ekeløf, S.V.; Halladin, N.L.; Jensen, S.E.; Zaremba, T.; Aarøe, J.; Kjærgaard, B.; Simonsen, C.W.; Rosenberg, J.; Gögenur, I.; Kjaergaard, B. Effects of intracoronary melatonin on ischemia–reperfusion injury in ST-elevation myocardial infarction. Heart Vessel. 2014, 31, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, J.; Slotwinska, D.; Olczak, S.; Tosik, D.; Pieniążek, A.; Matczak, K.; Koceva-Chyla, A.; Szczepanowska, A. Pharmacological doses of melatonin reduce the glycosaminoglycan level within the infarcted heart scar. J. Physiol. Pharmacol. 2011, 62, 29. [Google Scholar]
- Drobnik, J.; Tosik, D.; Piera, L.; Szczepanowska, A.; Olczak, S.; Zielinska, A. Melatonin-induced glycosaminoglycans augmentation in myocardium remote to infarction. J. Physiol Pharm. 2013, 64, 737–744. [Google Scholar]
- Song, Y.-J.; Zhong, C.-B.; Wu, W. Cardioprotective effects of melatonin: Focusing on its roles against diabetic cardiomyopathy. Biomed. Pharmacother. 2020, 128, 110260. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Yang, Y.; Guo, Y.; Fan, Y.; Zhang, M.; Man, W.; Gao, E.; Hu, W.; Reiter, R.J.; et al. Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J. Pineal Res. 2017, 62, e12368. [Google Scholar] [CrossRef]
- Singhanat, K.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Roles of melatonin and its receptors in cardiac ischemia–reperfusion injury. Cell. Mol. Life Sci. 2018, 75, 4125–4149. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Yu, L.; Liang, H.; Lu, Z.; Zhao, G.; Zhai, M.; Yang, Y.; Yang, J.; Yi, D.; Chen, W.; Wang, X. Membrane receptor-dependent Notch1 Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: In vivo and in vitro studies. J. Pineal Res. 2015, 59, 420–433. [Google Scholar] [CrossRef]
- Yeung, H.-M.; Hung, M.-W.; Lau, C.-F.; Fung, M.-L. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 2015, 58, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Deng, Y.; Pan, Y.Y.; Wang, Z.H.; Ren, J.; Guo, X.L.; Yuan, X.; Shang, J.; Liu, H. Melatonin protects against chronic intermittent hypoxia-induced cardiac hypertrophy by modulating autophagy through the 5′ adenosine monophosphate-activated protein kinase pathway. Biochem. Biophys. Res. Commun. 2015, 464, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Haghjooy Javanmard, S.; Ziaei, A.; Ziaei, S.; Ziaei, E.; Mirmohammad-Sadeghi, M. The effect of preoperative melatonin on nuclear erythroid 2-related factor 2 activation in patients undergoing coronary artery bypass grafting surgery. Oxidative Med. Cell. Longevity 2013, 2013, 676829. [Google Scholar] [CrossRef] [PubMed]
- Gögenur, I.; Kücükakin, B.; Panduro Jensen, L.; Reiter, R.J.; Rosenberg, J. Melatonin reduces cardiac morbidity and markers of myocardial ischemia after elective abdominal aortic aneurism repair: A randomized, placebo-controlled, clinical trial. J. Pineal Res. 2014, 57, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ghaeli, P.; Vejdani, S.; Ariamanesh, A.; Talasaz, A.H. Effect of Melatonin on Cardiac Injury after Primary Percutaneous Coronary Intervention: A Randomized Controlled Trial. Iran. J. Pharm. Res. IJPR 2015, 14, 851–855. [Google Scholar] [CrossRef]
- Dwaich, K.H.; Al-Amran, F.G.; Al-Sheibani, B.I.; Al-Aubaidy, H.A. Melatonin effects on myocardial ischemia-reperfusion injury: Impact on the outcome in patients undergoing coronary artery bypass grafting surgery. Int. J. Cardiol. 2016, 221, 977–986. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Jose, M.; Consuegra-Sanchez, L.; Piccolo, R.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; Mar Garcia-Saiz, M.; Aldea-Perona, A.; Reiter, R.J. Usefulness of early treatment with melatonin to reduce infarct size in patients with ST-segment elevation myocardial infarction receiving percutaneous coronary intervention (from the melatonin adjunct in the acute myocardial infarction treated with angioplasty trial). Am. J. Cardiol. 2017, 120, 522–526. [Google Scholar]
