Deuterated Arachidonic Acid Ameliorates Lipopolysaccharide-Induced Lung Damage in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. D6-ARA and D2-Lin Synthesis
2.2. In Vivo Model and Sampling Protocols
2.3. Bronchoalveolar Lavage (BAL) Fluid Collection
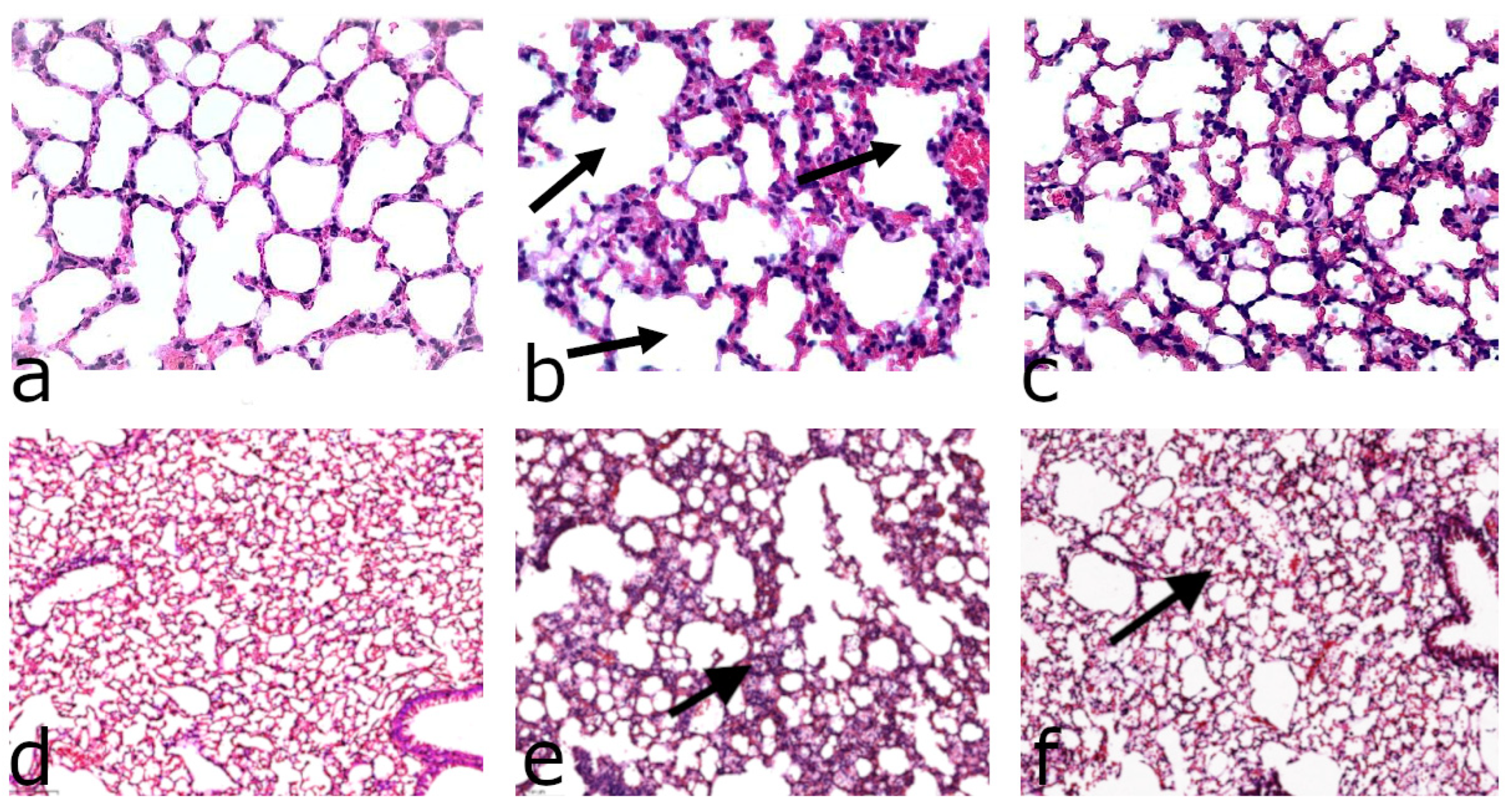
2.4. Histological Analysis
2.5. Cytokine Measurements with Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. ARA Isotopic Analysis (GC-MS/MS)
2.7. Statistical Analysis
3. Results
3.1. Effect of D-Linoleic Acid (D2-LIN) on the Lungs in LPS-Treated Mice
3.2. Pharmacokinetic Aspects of Dietary Supplementation of D-ARA in Healthy Mice
3.3. Effect of D-ARA on the Response to LPS
4. Discussion
4.1. Gender-Dependent Differences
4.2. LPS Model of ALI/ARDS
4.3. D2-LIN
4.4. D6-ARA
4.5. Role of Surfactant
4.6. Animal Models Are Imperfect
4.7. ARA, the Yin and Yang of Inflammation
4.8. Perspectives on Dietary D-ARA as a Drug
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fessler, M.B.; Summer, R.S. Surfactant Lipids at the Host–Environment Interface. Metabolic Sensors, Suppressors, and Effectors of Inflammatory Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, K.S.; Park, H.G.; Brenna, J.T. Polyunsaturated fatty acid biosynthesis pathway and genetics implications for interindividual variability in prothrombotic, inflammatory conditions such as COVID-19. Prostaglandins Leukot. Essent. Fat. Acids 2020, 162, 102183. [Google Scholar] [CrossRef] [PubMed]
- Navratil, A.R.; Shchepinov, M.S.; Dennis, E.A. Lipidomics Reveals Dramatic Physiological Kinetic Isotope Effects during the Enzymatic Oxygenation of Polyunsaturated Fatty Acids Ex Vivo. J. Am. Chem. Soc. 2017, 140, 235–243. [Google Scholar] [CrossRef]
- Hill, S.; Hirano, K.; Shmanai, V.V.; Marbois, B.N.; Vidovic, D.; Bekish, A.V.; Kay, B.; Tse, V.; Fine, J.; Clarke, C.F.; et al. Isotope-reinforced polyunsaturated fatty acids protect yeast cells from oxidative stress. Free Radic. Biol. Med. 2011, 50, 130–138. [Google Scholar] [CrossRef]
- Smarun, A.V.; Petković, M.; Shchepinov, M.S.; Vidović, D. Site-Specific Deuteration of Polyunsaturated Alkenes. J. Org. Chem. 2017, 82, 13115–13120. [Google Scholar] [CrossRef]
- Weiss, M.; Byrne, A.J.; Blazek, K.; Saliba, D.G.; Pease, J.E.; Perocheau, D.; Feldmann, M.; Udalova, I.A. IRF5 controls both acute and chronic inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Job, E.R.; Saelens, X.; Roose, K. Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration. J. Vis. Exp. 2017, 123, e55398. [Google Scholar] [CrossRef]
- Chen, Y.; Hanaoka, M.; Chen, P.; Droma, Y.; Voelkel, N.F.; Kubo, K. Protective effect of beraprost sodium, a stable prostacyclin analog, in the development of cigarette smoke extract-induced emphysema. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L648–L656. [Google Scholar] [CrossRef]
