Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Fluorescence Spectroscopy
2.4. Particle Size and ζ-Potential
2.5. Color Evaluation
2.6. Resveratrol Quantification
2.7. Loading Efficiency
2.8. Antioxidant Activity
2.9. Sulfhydryl Analysis
2.10. Carbonyl Analysis
2.11. Amino Acid Analysis
2.12. Statistical Analysis
3. Results
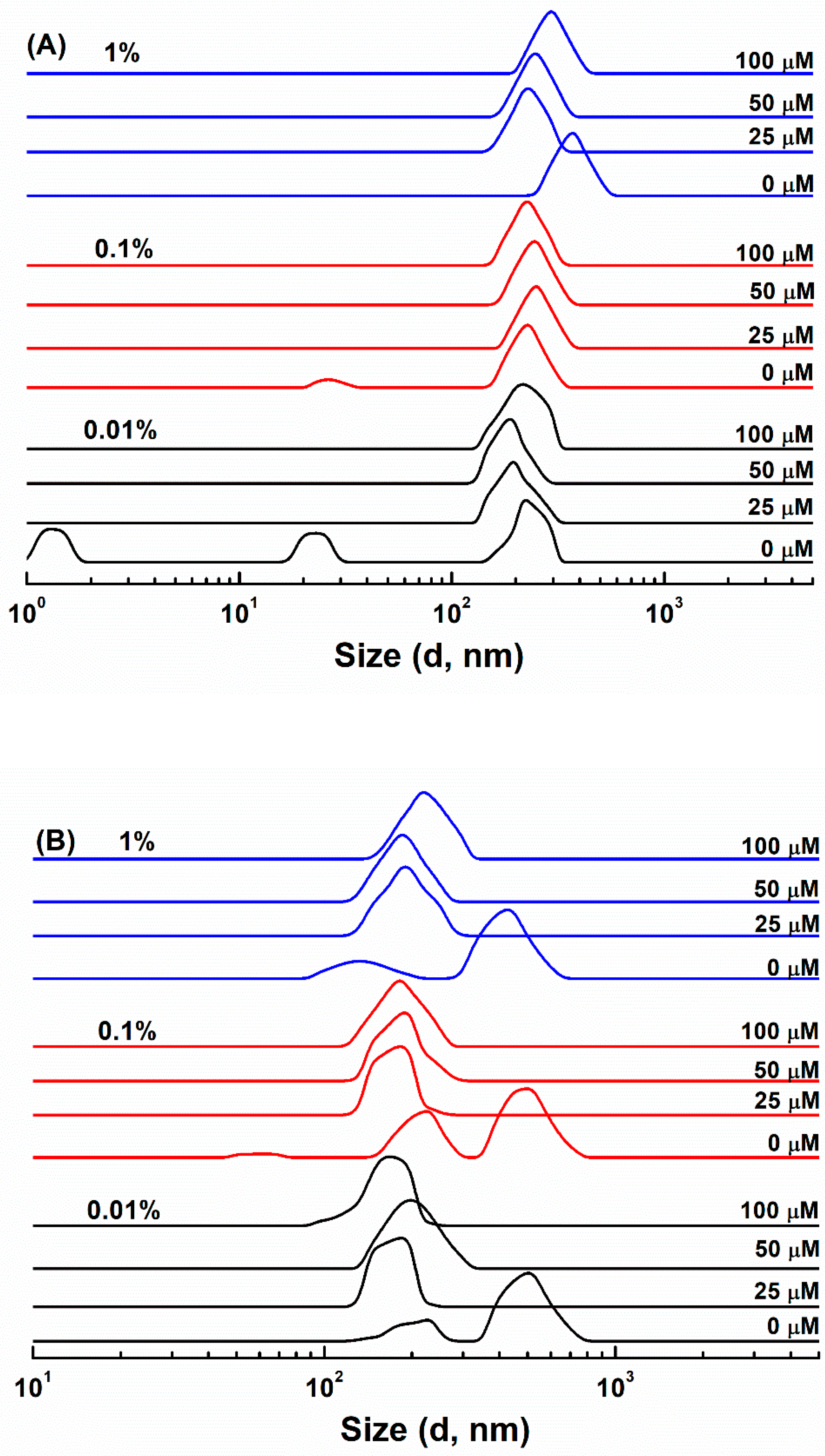
3.1. Particle Characterization
3.2. Resveratrol Loading
3.2.1. Microenvironment of Resveratrol
3.2.2. Loading Efficiency of Resveratrol
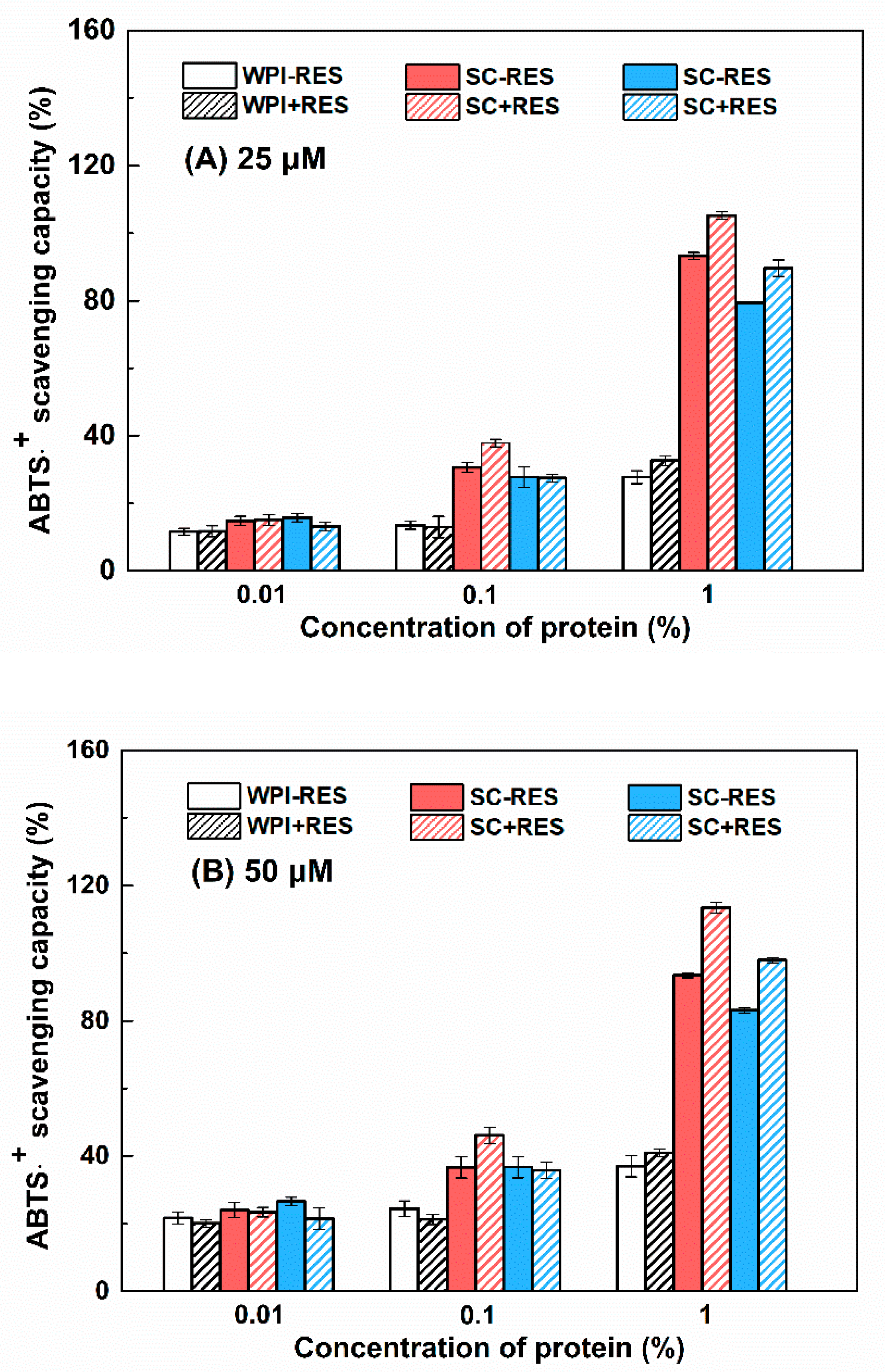
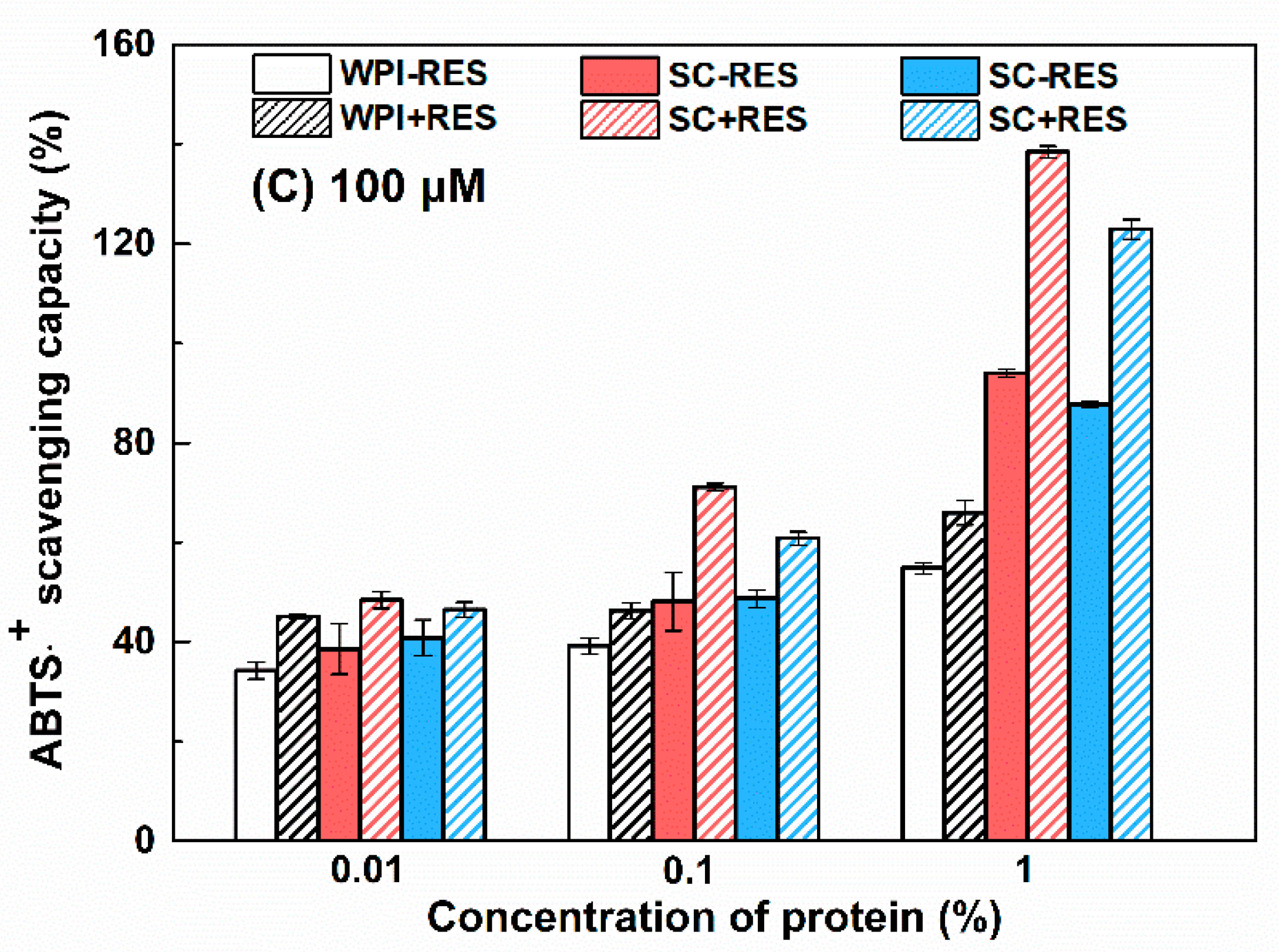
3.2.3. Antioxidant Activity
3.3. Protein Oxidation
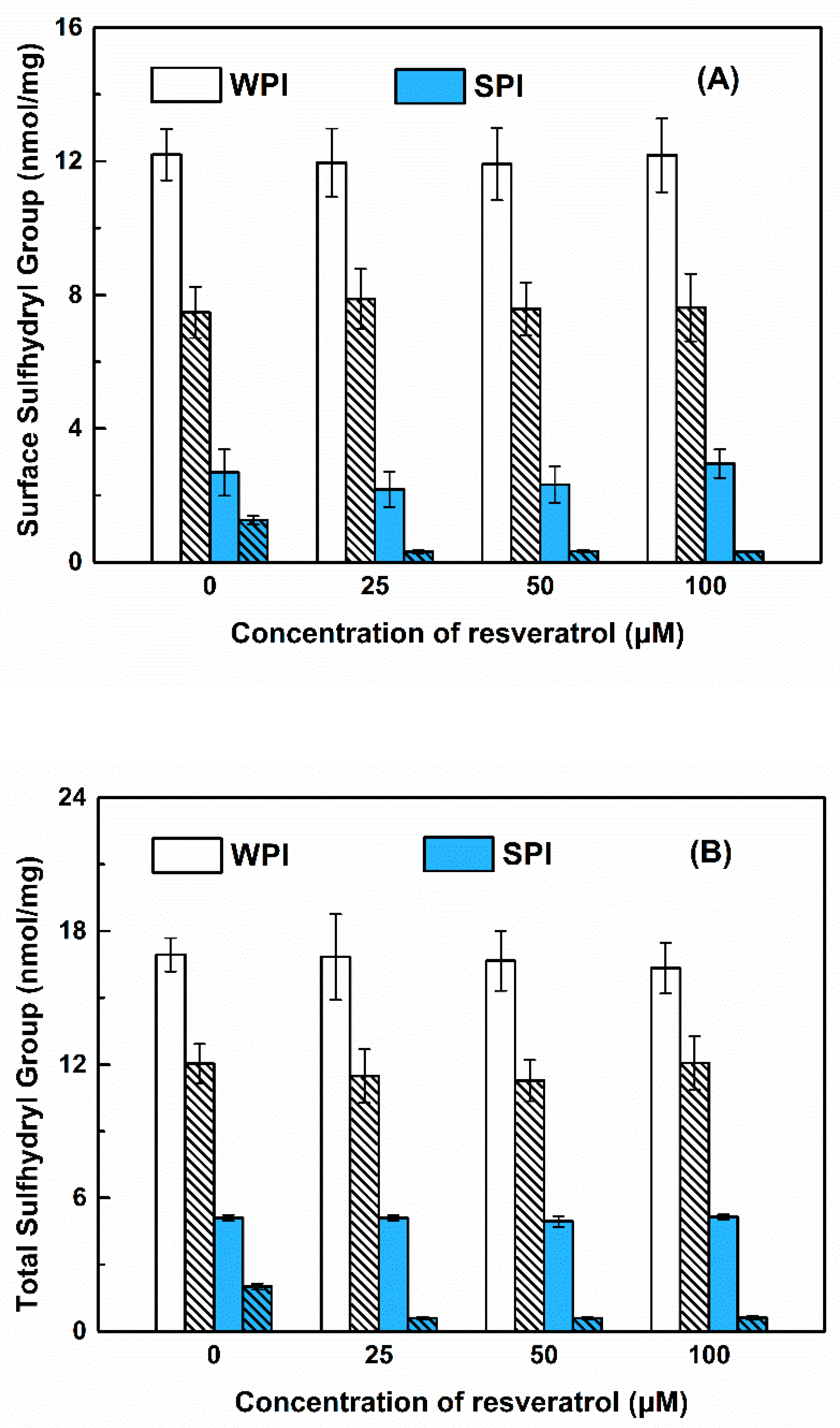
3.3.1. Sulfhydryl Groups
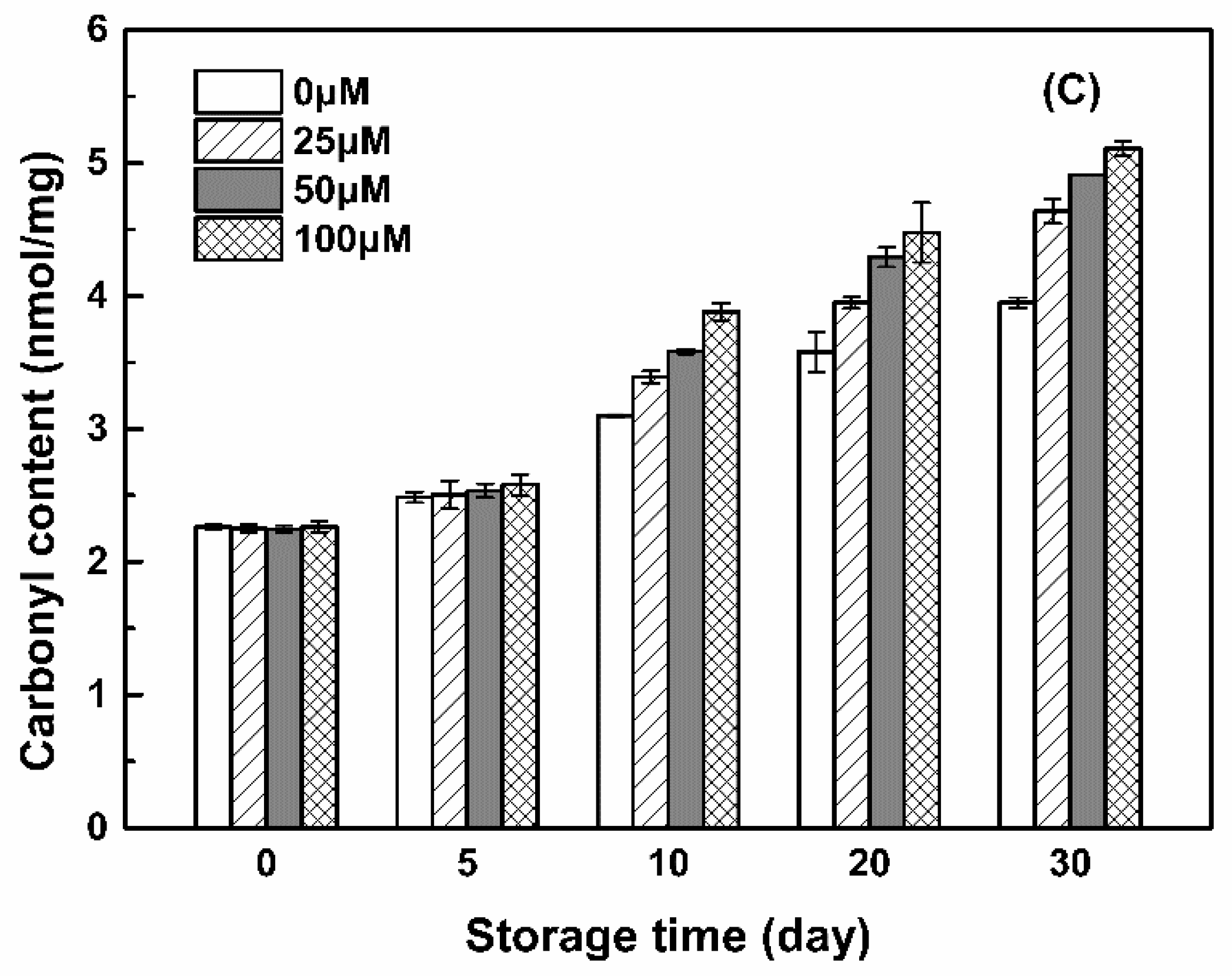
3.3.2. Carbonyl Groups
3.3.3. Amino Acid Composition
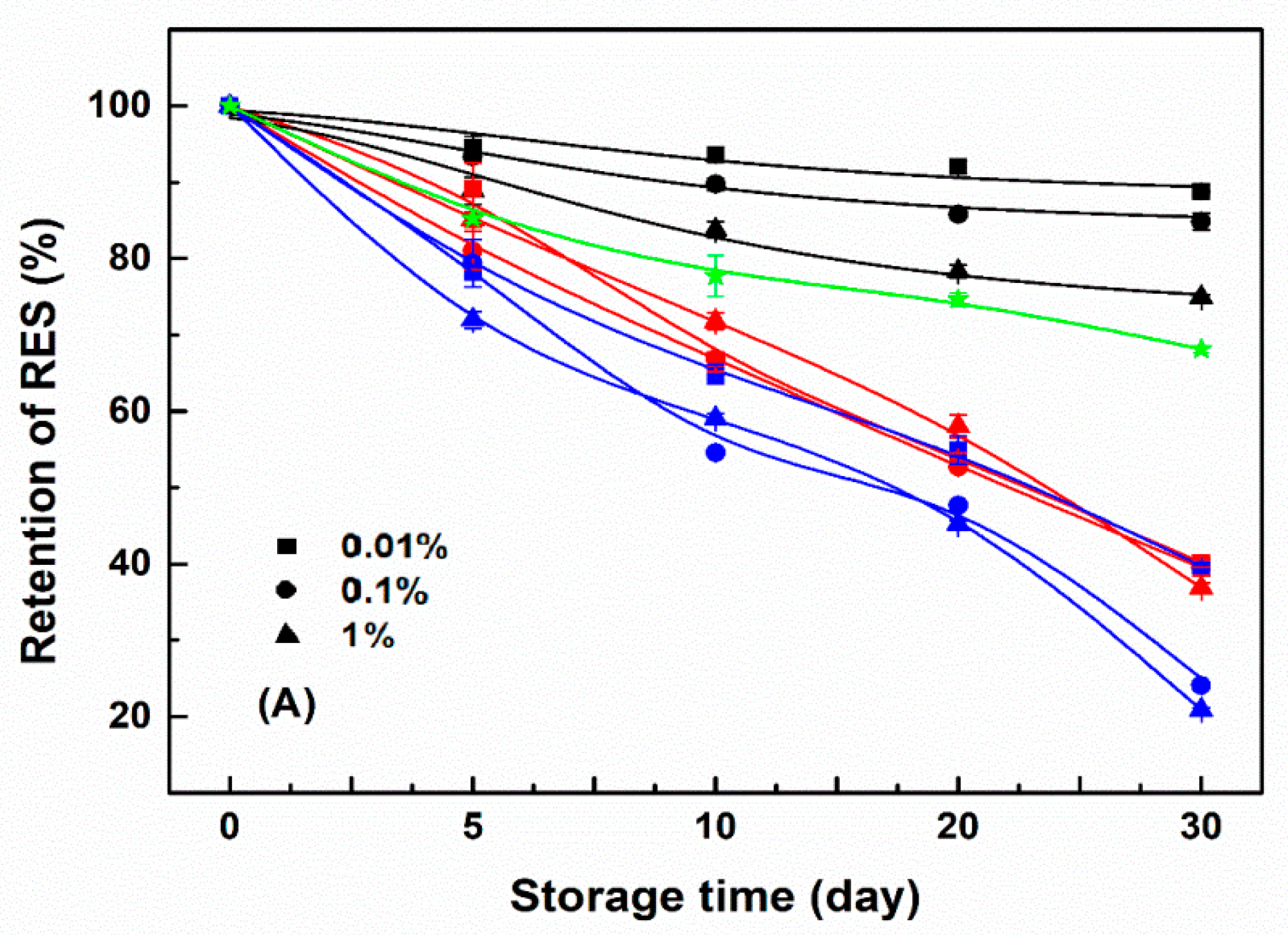
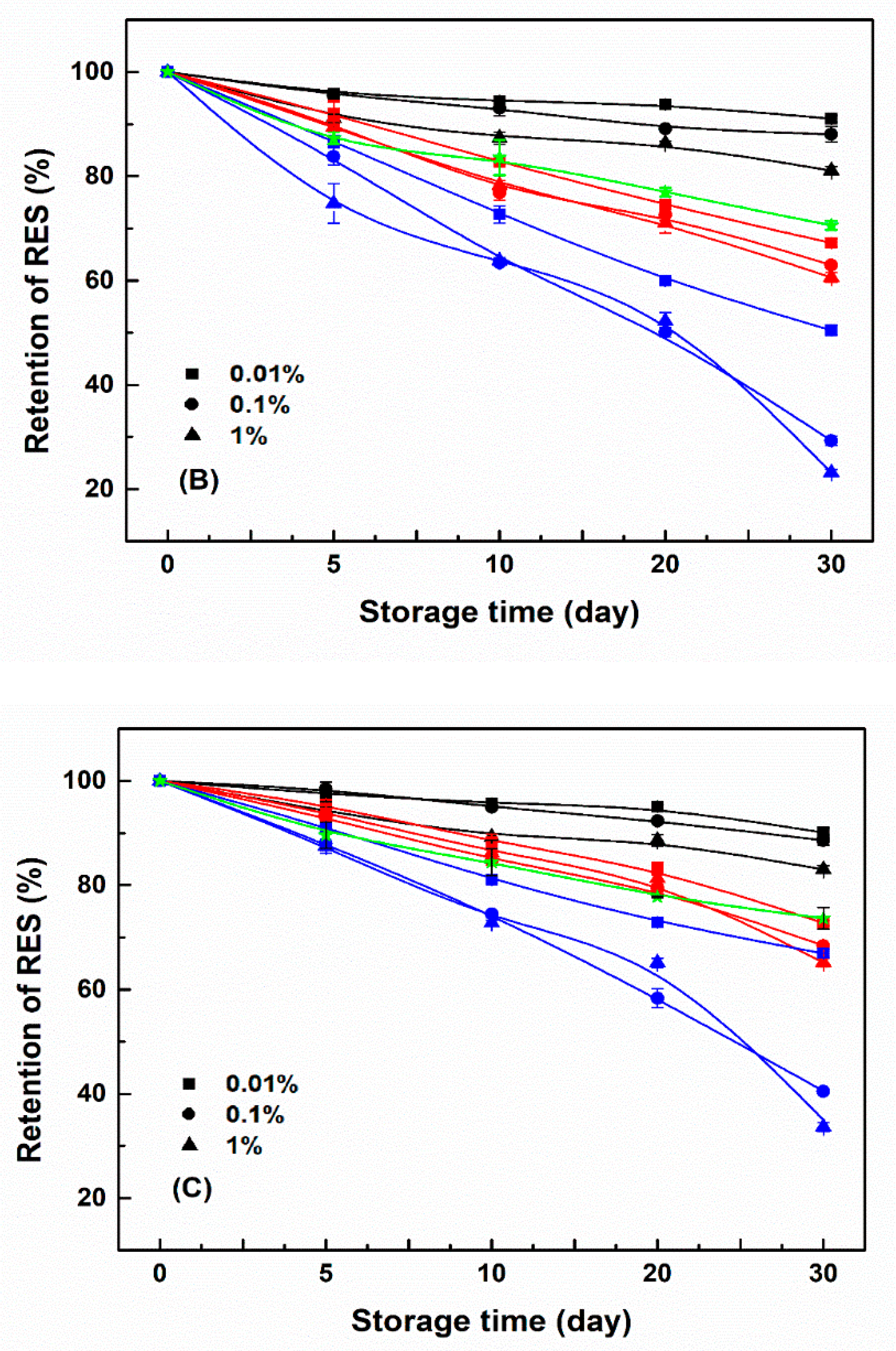
3.4. Storage Stability of Resveratrol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WPI | whey protein isolate |
| SC | sodium caseinate |
| SPI | soy protein isolate |
| BSA | bovine serum albumin |
| RES | resveratrol |
| GRAS | generally recognized as safe |
| ABTS | 2,2′-azino-bis-3- ethylbenzthiazoline-6-sulphonic acid |
| CMC | critical micelle concentration |
| β-LG | β-lactoglobulin |
| LOX | lipoxygenase |
| SH | sulfhydryl |
| ΔE | total color difference |
| ΔC* | chroma change |
References
- Zhang, J.; Field, C.J.; Vine, D.; Chen, L. Intestinal Uptake and Transport of Vitamin B12-loaded Soy Protein Nanoparticles. Pharm. Res. 2015, 32, 1288–1303. [Google Scholar] [CrossRef]
- McClements, D.J. The future of food colloids: Next-generation nanoparticle delivery systems. Curr. Opin. Colloid Interface Sci. 2017, 28, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Dalsgaard, T.K.; Otzen, D.; Nielsen, J.H.; Larsen, L.B. Changes in Structures of Milk Proteins upon Photo-oxidation. J. Agric. Food Chem. 2007, 55, 10968–10976. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wusigale; Cheng, H.; Fang, Z.; Liang, L. Mechanism for improved protection of whey protein isolate against the photodecomposition of folic acid. Food Hydrocoll. 2018, 79, 439–449. [Google Scholar] [CrossRef]