- Ekeloef, S.; Halladin, N.; Fonnes, S.; Jensen, S.E.; Zaremba, T.; Rosenberg, J.; Jonsson, G.; Aarøe, J.; Smidt Gasbjerg, L.; Marie Rosenkilde, M. Effect of intracoronary and intravenous melatonin on myocardial salvage index in patients with ST-elevation myocardial infarction: A randomized placebo controlled trial. J. Cardiovasc. Transl. Res. 2017, 10, 470–479. [Google Scholar] [CrossRef]
- Shafiei, E.; Bahtoei, M.; Raj, P.; Ostovar, A.; Iranpour, D.; Akbarzadeh, S.; Shahryari, H.; Anvaripour, A.; Tahmasebi, R.; Netticadan, T. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine 2018, 97, e11383. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, A.; Abreu-González, P.; Báez-Ferrer, N.; Reiter, R.J.; Avanzas, P.; Hernández-Vaquero, D. Melatonin and Cardioprotection in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 364. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ma, Q.; Zhu, P.; Ren, J.; Reiter, R.J.; Chen, Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J. Pineal Res. 2018, 64, e12471. [Google Scholar] [CrossRef] [PubMed]
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S., Jr. The Absolute Bioavailability of Oral Melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Sinicropi, M.S. Melatonergic drugs in development. Clin. Pharmacol. Adv. Appl. 2014, 6, 127–137. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. J. Cereb. Blood Flow Metab. 2017, 175, 3263–3280. [Google Scholar] [CrossRef]
- Paulis, L.; Simko, F.; Laudon, M. Cardiovascular effects of melatonin receptor agonists. Expert Opin. Investig. Drugs 2012, 21, 1661–1678. [Google Scholar] [CrossRef]
- Mailliet, F.; Ferry, G.; Vella, F.; Berger, S.; Cogé, F.; Chomarat, P.; Mallet, C.; Guénin, S.-P.; Guillaumet, G.; Viaud-Massuard, M.-C.; et al. Characterization of the melatoninergic MT3 binding site on the NRH:quinone oxidoreductase 2 enzyme. Biochem. Pharmacol. 2005, 71, 74–88. [Google Scholar] [CrossRef]
- Molinari, E.J.; North, P.C.; Dubocovich, M.L. 2-[125I]Iodo-5-methoxycarbonylamino-N-acetyltryptamine: A selective radioligand for the characterization of melatonin ML2 binding sites. Eur. J. Pharmacol. 1996, 301, 159–168. [Google Scholar] [CrossRef]
- Baltatu, O.C.; Senar, S.; Campos, L.A.; Cipolla-Neto, J. Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. Int. J. Mol. Sci. 2019, 20, 4342. [Google Scholar] [CrossRef]
- Stroethoff, M.; Behmenburg, F.; Spittler, K.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Mathes, A. Activation of Melatonin Receptors by Ramelteon Induces Cardioprotection by Postconditioning in the Rat Heart. Anesthesia Analg. 2018, 126, 2112–2115. [Google Scholar] [CrossRef]
- Yu, J.; Wei, J.; Ji, L.; Hong, X. Exploration on Mechanism of a New Type of Melatonin Receptor Agonist Neu-p11 in Hypoxia–Reoxygenation Injury of Myocardial Cells. Cell Biophys. 2014, 70, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Liu, C.; Wu, N.; Jia, D.; Sun, Y. Agomelatine protects against myocardial ischemia reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am. J. Transl. Res. 2018, 10, 1310. [Google Scholar] [PubMed]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. Bir melatonin derlemesi, reseptörleri ve ilaçları. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]


| Experimental Studies | ||||
|---|---|---|---|---|
| Experiment | Animals or Tissues Used | Melatonin Dosage | Outcome | Major Findings |