- Wang, Z.; Park, H.G.; Wang, D.; Kitano, R.; Kothapalli, K.S.; Brenna, J.T. Fatty acid desaturase 2 (FADS2) but not FADS1 desaturates branched chain and odd chain saturated fatty acids. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2019, 1865, 158572. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lee, S.H.; Kim, J.Y.; Lee, W.S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. J. Thorac. Dis. 2019, 11, 1287–1302. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, Y.-H.; Liu, D.-X.; Li, J.; Zhao, P.-C.; Zhong, Y.-P.; Chen, Y.-Q.; Xu, W.; Zhu, Z.-Q. Intranasal Application of Budesonide Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Suppressing Nucleotide-Binding Oligomerization Domain-Like Receptor Family, Pyrin Domain-Containing 3 Inflammasome Activation in Mice. J. Immunol. Res. 2019, 2019, 7264383. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, Y.; Qu, S.; Cao, Y.; Li, S.; Zhang, W.; Zhang, Z.; Zhang, N.; Fu, Y. Protective effect of TM6 on LPS-induced acute lung injury in mice. Sci. Rep. 2017, 7, 572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Z.; Jiang, A.; Wu, D.; Li, S.; Liu, Z.; Wei, Z.; Yang, Z.; Guo, C. Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-κB and Activating Nrf2/HO-1 Signaling Pathways. Front. Pharmacol. 2021, 11, 591836. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Peng, Y.; Gu, Y.; Zhong, Y.; Su, C.; Liu, L.; Chai, D.; Song, T.; Zhao, N.; Yan, X.; et al. Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function. Open Life Sci. 2021, 16, 1064–1081. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yamashita, H.; Oka, N.; Ueda, T.; Kohama, K.; Nakao, A.; Kotani, J. Antithrombin III improved neutrophil extracellular traps in lung after the onset of endotoxemia. J. Surg. Res. 2017, 208, 140–150. [Google Scholar] [CrossRef]
- Costola-De-Souza, C.; Ribeiro, A.; Ferraz-De-Paula, V.; Calefi, A.S.; Aloia, T.P.A.; Junior, J.A.G.; De Almeida, V.I.; Pinheiro, M.L.; Palermo-Neto, J. Monoacylglycerol Lipase (MAGL) Inhibition Attenuates Acute Lung Injury in Mice. PLoS ONE 2013, 8, e77706. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.D.; Mei, S.H.J.; Lai, P.F.H.; Zhang, Q.W.; Parker, C.H.; Suen, R.S.; Hood, R.D.; Zhao, Y.D.; Deng, Y.; Han, R.N.N.; et al. Cell-based Angiopoietin-1 Gene Therapy for Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2007, 175, 1014–1026. [Google Scholar] [CrossRef]
- Guilbault, C.; Stotland, P.; Lachance, C.; Tam, M.; Keller, A.; Thompson-Snipes, L.; Cowley, E.; Hamilton, T.A.; Eidelman, D.H.; Stevenson, M.M.; et al. Influence of gender and interleukin-10 deficiency on the inflammatory response during lung infection withPseudomonas aeruginosain mice. Immunology 2002, 107, 297–305. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimizu, T.; Yu, H.-P.; Hsieh, Y.-C.; Choudhry, M.A.; Chaudry, I.H. Salutary effects of 17β-estradiol on T-cell signaling and cytokine production after trauma-hemorrhage are mediated primarily via estrogen receptor-α. Am. J. Physiol. Physiol. 2007, 292, C2103–C2111. [Google Scholar] [CrossRef] [PubMed]
