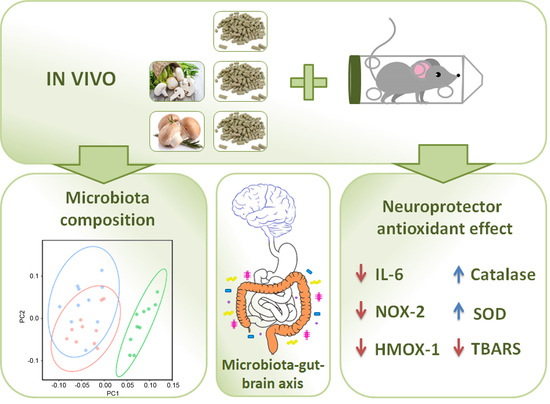
Agaricus Mushroom-Enriched Diets Modulate the Microbiota-Gut-Brain Axis and Reduce Brain Oxidative Stress in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom-Enriched Diets
2.2. Experimental Animals, Feces Collection, and DNA Extraction
2.3. Bacterial 16S rDNA Massive Sequencing and Sequence Postprocessing
2.4. Restraint Stress Model
2.5. RNA Extraction and qRT-PCR
2.6. TBARS, SOD, and Catalase Activity
2.7. Statistical Analysis
3. Results
3.1. Mushroom-Enriched Diets Do Not Modify Mouse Body Weight in the Long Term
3.2. Mushroom-Enriched Diets Induced a More Beneficial Microbiota Composition
3.3. Mushroom-Enriched Diets Reduced the Expression of Stress-Related Genes
3.4. Mushroom-Enriched Diets Normalized Stress-Induced Changes in Enzyme Activities and Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villain, N.; Dubois, B. Alzheimer’s Disease Including Focal Presentations. Semin. Neurol. 2019, 39, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of Wine Consumption with Alzheimer’s Disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76 Pt A, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Perl, D.P.; Carlson, G.A. Neurodegenerative Disease Transmission and Transgenesis in Mice. Cold Spring Harb. Perspect. Biol. 2017, 9, a023549. [Google Scholar] [CrossRef] [Green Version]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Kamal, M.A.; Velluto, L.; Gambi, D.; Di, N.M.; Greig, N.H. Relationship between inflammatory mediators, Abeta levels and ApoE genotype in Alzheimer disease. Curr. Alzheimer Res. 2012, 9, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Bartolome, B.; Penalvo, J.L.; Perez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. [Google Scholar] [CrossRef]
- Bobadilla, M.; García-Sanmartín, J.; Martínez, A. Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants 2021, 10, 46. [Google Scholar] [CrossRef]
- Bobadilla, M.; Hernández, C.; Ayala, M.; Alonso, I.; Iglesias, A.; García-Sanmartín, J.; Mirpuri, E.; Barriobero, J.I.; Martínez, A. A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress. Antioxidants 2021, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; La, F.G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.Y.; Yeo, X.Y.; Bae, H.G.; Lee, D.P.S.; Ho, R.C.; Kim, J.E.; Jo, D.G.; Jung, S. Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life 2021, 11, 698. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef] [PubMed]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2021, 164, 105314. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Dinu, L.D.; Pelinescu, D.R.; Gatea, F. Therapeutic Properties of Edible Mushrooms and Herbal Teas in Gut Microbiota Modulation. Microorganisms 2021, 9, 1262. [Google Scholar] [CrossRef]
- Mushroom Production in La Rioja. Available online: https://larioja.org/larioja-client/cm/agricultura/images?idMmedia=600508 (accessed on 1 March 2022).