| After melatonin pretreatment, rats hearts were excised and perfused retrogradely using the Langendorff technique, then ischemia was induced by left coronary artery ligation [28] | Male Wistar rat hearts | 10 mg/kg | Pretreatment with melatonin conferred protection against arrhythmias caused by the infarction, as well as reduced the damaged area | Melatonin and 5-MCA-NAT have similar protective functions when administered at doses of 10 mg/kg against myocardial ischemia-reperfusion injury |
| Rats hearts were excised and perfused retrogradely using the Langendorff technique, then ischemia was induced followed by 30 min of reperfusion with melatonin [29] | Male Sprague-Dawley rats | 100 µmol | Melatonin had a protective effect against myocardial reperfusion injury | Melatonin decreases reperfusion arrhythmia by decreasing lipid peroxidation and scavenging. The use of melatonin decreased ventricular tachycardia and ventricular fibrillation |
| Regional ischemia induced by left anterior descending coronary artery occlusion [30] | Adult male Wistar rats | 10 µmol/L | Melatonin decreased the incidence and severity of ventricular arrhythmias | Melatonin mainly reduced ventricular fibrillation and improved post-ischemic contractile function |
| Rat hearts were connected to perfusion cannulas of Langendorff apparatus, this cannula will be closed for the ischemia process for 30 min and then opened for reperfusion for 45 min [16] | Male Wistar rats | 50 and 100 mM of melatonin in KHB solution | Melatonin reduced dangerous oxidation | Melatonin does not have an oxidation-reduction cycle and therefore prevents oxidative damage to cellular components |
| Mouse coronary artery occlusion (Left coronary artery ligation) [33] | In vitro cultures of mouse cardiac myofibroblasts | 300 µg/100 g | Melatonin modified cardiac remodeling | Exogen melatonin decreases GAG´s concentrations but does not modify collagen concentrations |
| Myocardial infarction/reperfusion surgery [40] | Male Sprague Dawley rats | 10 mg/kg/day for 4 weeks | Prophylactic use of melatonin improved cardiac function | Melatonin reduces apoptosis by reducing caspase 3 expression when Notch1/Hes1 signaling is stimulated |
| Myocardial ischemia/reperfusion (MI/R) induced injury exacerbated by CIH [41] | Adult male Sprague Dawley rats | 10 mg/kg | Melatonin reduced myocardial inflammation, fibrosis and exacerbated MI/R injury | Systolic pressure, heart weights, and malondialdehyde were significantly increased in hypoxic rats but not in the melatonin-treated group Rats treated with melatonin have reduced levels of inflammatory cytokines (TNF-α, IL-6, and COX-2) and fibrotic markers (PC1 and TGF-β) |
| Chronic intermittent hypoxia [42] | Rats | 10 mg/kg melatonin or saline solution daily for six weeks | Melatonin reversed myocardial hypertrophy | The activation of AMPK signaling activates autophagy reducing myocardium apoptosis |
| Closed-chest porcine model of myocardial ischemia and reperfusion [32] | Female Danish Landrace pigs | 200 mg (0.4 mg/mL) | The combination of intravenous and intracoronary melatonin did not reduce myocardial reperfusion injury. | The use of melatonin does not significantly increase the myocardial salvage index, nor does it significantly reduce the high-sensitive troponin T release |
| MI model using Mst1 transgenic (Mst1 Tg) and Mst1 knockout (Mst1−/−) mice [37] | Mice | 10 mg/kg/day of melatonin for 3 weeks | Melatonin reduced cardiomyocyte apoptosis | Melatonin was found to alleviate intracellular stress by stabilizing mitochondrial dysfunction |