- Al-Lami, R.A.; Urban, R.J.; Volpi, E.; Algburi, A.M.A.; Baillargeon, J. Sex Hormones and Novel Corona Virus Infectious Disease (COVID-19). Mayo Clin. Proc. 2020, 95, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Asti, C.; Ruggieri, V.; Porzio, S.; Chiusaroli, R.; Melillo, G.; Caselli, G. Lipopolysaccharide-induced Lung Injury in Mice. I. Concomitant Evaluation of Inflammatory Cells and Haemorrhagic Lung Damage. Pulm. Pharmacol. Ther. 2000, 13, 61–69. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Y.; Wang, Y.; Jiang, Z.; Xu, M.; Bo, L.; Deng, X. Ouabain Protects Mice Against Lipopolysaccharide-Induced Acute Lung Injury. Med. Sci. Monit. 2018, 24, 4455–4464. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-L.; Fang, S.-H.; Wang, S.-C.; Cheng, W.-C.; Liu, P.-L.; Su, C.-C.; Chen, C.-S.; Huang, M.-Y.; Hua, K.-F.; Shen, K.-H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, srep46299. [Google Scholar] [CrossRef] [PubMed]
- Firsov, A.M.; Fomich, M.A.; Bekish, A.V.; Sharko, O.L.; Kotova, E.A.; Saal, H.J.; Vidovic, D.; Shmanai, V.V.; Pratt, D.A.; Antonenko, Y.N.; et al. Threshold protective effect of deuterated polyunsaturated fatty acids on peroxidation of lipid bilayers. FEBS J. 2019, 286, 2099–2117. [Google Scholar] [CrossRef]
- Shchepinov, M.S. Reactive Oxygen Species, Isotope Effect, Essential Nutrients, and Enhanced Longevity. Rejuvenation Res. 2007, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Shchepinov, M.S. Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends Pharmacol. Sci. 2020, 41, 236–248. [Google Scholar] [CrossRef]
- Brenna, J.T.; James, G.; Midei, M.; Heerinckx, F.; Atwal, P.; Milner, P.; Schmidt, K.; van der Ploeg, L.; Fielding, R.; Shchepinov, M.S. Plasma and Red Blood Cell Membrane Accretion and Pharmacokinetics of RT001 (bis-Allylic 11,11-D2-Linoleic Acid Ethyl Ester) during Long Term Dosing in Patients. J. Pharm. Sci. 2020, 109, 3496–3503. [Google Scholar] [CrossRef]
- Brenna, J.T. Long-chain polyunsaturated fatty acids and the preterm infant: A case study in developmentally sensitive nutrient needs in the United States1–4. Am. J. Clin. Nutr. 2016, 103, 606S–615S. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Li, H.; Tian, Y.; Han, J.; Wang, X.-Y.; Li, X.-Y.; Tian, C.; Zhang, P.-H.; Hao, Y.; Gao, F.; et al. PCTR1 ameliorates lipopolysaccharide-induced acute inflammation and multiple organ damage via regulation of linoleic acid metabolism by promoting FADS1/FASDS2/ELOV2 expression and reducing PLA2 expression. Lab. Investig. 2020, 100, 904–915. [Google Scholar] [CrossRef]
- Di Gioia, M.; Spreafico, R.; Springstead, J.R.; Mendelson, M.M.; Joehanes, R.; Levy, D.; Zanoni, I. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat. Immunol. 2019, 21, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.S.; Galano, J.-M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.-Y.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Caron, E.; Desseyn, J.-L.; Sergent, L.; Bartke, N.; Husson, M.-O.; Duhamel, A.; Gottrand, F. Impact of fish oils on the outcomes of a mouse model of acutePseudomonas aeruginosapulmonary infection. Br. J. Nutr. 2015, 113, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, X.; Lin, C.; He, X. Protective Effects of Arachidonic Acid against Paraquat-Induced Pulmonary Injury. Inflammation 2015, 38, 1458–1463. [Google Scholar] [CrossRef]