- Xu, D.; Wang, X.; Jiang, J.; Yuan, F.; Decker, E.A.; Gao, Y. Influence of pH, EDTA, α-tocopherol, and WPI oxidation on the degradation of β-carotene in WPI-stabilized oil-in-water emulsions. LWT-Food Sci. Technol. 2013, 54, 236–241. [Google Scholar] [CrossRef]
- Salminen, H.; Heinonen, M. Plant Phenolics Affect Oxidation of Tryptophan. J. Agric. Food Chem. 2008, 56, 7472–7481. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and their impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, X.; Liang, L.; Xu, X. Gallic Acid-Aided Cross-Linking of Myofibrillar Protein Fabricated Soluble Aggregates for Enhanced Thermal Stability and a Tunable Colloidal State. J. Agric. Food Chem. 2020, 68, 11535–11544. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; McClements, D. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Ren, C.; Li, J.; Li, B. Enhancing the photostability and bioaccessibility of resveratrol using ovalbumin–carboxymethylcellulose nanocomplexes and nanoparticles. Food Funct. 2018, 9, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, H.; Chen, Y.; Chen, L.; Fang, Z.; Liang, L. Formation of a multiligand complex of bovine serum albumin with retinol, resveratrol, and (−)-epigallocatechin-3-gallate for the protection of bioactive components. J. Agric. Food. Chem. 2017, 65, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Liyanaarachchi, W.S.; Chandrapala, J.; Dissanayake, M.; Vasiljevic, T. Utilizing unique properties of caseins and the casein micelle for delivery of sensitive food ingredients and bioactives. Trends Food Sci. Technol. 2016, 57, 178–187. [Google Scholar] [CrossRef]