| Hypoxia/reoxygenation process [38] | Cardiomyocytes from neonatal rats and H9C2 cells | - | Melatonin activated some signaling pathways that protect cardiomyocytes from oxidative stress | Melatonin protects heart cells by activating survival via JAK/STAT, STAT3, Notch1/Hes1, PKG1α, PI3K/Akt, ERK1/2, and AMPKα reducing mitochondrial and cellular oxidative stress, mitochondrial fission, endoplasmic reticulum stress, and apoptosis |
| Clinical Studies | |||||||
|---|---|---|---|---|---|---|---|
| Study Model | Sample Size | Melatonin Administration | Reperfusion Medical Strategies | Major Findings | Interpretation | ||
| Lv Function/Hemodynamic Parameters | Infarct Size | Biomarkers | |||||
| Elective CABG None with AMI [39]. | 30 | Before bedtime, orally 10 mg of melatonin 1 month before the procedure | CABG | - | - | ↑ Melatonin ↑ Nrf2 | Melatonin, through the Nrf2 pathway, may have a key role in the potentiation of antioxidant defense and mitigation of cellular damages caused by CABG surgery. |
| Elective surgery for abdominal aortic aneurysm [43]. | 50 | Intraoperatively and intravenously 50 mg melatonin over 2 h; and orally 10 mg of melatonin throughout the first 3 nights after the procedure | CABG | - | - | ↓ Troponin-I | Clinical cardiac morbidity, troponin I levels, the frequency of ST-segment deviations, and the incidence of myocardial ischemia were reduced following the procedure. |
| Patients with STEMI [44]. | 40 | The night following PCI melatonin 3 mg was orally given and maintained daily in the hospital | PCI | - | - | ↓ CK-MB hs-TnT hs-TnT | There is no impact. The length of the trial, the sample size, and the low doses of melatonin used were all limitations. |
| Ischemic heart disease patients undergoing elective CABG [45]. | 45 | From the fifth day before surgery, a low dosage melatonin therapy group (10 mg/day) and a high dosage melatonin treatment group (20 mg/day) were used | CABG | ↑ LVEF ↓ HR | - | ↓ cTn-I ↓ Interleukin-1β ↓ Inducible nitric oxide synthase ↓ Caspase-3 enzymes. | Melatonin reduced oxidative stress, inflammation, and apoptosis in ischemic heart disease patients following CABG, decreasing MI/R injury. |
| ST-elevation myocardial infarction patients [46]. | 146 | An intravenous bolus of 51.7 μmol melatonin was given 60 min before reperfusion, followed by an intracoronary bolus of 8.6 μmol (total 14 mg) melatonin at the start of reperfusion. | Primary PCI |  LVEDV LVEDV LVESV LVESV Total LV mass Total LV mass | ↓ Infarct size with symptoms starting 136 ± 23 min later Infarct size with symptoms starting between 196 ± 19 min and 249 ± 41 min later Infarct size with symptoms starting between 196 ± 19 min and 249 ± 41 min later | - | In STEMI individuals, early melatonin treatment decreased infarct size. |
| ST-elevation myocardial infarction patients [47]. | 48 | 0.1 mg/mL melatonin intracoronary and 0.1 mg/mL melatonin intravenous injection (total 50 mg) | Primary PCI |  LVEDV LVEDV LVESV LVESV LVEF LVEF |  Infarct size Infarct size |  hs-TnT, CK-MB hs-TnT, CK-MB | Melatonin did not affect LV function or clinical outcomes in STEMI patients. |
| Elective CABG [48]. | 88 | Melatonin 5 mg given orally (3 times beginning from 24 h before the procedure and a single dose [15 mg] 1 h before the surgery) | CABG | - | - | ↓ Troponin I ↓ Lactate ↓ MDA ↓ TNF-α | NAC and melatonin are powerful antioxidants that have almost equal effectiveness in decreasing CABG-related heart damage and oxidative stress at the doses used in the study. |
| Experimental Studies of Melatonin Analogs | ||||
|---|---|---|---|---|
| Melatonin Analog | Animals or Tissues Used | Conduct of the Experiment | Outcome | Major Findings |