- Hetland, G.; Tangen, J.M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjonnfjord, G.E.; Johnson, E. Antitumor, Anti-Inflammatory and Antiallergic Effects of Agaricus blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifola frondosa: A Review of Preclinical and Clinical Studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Wang, Q.; Gould, T.; Slavin, J. Impact of Agaricus bisporus Mushroom Consumption on Gut Health Markers in Healthy Adults. Nutrients 2018, 10, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano-Aguilar, G.I.; Jang, S.; Lakshman, S.; Gupta, R.; Beshah, E.; Sikaroodi, M.; Vinyard, B.; Molokin, A.; Gillevet, P.M.; Urban, J.F. The Effect of Dietary Mushroom Agaricus bisporus on Intestinal Microbiota Composition and Host Immunological Function. Nutrients 2018, 10, 1721. [Google Scholar] [CrossRef] [Green Version]
- Iniguez, M.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Recio-Fernández, E.; Roncero-Ramos, I.; Pérez-Clavijo, M.; Oteo, J.A. Agaricus bisporus supplementation reduces high-fat diet-induced body weight gain and fatty liver development. J. Physiol. Biochem. 2018, 74, 635–646. [Google Scholar] [CrossRef]
- Martínez-Herrero, S.; Larrayoz, I.M.; Narro-Iñiguez, J.; Villanueva-Millán, M.J.; Recio-Fernández, E.; Pérez-Matute, P.; Oteo, J.A.; Martínez, A. Lack of Adrenomedullin Results in Microbiota Changes and Aggravates Azoxymethane and Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Physiol. 2016, 7, 595. [Google Scholar] [CrossRef] [Green Version]
- Initial Readquality. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 March 2022).
- Qiime2 Protocol. Available online: https://qiime2.org/ (accessed on 1 March 2022).
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef]
- Silva Database. Available online: https://www.arb-silva.de/ (accessed on 1 March 2022).
- Mandal, S.; van, T.W.; White, R.A.; Eggesbo, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [Green Version]
- Bioproject 814431. Available online: http://www.ncbi.nlm.nih.gov/bioproject/814431 (accessed on 1 March 2022).
- Di, P.F. Gut Microbiota Parameters Potentially Useful in Clinical Perspective. Microorganisms 2021, 9, 2402. [Google Scholar] [CrossRef]
- Tilocca, B.; Pieroni, L.; Soggiu, A.; Britti, D.; Bonizzi, L.; Roncada, P.; Greco, V. Gut-Brain Axis and Neurodegeneration: State-of-the-Art of Meta-Omics Sciences for Microbiota Characterization. Int. J. Mol. Sci. 2020, 21, 4045. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Chiu, P.W.Y.; Lau, C.B.S. A Review of the Effects of Natural Compounds, Medicinal Plants, and Mushrooms on the Gut Microbiota in Colitis and Cancer. Front. Pharmacol. 2020, 11, 744. [Google Scholar] [CrossRef]
- Lyu, M.; Wang, Y.F.; Fan, G.W.; Wang, X.Y.; Xu, S.Y.; Zhu, Y. Balancing Herbal Medicine and Functional Food for Prevention and Treatment of Cardiometabolic Diseases through Modulating Gut Microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yu, Z.; Jia, W.; Wu, Y.; Wu, D.; Zhang, H.; Liu, Z.; Yang, Y.; Zhang, J.; Liu, Y.; et al. A Review on Linking the Medicinal Functions of Mushroom Prebiotics with Gut Microbiota. Int. J. Med. Mushrooms 2020, 22, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Ruthes, A.C.; Cantu-Jungles, T.M.; Cordeiro, L.M.C.; Iacomini, M. Prebiotic potential of mushroom d-glucans: Implications of physicochemical properties and structural features. Carbohydr. Polym. 2021, 262, 117940. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arévalo, O.; Choi, H.A.; Savarraj, J.; et al. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef]