- Insuela, D.B.R.; Ferrero, M.R.; Coutinho, D.D.S.; Martins, M.A.; Carvalho, V.F. Could Arachidonic Acid-Derived Pro-Resolving Mediators Be a New Therapeutic Strategy for Asthma Therapy. Front. Immunol. 2020, 11, 580598. [Google Scholar] [CrossRef] [PubMed]
- Aldrovandi, M.; Banthiya, S.; Meckelmann, S.; Zhou, Y.; Heydeck, D.; O’Donnell, V.B.; Kuhn, H. Specific oxygenation of plasma membrane phospholipids by Pseudomonas aeruginosa lipoxygenase induces structural and functional alterations in mammalian cells. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2017, 1863, 152–164. [Google Scholar] [CrossRef]
- van der Does, A.M.; Heijink, M.; Mayboroda, O.A.; Persson, L.J.; Aanerud, M.; Bakke, P.; Eagan, T.M.; Hiemstra, P.S.; Giera, M. Dynamic differences in dietary polyunsaturated fatty acid metabolism in sputum of COPD patients and controls. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2018, 1864, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Pawliczak, R.; Huang, X.-L.; Nanavaty, U.B.; Lawrence, M.; Madara, P.; Shelhamer, J.H. Oxidative Stress Induces Arachidonate Release from Human Lung Cells through the Epithelial Growth Factor Receptor Pathway. Am. J. Respir. Cell Mol. Biol. 2002, 27, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Meier, U.; Yabut-Perez, M.; Walmrath, D.; Grimminger, F.; Seeger, W.; Günther, A. Alteration of Fatty Acid Profiles in Different Pulmonary Surfactant Phospholipids in Acute Respiratory Distress Syndrome and Severe Pneumonia. Am. J. Respir. Crit. Care Med. 2001, 163, 95–100. [Google Scholar] [CrossRef]
- Cole, B.K.; Lieb, D.C.; Dobrian, A.D.; Nadler, J.L. 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2012, 104–105, 84–92. [Google Scholar] [CrossRef]
- Yu, Z.; Schneider, C.; Boeglin, W.E.; Brash, A.R. Human and mouse eLOX3 have distinct substrate specificities: Implications for their linkage with lipoxygenases in skin. Arch. Biochem. Biophys. 2006, 455, 188–196. [Google Scholar] [CrossRef][Green Version]
- Bender, G.; Schexnaydre, E.E.; Murphy, R.C.; Uhlson, C.; Newcomer, M.E. Membrane-Dependent Activities of Human 15-LOX-2 and Its Murine Counterpart: Implications for Murine Models of Atherosclerosis. J. Biol. Chem. 2016, 291, 19413–19424. [Google Scholar] [CrossRef] [PubMed]
- Blevitt, J.M.; Hack, M.D.; Herman, K.; Chang, L.; Keith, J.M.; Mirzadegan, T.; Rao, N.L.; Lebsack, A.D.; Milla, M.E. A Single Amino Acid Difference between Mouse and Human 5-Lipoxygenase Activating Protein (FLAP) Explains the Speciation and Differential Pharmacology of Novel FLAP Inhibitors. J. Biol. Chem. 2016, 291, 12724–12731. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.P.; Monick, M.M.; Hunninghake, G.W. Human alveolar macrophage arachidonic acid metabolism. Am. J. Physiol. Physiol. 1988, 254, C809–C815. [Google Scholar] [CrossRef]