- Cheng, H.; Dong, H.; Liang, L. A comparison of beta-casein complexes and micelles as vehicles for trans-/cis-resveratrol. Food Chem. 2020, 330, 127209. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Morr, C.V.; Ha, E.Y.W. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Cellular Uptake of β-Carotene from Protein Stabilized Solid Lipid Nanoparticles Prepared by Homogenization–Evaporation Method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, L.; Cai, Q.; Zou, L.; Liu, C.; Liu, W.; McClements, D.J. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocoll. 2020, 107, 105963. [Google Scholar] [CrossRef]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.-M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Fang, Z.; Liu, T.; Gao, Y.; Liang, L. A study on β-lactoglobulin-triligand-pectin complex particle: Formation, characterization and protection. Food Hydrocoll. 2018, 84, 93–103. [Google Scholar] [CrossRef]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Wu, C. Fluorescence and light-scattering studies on the formation of stable colloidal nanoparticles made of sodium sulfonated polystyrene ionomers. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1593–1599. [Google Scholar] [CrossRef]
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- Almajano, M.; Delgado, M.E.; Gordon, M.H. Changes in the antioxidant properties of protein solutions in the presence of epigallocatechin gallate. Food Chem. 2007, 101, 126–130. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.-R.; Gahruie, H.H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- Webb, M.; Naeem, H.; Schmidt, K. Food Protein Functionality in a Liquid System: A Comparison of Deamidated Wheat Protein with Dairy and Soy Proteins. J. Food Sci. 2002, 67, 2896–2902. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.D.; Cheng, Z.; Selomulya, C. On enhancing the solubility of curcumin by microencapsulation in whey protein isolate via spray drying. J. Food Eng. 2016, 169, 189–195. [Google Scholar] [CrossRef]