- Rigsbee, L.; Agans, R.; Shankar, V.; Kenche, H.; Khamis, H.J.; Michail, S.; Paliy, O. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am. J. Gastroenterol. 2012, 107, 1740–1751. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Zheng, J.; Hu, G.; Wang, J.; Huang, C.; Lou, L.; Wang, X.; Zeng, Y. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Sci. Rep. 2016, 6, 26337. [Google Scholar] [CrossRef]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Luglie, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; van, H.M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, B.; Wesierska, A.; Zalewska, K.; Sokolowska, M.M.; Bursiewicz, W.; Socha, M.; Ozorowski, M.; Pawlak-Osinska, K.; Wicinski, M. The role of Tannerella forsythia and Porphyromonas gingivalis in pathogenesis of esophageal cancer. Infect. Agents Cancer 2019, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; He, Y.; Tang, J.; Ou, Q.; Lin, J. Alteration of the gut microbiota in tumor necrosis factor-alpha antagonist-treated collagen-induced arthritis mice. Int. J. Rheum. Dis. 2020, 23, 472–479. [Google Scholar] [CrossRef]
- Marietta, E.V.; Gomez, A.M.; Yeoman, C.; Tilahun, A.Y.; Clark, C.R.; Luckey, D.H.; Murray, J.A.; White, B.A.; Kudva, Y.C.; Rajagopalan, G. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS ONE 2013, 8, e78687. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Jin, Z.; Wu, W.; Gao, R.; Guo, B.; Gao, Z.; Yang, Y.; Qin, H. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS ONE 2014, 9, e90849. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martinez-Herrero, S.; Rubio-Mediavilla, S.; Narro-Iniguez, J.; Martinez, A. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci. Rep. 2017, 7, 17488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.D.; Yin, J.H.; Hwang, C.S.; Tang, C.M.; Yang, D.I. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: Implication in Alzheimer’s disease and cerebral ischemia. Free Radic. Res. 2012, 46, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.K.; Gray, J.J.; Winter, A.N.; Linseman, D.A. Immunocal(R) and preservation of glutathione as a novel neuroprotective strategy for degenerative disorders of the nervous system. Recent Pat. CNS Drug Discov. 2012, 7, 230–235. [Google Scholar] [CrossRef]
- Kumar, A.; Garg, R.; Gaur, V.; Kumar, P. Possible role of NO modulators in protective effect of trazodone and citalopram (antidepressants) in acute immobilization stress in mice. Indian J. Exp. Biol. 2010, 48, 1131–1135. [Google Scholar] [PubMed]
- Duval, C.; Cantero, A.V.; Auge, N.; Mabile, L.; Thiers, J.C.; Negre-Salvayre, A.; Salvayre, R. Proliferation and wound healing of vascular cells trigger the generation of extracellular reactive oxygen species and LDL oxidation. Free Radic. Biol. Med. 2003, 35, 1589–1598. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Agundez, J.A.; Garcia-Martin, E.; Martinez, C.; Benito-Leon, J.; Millan-Pascual, J.; Diaz-Sanchez, M.; Calleja, P.; Pisa, D.; Turpin-Fenoll, L.; Alonso-Navarro, H.; et al. Heme Oxygenase-1 and 2 Common Genetic Variants and Risk for Multiple Sclerosis. Sci. Rep. 2016, 6, 20830. [Google Scholar] [CrossRef] [Green Version]
- Emerson, M.R.; LeVine, S.M. Heme oxygenase-1 and NADPH cytochrome P450 reductase expression in experimental allergic encephalomyelitis: An expanded view of the stress response. J. Neurochem. 2000, 75, 2555–2562. [Google Scholar] [CrossRef]
- Choi, H.I.; Lee, H.W.; Eom, T.M.; Lim, S.A.; Ha, H.Y.; Seol, I.C.; Kim, Y.S.; Oh, D.S.; Yoo, H.R. A traditional Korean multiple herbal formulae (Yuk-Mi-Jihwang-Tang) attenuates acute restraint stress-induced brain tissue oxidation. Drug Chem. Toxicol. 2017, 40, 125–133. [Google Scholar] [CrossRef]