- Fall, F.; Lamy, E.; Brollo, M.; Naline, E.; Lenuzza, N.; Thévenot, E.; DeVillier, P.; Grassin-Delyle, S. Metabolic reprograming of LPS-stimulated human lung macrophages involves tryptophan metabolism and the aspartate-arginosuccinate shunt. PLoS ONE 2020, 15, e0230813. [Google Scholar] [CrossRef]
- Coffey, M.J.; Phare, S.M.; Peters-Golden, M. Prolonged Exposure to Lipopolysaccharide Inhibits Macrophage 5-Lipoxygenase Metabolism via Induction of Nitric Oxide Synthesis. J. Immunol. 2000, 165, 3592–3598. [Google Scholar] [CrossRef]
- Fussbroich, D.; Zimmermann, K.; Göpel, A.; Eickmeier, O.; Trischler, J.; Zielen, S.; Schubert, R.; Beermann, C. A specific combined long-chain polyunsaturated fatty acid supplementation reverses fatty acid profile alterations in a mouse model of chronic asthma. Lipids Health Dis. 2019, 18, 16. [Google Scholar] [CrossRef]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Ohara, O.; Kawana, A.; Asano, K.; Arita, M. Dysregulated metabolism of polyunsaturated fatty acids in eosinophilic allergic diseases. Prostaglandins Other Lipid Mediat. 2020, 150, 106477. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.; Childs, C.; Calder, P. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Whelan, C.J. Inhibition of PAF-, LPS-, and cytokine-induced granulocyte accumulation in guinea pig lung by dexamethasone: Evidence that inhibition of IL-5 release is responsible for the selective inhibition of eosinophilia by glucocorticoids in guinea-pigs. Agents Actions 1996, 45, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.R.; Quinlan, M.F.; Schwartz, L.W.; Wheeldon, E.B. Therapeutic intervention in a rat model of ARDS: I. Dual inhibition of arachidonic acid metabolism. Circ. Shock. 1990, 32, 231–242. [Google Scholar] [PubMed]
- Lee, I.; Dodia, C.; Chatterjee, S.; Feinstein, S.I.; Fisher, A.B. Protection against LPS-induced acute lung injury by a mechanism-based inhibitor of NADPH oxidase (type 2). Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L635–L644. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sánchez-Alonso, S.; Sánchez-Azofra, A.; Marcos-Jiménez, A.; Ávalos, E.; Alcaraz-Serna, A.; Santos, I.D.L.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020, 130, 6290–6300. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Domínguez-Cherit, J.G.; Herrera-Bello, H.; Castillejos-Suastegui, H.; Moreno-Castañeda, L.; et al. Alteration in the Lipid Profile and the Desaturases Activity in Patients With Severe Pneumonia by SARS-CoV-2. Front. Physiol. 2021, 12, 667024. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Ronconi, G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost Agents 2020, 34, 1971–1975. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Gawish, A.; El-Dahab, M.A.; Tallima, H.; El Ridi, R. Is arachidonic acid an endoschistosomicide? J. Adv. Res. 2018, 11, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.A.; Dazy, K.M.; Waldram, J.D. Aspirin-exacerbated respiratory disease: Characteristics and management strategies. Expert Rev. Clin. Immunol. 2015, 11, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Midei, M.; Dastgir, J.; Flora, C.; Molinari, R.J.; Heerinckx, F.; Endemann, S.; Atwal, P.; Milner, P.; Shchepinov, M.S. Treatment of infantile neuroaxonal dystrophy with RT001: A di-deuterated ethyl ester of linoleic acid: Report of two cases. JIMD Rep. 2020, 54, 54–60. [Google Scholar] [CrossRef]
- Angelova, P.; Andruska, K.; Midei, M.; Barilani, M.; Atwal, P.; Tucher, O.; Milner, P.; Heerinckx, F.; Shchepinov, M. RT001 in Progressive Supranuclear Palsy—Clinical and In-Vitro Observations. Antioxidants 2021, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Zhang, W.; Cai, Y.; Shan, P.; Wu, D.; Zhang, B.; Liu, H.; Khan, Z.A.; Liang, G. Arachidonic acid inhibits inflammatory responses by binding to myeloid differentiation factor-2 (MD2) and preventing MD2/toll-like receptor 4 signaling activation. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165683. [Google Scholar] [CrossRef] [PubMed]





| Fat Composition of Research Diets | H-ARA (Green Dye) | D-ARA (Pink Dye) |
|---|---|---|
| Saturated fat, % | 7.75 | 7.75 |
| High oleic sunflower, % | 3.1 | 3.1 |
| H-linolenic (ethyl linolenate), % | 0.2 | 0.2 |
| ARA ethyl ester, % | 0.25 (H-ARA) | 0.25 (D-ARA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molchanova, A.Y.; Rjabceva, S.N.; Melik-Kasumov, T.B.; Pestov, N.B.; Angelova, P.R.; Shmanai, V.V.; Sharko, O.L.; Bekish, A.V.; James, G.; Park, H.G.; et al. Deuterated Arachidonic Acid Ameliorates Lipopolysaccharide-Induced Lung Damage in Mice. Antioxidants 2022, 11, 681. https://doi.org/10.3390/antiox11040681
Molchanova AY, Rjabceva SN, Melik-Kasumov TB, Pestov NB, Angelova PR, Shmanai VV, Sharko OL, Bekish AV, James G, Park HG, et al. Deuterated Arachidonic Acid Ameliorates Lipopolysaccharide-Induced Lung Damage in Mice. Antioxidants. 2022; 11(4):681. https://doi.org/10.3390/antiox11040681
Chicago/Turabian StyleMolchanova, Alla Y., Svetlana N. Rjabceva, Tigran B. Melik-Kasumov, Nikolay B. Pestov, Plamena R. Angelova, Vadim V. Shmanai, Olga L. Sharko, Andrei V. Bekish, Genevieve James, Hui Gyu Park, and et al. 2022. "Deuterated Arachidonic Acid Ameliorates Lipopolysaccharide-Induced Lung Damage in Mice" Antioxidants 11, no. 4: 681. https://doi.org/10.3390/antiox11040681
APA StyleMolchanova, A. Y., Rjabceva, S. N., Melik-Kasumov, T. B., Pestov, N. B., Angelova, P. R., Shmanai, V. V., Sharko, O. L., Bekish, A. V., James, G., Park, H. G., Udalova, I. A., Brenna, J. T., & Shchepinov, M. S. (2022). Deuterated Arachidonic Acid Ameliorates Lipopolysaccharide-Induced Lung Damage in Mice. Antioxidants, 11(4), 681. https://doi.org/10.3390/antiox11040681