- Yin, X.; Fu, X.; Cheng, H.; Liang, L. α-Tocopherol and naringenin in whey protein isolate particles: Partition, antioxidant activity, stability and bioaccessibility. Food Hydrocoll. 2020, 106, 105895. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, G.; Cao, W.; Li, J.; Taha, A.; Hu, H.; Pan, S. Interaction between pH-shifted beta-conglycinin and flavonoids hesperetin/hesperidin: Characterization of nanocomplexes and binding mechanism. LWT-Food Sci. Technol. 2021, 140, 110698. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, R.; Fu, R.; Xiong, J.; Hu, Y. Cytotoxicity and inhibition of lipid peroxidation activity of resveratrol/cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 313–320. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X. Binding, stability, and antioxidant activity of quercetin with soy protein isolate particles. Food Chem. 2015, 188, 24–29. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Z.; Bakry, A.M.; Chen, Y.; Liang, L. Complexation of trans-and cis-resveratrol with bovine serum albumin, β-lactoglobulin or α-lactalbumin. Food Hydrocoll. 2018, 81, 242–252. [Google Scholar] [CrossRef]
- Ahmed, A.S.; El-Bassiony, T.; Elmalt, L.M.; Ibrahim, H.R. Identification of potent antioxidant bioactive peptides from goat milk proteins. Food Res. Int. 2015, 74, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Velickovic, T.C. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y. Eugenol Nanoemulsion Stabilized with Zein and Sodium Caseinate by Self-Assembly. J. Agric. Food Chem. 2017, 65, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Amamcharla, J.K.; Metzger, L.E. Modification of the ferric reducing antioxidant power (FRAP) assay to determine the susceptibility of raw milk to oxidation. Int. Dairy J. 2014, 34, 177–179. [Google Scholar] [CrossRef]