- Kowalczuk, K.; Stryjecka-Zimmer, M. The influence of oxidative stress on the level of malondialdehyde (MDA) in different areas of the rabbit brain. Ann. Univ. Mariae Curie Sklodowska Med. 2002, 57, 160–164. [Google Scholar] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De, A.M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Rodriguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Chee, W.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Kolimar, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abranko, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. The Anti-Inflammatory and Antioxidant Effects of Sodium Propionate. Int. J. Mol. Sci. 2020, 21, 3026. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Harding, A.; Poole, S.; Kesavalu, L.; Crean, S. Porphyromonas gingivalis Periodontal Infection and Its Putative Links with Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 137357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragon-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Alvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef] [PubMed]





| Basic Diet (D11112201) | White Button Suppl. | Portobello Suppl. | ||||
|---|---|---|---|---|---|---|
| grams (%) | kcal (%) | grams (%) | kcal (%) | grams (%) | kcal (%) | |
| Protein | 19 | 20 | 18 | 20 | 18 | 20 |
| Carbohydrate | 63 | 65 | 60 | 65 | 60 | 65 |
| Fat | 7 | 15 | 6 | 15 | 6 | 15 |
| Total | 100 | 100 | 100 | |||
| kcal/gram | 3.81 | 3.62 | 3.62 | |||
| Ingredients | grams | kcal | grams | kcal | grams | kcal |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 |
| L-cystine | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 381 | 1524 | 381 | 1524 | 381 | 1524 |
| Maltodextrin 10 | 110 | 440 | 110 | 440 | 110 | 440 |
| Dextrose | 150 | 600 | 150 | 600 | 150 | 600 |
| Cellulose, BW200 | 75 | 0 | 75 | 0 | 75 | 0 |
| Inulin | 25 | 37.5 | 25 | 37.5 | 25 | 37.5 |
| Soybean oil | 70 | 630 | 70 | 630 | 70 | 630 |
| Mineral mix S10028 (w/o Ca, P, or K) | 10 | 0 | 10 | 0 | 10 | 0 |
| CaKPO4 | 13 | 0 | 13 | 0 | 13 | 0 |
| CaCO3, Light, USP | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 |
| Vitamin mix V10001 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 | 2 | 0 |
| White button powder | 0 | 56.5 | 0 | |||
| Portobello powder | 0 | 0 | 56.5 | |||
| Total | 1071.05 | 4084 | 1127.55 | 4084 | 1127.55 | 4084 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession No. |
|---|---|---|---|
| Nox-2 | GCTGGGATCACAGGAATTGT | CTTCCAAACTCTCCGCAGTC | NM_007807 |
| Hmox-1 | TGCTCGAATGAACACTCTGG | TAGCAGGCCTCTGACGAAGT | NM_010442 |
| Il-6 | ATGGATGCTACCAAACTGGAT | TGAAGGACTCTGGCTTTGTCT | NM_031168 |
| Tnfα | CCACCACGCTCTTCTGTCTA | CACTTGGTGGTTTGCTACGA | NM_001278601 |
| Nrf2 | AGCGAGCAGGCTATCTCCTA | TCTTGCCTCCAAAGGATGTC | NM_010902 |
| Gapdh | CATGGCCTTCCGTGTTCCTA | GCGGCACGTCAGATCCA | NM_008084 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sanmartín, J.; Bobadilla, M.; Mirpuri, E.; Grifoll, V.; Pérez-Clavijo, M.; Martínez, A. Agaricus Mushroom-Enriched Diets Modulate the Microbiota-Gut-Brain Axis and Reduce Brain Oxidative Stress in Mice. Antioxidants 2022, 11, 695. https://doi.org/10.3390/antiox11040695
García-Sanmartín J, Bobadilla M, Mirpuri E, Grifoll V, Pérez-Clavijo M, Martínez A. Agaricus Mushroom-Enriched Diets Modulate the Microbiota-Gut-Brain Axis and Reduce Brain Oxidative Stress in Mice. Antioxidants. 2022; 11(4):695. https://doi.org/10.3390/antiox11040695
Chicago/Turabian StyleGarcía-Sanmartín, Josune, Miriam Bobadilla, Eduardo Mirpuri, Vanessa Grifoll, Margarita Pérez-Clavijo, and Alfredo Martínez. 2022. "Agaricus Mushroom-Enriched Diets Modulate the Microbiota-Gut-Brain Axis and Reduce Brain Oxidative Stress in Mice" Antioxidants 11, no. 4: 695. https://doi.org/10.3390/antiox11040695