| 5-MCA-NAT [27] | Wistar rats | Rats were treated with either melatonin or 5-MCA-NAT (10 mg/kg) and divided into two groups: regional ischemia for 5 min and reperfused for 30 min, and regional ischemia for 30 min and reperfused for 120 min | Pretreatment with either melatonin or 5-MCA-NAT conferred protection against arryhtmias caused by the infarction and reduced the infarction size | 5-MCA-NAT shows a significant protection against ischemia-reperfusion injury. These protection is very similar to that of melatonin |
| Ramelteon [60] | Male Wistar rats | Six sets of rats that underwent ischemia-reperfusion: Treatment with melatonin and ramelteon, treatment with melatonin and luzindazole, and treatment with ramelteon and luzindazole | Ramelteon reduces infarct size to the same degree as melatonin and that luzindazole fully eliminates the effects od ramelteon | Ramelteon can decrease MI/R injury by approximately 50%, and because this compound has selectivity for MT1 and MT2 receptors and a favorable side effect profile, it may be of special therapeutic significance |
| Piromelatine [61] | H9c2 cardiac cells | H9c2 cardiac cells were used to create a hypoxia/reoxigenation model and cells were split into three: control, hypoxia/reoxigenation and Piromelatine. CK, LDH and SOD were compared | CK, LDH and MDA were lower, and SOD was raised in the Piromelatine group | Piromelatine protects cardaic cells from hypoxia-reoxigenation damage. It reduces lipid peroxidation and protects the mitochondria from MI/R injury, inhibits apoptosis, improves rhytm and morphology of cardiac cells, and reduces membrane permeability |
| Agomelatine [62] | Rats | Rat hearts isolated and and submitted to 30 min of ischemia. After 120 min, they were reperfused. Rats were given an intraperitoneal injection of 10, 20 or 40 mg/kg of agomelatine | Agomelatine improved cardiac function, alleviated changes in ischemic myocardium, reduced infarct size, decreased CK-MB and LDH, inhibited de MPTP. Agomelatine also decreased cytochrome C, caspases and enhaced GSK-3β phosphorylation | The data suggest that agomelatine protects against MI/R by blocking the opening of mitochondrial permeability transition pores |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermudez-Gonzalez, J.L.; Sanchez-Quintero, D.; Proaño-Bernal, L.; Santana-Apreza, R.; Jimenez-Chavarria, M.A.; Luna-Alvarez-Amezquita, J.A.; Straface, J.I.; Perez-Partida, A.M.; Berarducci, J.; Armenta-Moreno, J.I.; et al. Role of the Antioxidant Activity of Melatonin in Myocardial Ischemia-Reperfusion Injury. Antioxidants 2022, 11, 627. https://doi.org/10.3390/antiox11040627
Bermudez-Gonzalez JL, Sanchez-Quintero D, Proaño-Bernal L, Santana-Apreza R, Jimenez-Chavarria MA, Luna-Alvarez-Amezquita JA, Straface JI, Perez-Partida AM, Berarducci J, Armenta-Moreno JI, et al. Role of the Antioxidant Activity of Melatonin in Myocardial Ischemia-Reperfusion Injury. Antioxidants. 2022; 11(4):627. https://doi.org/10.3390/antiox11040627
Chicago/Turabian StyleBermudez-Gonzalez, Jorge Luis, Denya Sanchez-Quintero, Leonardo Proaño-Bernal, Rafael Santana-Apreza, Marco Antonio Jimenez-Chavarria, Jose Antonio Luna-Alvarez-Amezquita, Juan Ignacio Straface, Arantza Marie Perez-Partida, Joaquin Berarducci, Javier Ivan Armenta-Moreno, and et al. 2022. "Role of the Antioxidant Activity of Melatonin in Myocardial Ischemia-Reperfusion Injury" Antioxidants 11, no. 4: 627. https://doi.org/10.3390/antiox11040627
APA StyleBermudez-Gonzalez, J. L., Sanchez-Quintero, D., Proaño-Bernal, L., Santana-Apreza, R., Jimenez-Chavarria, M. A., Luna-Alvarez-Amezquita, J. A., Straface, J. I., Perez-Partida, A. M., Berarducci, J., Armenta-Moreno, J. I., Garza-Cruz, K. J., Espinola-Zavaleta, N., & Alexanderson-Rosas, E. (2022). Role of the Antioxidant Activity of Melatonin in Myocardial Ischemia-Reperfusion Injury. Antioxidants, 11(4), 627. https://doi.org/10.3390/antiox11040627