- Eaton, P. Protein thiol oxidation in health and disease: Techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic. Biol. Med. 2006, 40, 1889–1899. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Wu, J.; Li-Chan, E.C.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; Kyriakopoulou, K.; de Groot, W.; van der Goot, A.J.; Berton-Carabin, C.C. Oxidative stability of soy proteins: From ground soybeans to structured products. Food Chem. 2020, 318, 126499. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.L.; Chen, J. Oxidation-induced unfolding facilitates myosin cross-linking in myofibrillar protein by microbial transglutaminase. J. Agric. Food. Chem. 2012, 60, 8020–8027. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Zhang, K.; Yao, C.; Yan, C.; Bai, Y.; Jiang, S. Determination of trans-Resveratrol in China Great Wall ‘‘Fazenda’’Red Wine by Use of Micellar Electrokinetic Chromatography. Chromatographia 2005, 62, 289–294. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of emulsifiers and antioxidants on physical and chemical stability. Food Chem. 2018, 242, 395–403. [Google Scholar] [CrossRef]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of β-lactoglobulin with resveratrol and its biological implications. Biomacromolecules 2007, 9, 50–56. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Pérez-Gilabert, M.; García-Carmona, F. Effect of Protonation and Aggregation State of (E)-Resveratrol on Its Hydroperoxidation by Lipoxygenase. J. Agric. Food Chem. 2009, 57, 4630–4635. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ma, L.; Yang, L.; Cong, P.; Lan, H.; Xue, C.; Xu, J. Co-oxidation of Antarctic krill oil with whey protein and myofibrillar protein in oil-in-water emulsions. J. Food Sci. 2020, 85, 3797–3805. [Google Scholar] [CrossRef]
- Hellwig, M. Analysis of Protein Oxidation in Food and Feed Products. J. Agric. Food Chem. 2020, 68, 12870–12885. [Google Scholar] [CrossRef]
- Zoldners, J.; Kiseleva, T.; Kaiminsh, I. Influence of ascorbic acid on the stability of chitosan solutions. Carbohydr. Polym. 2005, 60, 215–218. [Google Scholar] [CrossRef]
- Yang, N.C.; Lee, C.H.; Song, T.Y. Evaluation of resveratrol oxidation in vitro and the crucial role of bicarbonate ions. Biosci. Biotechnol. Biochem. 2010, 74, 63–68. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, W.; Fan, R.; Yuan, F.; Gao, Y. Evaluation of structural and functional properties of protein–EGCG complexes and their ability of stabilizing a model β-carotene emulsion. Food Hydrocoll. 2015, 45, 337–350. [Google Scholar] [CrossRef]






| Amino Acid | Content of Amino Acid (μg/mL) | |||
|---|---|---|---|---|
| WPI (0) | WPI-RES (0) | WPI (30) | WPI-RES (30) | |
| Cys | 121 ± 4 a | 119 ± 4 a | 106 ± 4 b | 104 ± 3 b |
| Trp | 198 ± 7 a | 195 ± 9 a | 172 ± 8 b | 179 ± 6 b |
| Tyr | 298 ± 9 a | 301 ± 7 a | 277 ± 6 b | 280 ± 6 b |
| Thr | 433 ± 5 a | 430 ± 7 a | 417 ± 8 b | 410 ± 8 b |
| Lys | 942 ± 17 a | 947 ± 12 a | 933 ± 11 b | 928 ± 9 b |
| Met | 194 ± 5 a | 192 ± 7 a | 165 ± 5 b | 163 ± 8 b |
| Phe | 346 ± 2 a | 351 ± 6 a | 324 ± 8 b | 320 ± 5 b |
| Asp | 1195 ± 16 a | 1203 ± 11 a | 1196 ± 38 a | 1158 ± 43 a |
| Arg | 233 ± 11 a | 235 ± 8 a | 239 ± 8 a | 235 ± 9 a |
| Glu | 1790 ± 17 a | 1784 ± 29 a | 1777 ± 17 a | 1783 ± 26 a |
| Ser | 296 ± 7 a | 292 ± 5 a | 295 ± 8 a | 299 ± 9 a |
| Gly | 161 ± 2 a | 158 ± 4 a | 160 ± 3 a | 158 ± 3 a |
| His | 172 ± 2 a | 169 ± 3 a | 172 ± 5 a | 173 ± 6 a |
| Val | 506 ± 14 a | 496 ± 29 a | 508 ± 13 a | 495 ± 10 a |
| Ala | 474 ± 7 a | 474 ± 5 a | 475 ± 5 a | 472 ± 9 a |
| Ile | 586 ± 18 a | 579 ± 16 a | 573 ± 10 a | 575 ± 15 a |
| Leu | 991 ± 16 a | 981 ± 22 a | 980 ± 12 a | 976 ± 20 a |
| Pro | 399 ± 19 a | 393 ± 7 a | 398 ± 28 a | 406 ± 10 a |
| Total | 9335 ± 74 a | 9299 ± 53 a | 9167 ± 43 b | 9114 ± 64 b |
| Amino Acid | Content of Amino Acid (μg/mL) | |||
|---|---|---|---|---|
| SC (0) | SC-RES (0) | SC (30) | SC-RES (30) | |
| Cys | 5 ± 0 a | 5 ± 0 a | 5 ± 0 a | 4 ± 1 a |
| Trp | 571 ± 9 a | 566 ± 12 a | 119 ± 14 b | 74 ± 10 c |
| Tyr | 451 ± 9 a | 448 ± 12 a | 409 ± 10 b | 384 ± 6 c |
| Thr | 344 ± 5 a | 350 ± 8 a | 330 ± 3 b | 318 ± 2 c |
| Lys | 744 ± 7 a | 737 ± 11 a | 690 ± 9 b | 629 ± 7 c |
| Met | 226 ± 8 a | 222 ± 2 a | 185 ± 2 b | 184 ± 2 b |
| Phe | 443 ± 8 a | 439 ± 6 a | 427 ± 10 a | 433 ± 10 a |
| Asp | 548 ± 14 a | 556 ± 12 a | 493 ± 18 b | 460 ± 13 c |
| Arg | 337 ± 10 a | 345 ± 9 a | 313 ± 12 b | 294 ± 3 c |
| Glu | 2053 ± 87 a | 2014 ± 79 a | 2048 ± 57 a | 1998 ± 50 b |
| Ser | 380 ± 7 a | 378 ± 5 a | 384 ± 8 a | 375 ± 8 b |
| Gly | 159 ± 4 a | 153 ± 9 a | 160 ± 2 a | 152 ± 3 b |
| His | 293 ± 3 a | 290 ± 9 a | 299 ± 7 a | 288 ± 7 a |
| Val | 600 ± 10 a | 593 ± 19 a | 588 ± 15 a | 589 ± 11 a |
| Ala | 260 ± 7 a | 263 ± 7 a | 252 ± 4 a | 252 ± 4 a |
| Ile | 487 ± 12 a | 479 ± 12 a | 473 ± 10 a | 467 ± 16 a |
| Leu | 788 ± 14 a | 795 ± 22 a | 783 ± 20 a | 780 ± 17 a |
| Pro | 727 ± 16 a | 722 ± 10 a | 720 ± 18 a | 735 ± 11 a |
| Total | 9416 ± 106 a | 9355 ± 99 a | 8678 ± 70 b | 8416 ± 68 c |
| Amino Acid | Content of Amino Acid (μg/mL) | |||
|---|---|---|---|---|
| SPI (0) | SPI-RES (0) | SPI (30) | SPI-RES (30) | |
| Cys | 20 ± 2 a | 19 ± 4 a | 13 ± 2 b | 5 ± 2 c |
| Trp | 160 ± 6 a | 158 ± 8 a | 93 ± 4 b | 78 ± 3 c |
| Tyr | 281 ± 8 a | 276 ± 6 a | 231 ± 4 b | 200 ± 1 c |
| Thr | 209 ± 3 a | 211 ± 5 a | 177 ± 8 b | 170 ± 3 c |
| Lys | 481 ± 7 a | 476 ± 8 a | 403 ± 10 b | 382 ± 8 c |
| Met | 84 ± 3 a | 89 ± 8 a | 79 ± 4 b | 63 ± 6 c |
| Phe | 418 ± 6 a | 421 ± 9 a | 358 ± 10 b | 347 ± 7 c |
| Asp | 735 ± 14 a | 735 ± 13 a | 685 ± 21 b | 554 ± 10 c |
| Arg | 585 ± 5 a | 591 ± 6 a | 584 ± 5 a | 591 ± 8 a |
| Glu | 1520 ± 67 a | 1479 ± 67 a | 1335 ± 79 a | 1192 ± 106 b |
| Ser | 371 ± 9 a | 365 ± 8 a | 325 ± 9 b | 300 ± 4 c |
| Gly | 352 ± 8 a | 346 ± 9 a | 328 ± 8 b | 280 ± 9 c |
| His | 213 ± 6 a | 215 ± 3 a | 195 ± 6 b | 167 ± 3 c |
| Val | 431 ± 8 a | 442 ± 13 a | 399 ± 6 b | 352 ± 7 c |
| Ala | 356 ± 4 a | 350 ± 9 a | 355 ± 11 a | 349 ± 6 a |
| Ile | 416 ± 11 a | 409 ± 7 a | 414 ± 3 a | 402 ± 8 a |
| Leu | 623 ± 12 a | 627 ± 7 a | 614 ± 10 a | 606 ± 19 a |
| Pro | 366 ± 6 a | 357 ± 11 a | 358 ± 8 a | 347 ± 10 a |
| Total | 7621 ± 90 a | 7566 ± 89 a | 6946 ± 98 b | 6385 ± 121 c |
| Protein | Concentration of Resveratrol (μM) | |||
|---|---|---|---|---|
| 0 | 25 | 50 | 100 | |
| ΔE | ||||
| 1.24 ± 0.19 Aa | 1.85 ± 0.45 Aa | 3.06 ± 0.27 Bb | ||
| WPI | 0.74 ± 0.65 Aa | 0.59 ± 0.31 Aa | 0.58 ± 0.47 Aa | 0.87 ± 0.92 Aa |
| SPI | 1.16 ± 0.70 Aa | 3.79 ± 0.83 Bb | 6.95 ± 0.96 Cb | 9.16 ± 1.54 Dc |
| SC | 0.30 ± 0.20 Aa | 2.82 ± 1.19 Bb | 6.04 ± 1.49 Cb | 8.59 ± 0.71 Dc |
| ΔC* | ||||
| 1.17 ± 0.15 Aa | 1.73 ± 0.35 Ba | 2.94 ± 0.23 Cb | ||
| WPI | 0.29 ± 0.22 Aa | 0.56 ± 0.31 Aa | 0.42 ± 0.25 Aa | 0.30 ± 0.12 Aa |
| SPI | 0.58 ± 0.47 Aa | 3.30 ± 0.46 Bb | 5.94 ± 0.43 Cb | 8.31 ± 1.39 Dd |
| SC | 0.14 ± 0.09 Aa | 2.63 ± 1.02 Bb | 4.44 ± 1.23 Cb | 6.19 ± 0.50 Dc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Cheng, H.; Wusigale; Dong, H.; Huang, W.; Liang, L. Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability. Antioxidants 2022, 11, 647. https://doi.org/10.3390/antiox11040647
Yin X, Cheng H, Wusigale, Dong H, Huang W, Liang L. Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability. Antioxidants. 2022; 11(4):647. https://doi.org/10.3390/antiox11040647
Chicago/Turabian StyleYin, Xin, Hao Cheng, Wusigale, Huanhuan Dong, Weining Huang, and Li Liang. 2022. "Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability" Antioxidants 11, no. 4: 647. https://doi.org/10.3390/antiox11040647
APA StyleYin, X., Cheng, H., Wusigale, Dong, H., Huang, W., & Liang, L. (2022). Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability. Antioxidants, 11(4), 647. https://doi.org/10.3390/antiox11040647









