Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Zn and Water Hardness Treatment and Sampling
2.3. Zn Accumulation in Fish Body
2.4. Expression of the Metallothionein Gene and iNOS mRNA
2.5. NO Activity and Total Antioxidant Capacity (TAC) in Plasma and Caspase-3 Activity in Liver
2.6. MT mRNA In Situ Hybridization
2.7. Terminal Transferase dUTP Nick End Labeling (TUNEL) Assay
2.8. Statistical Analysis
3. Results
3.1. Changes in Water Hardness
3.2. Changes in Zn Accumulation in Fish
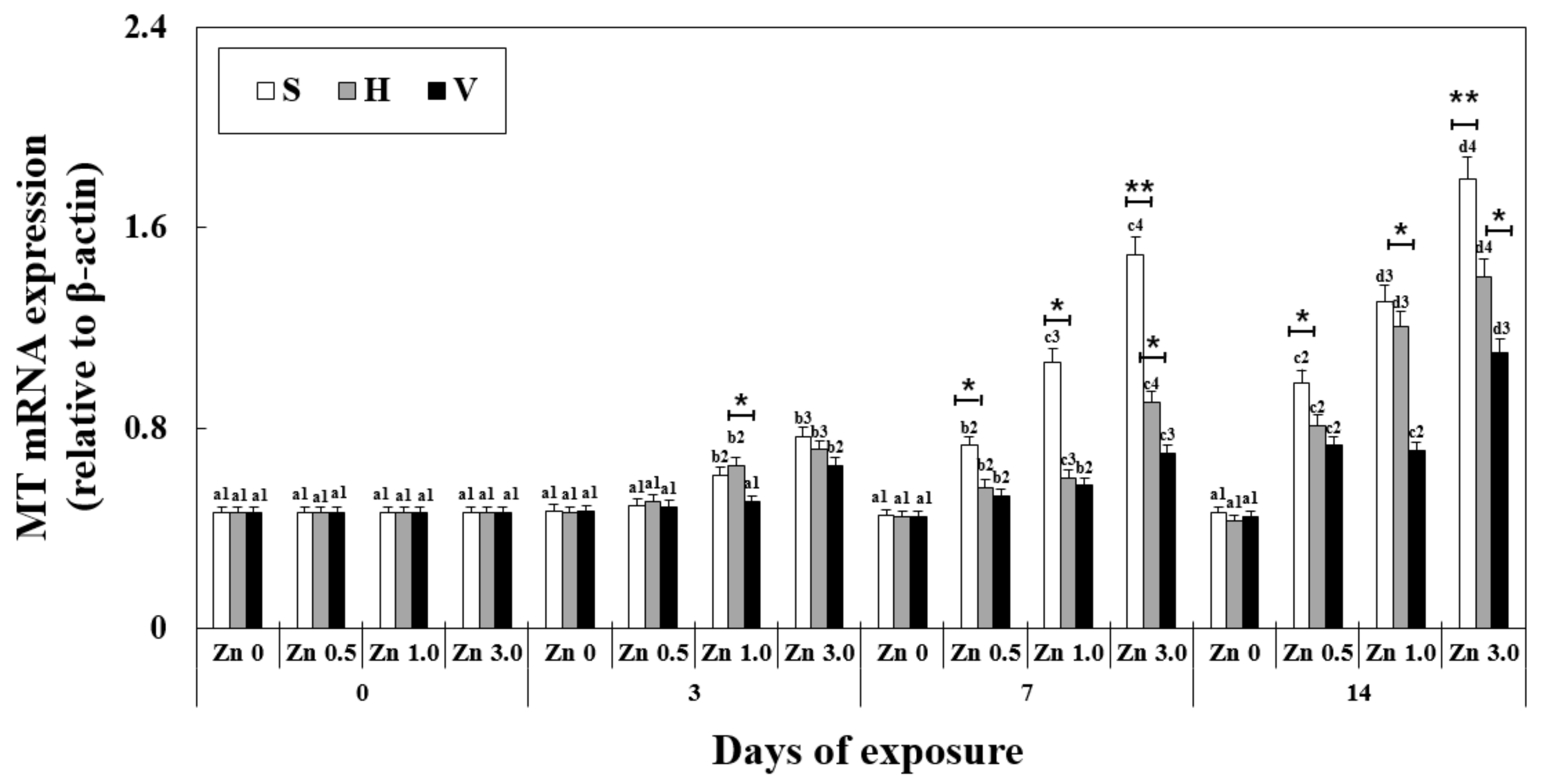
3.3. Changes in MT mRNA Expression in the Liver
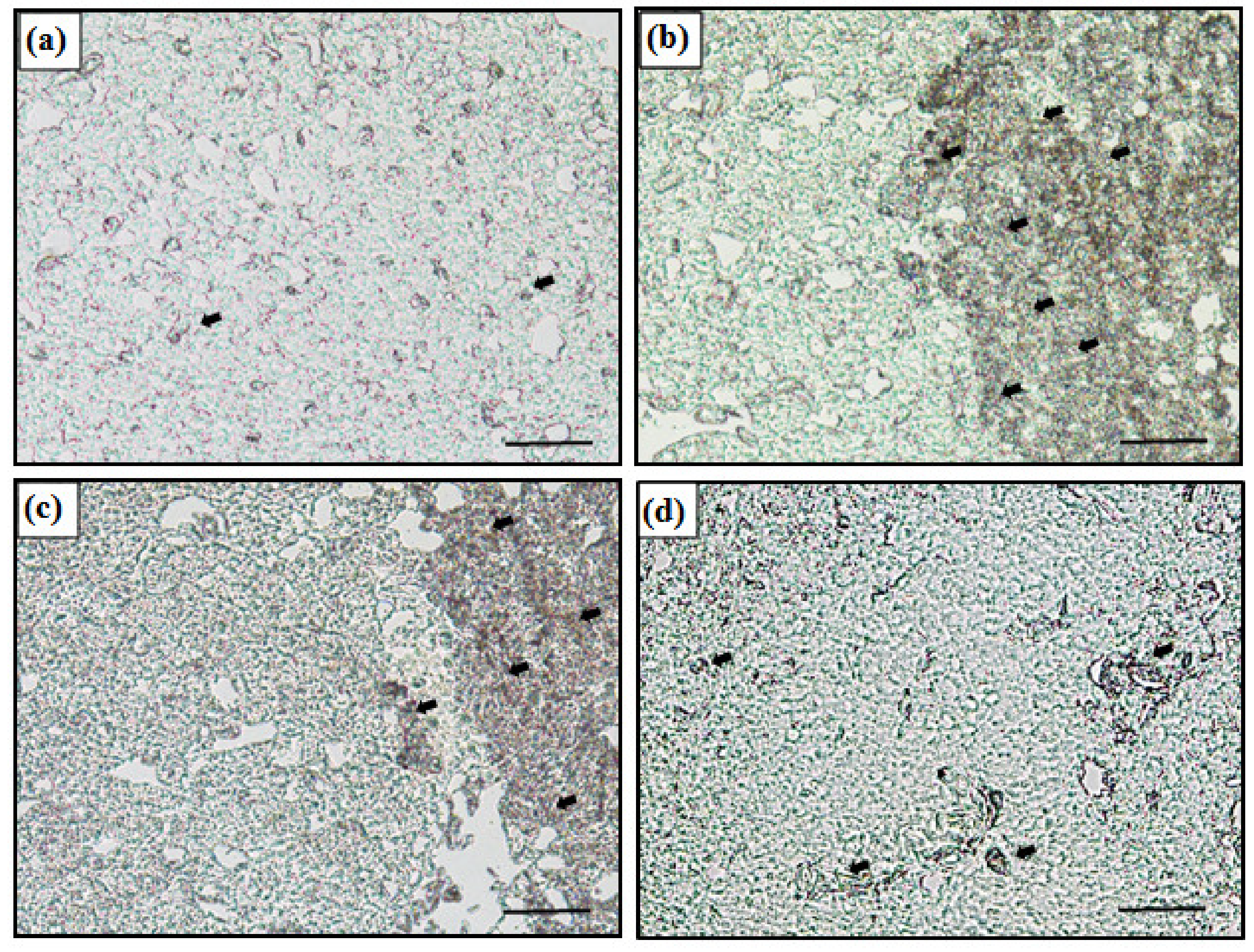
3.4. MT mRNA Expression in Liver Using In Situ Hybridization
3.5. Changes in iNOS mRNA Expression in the Liver
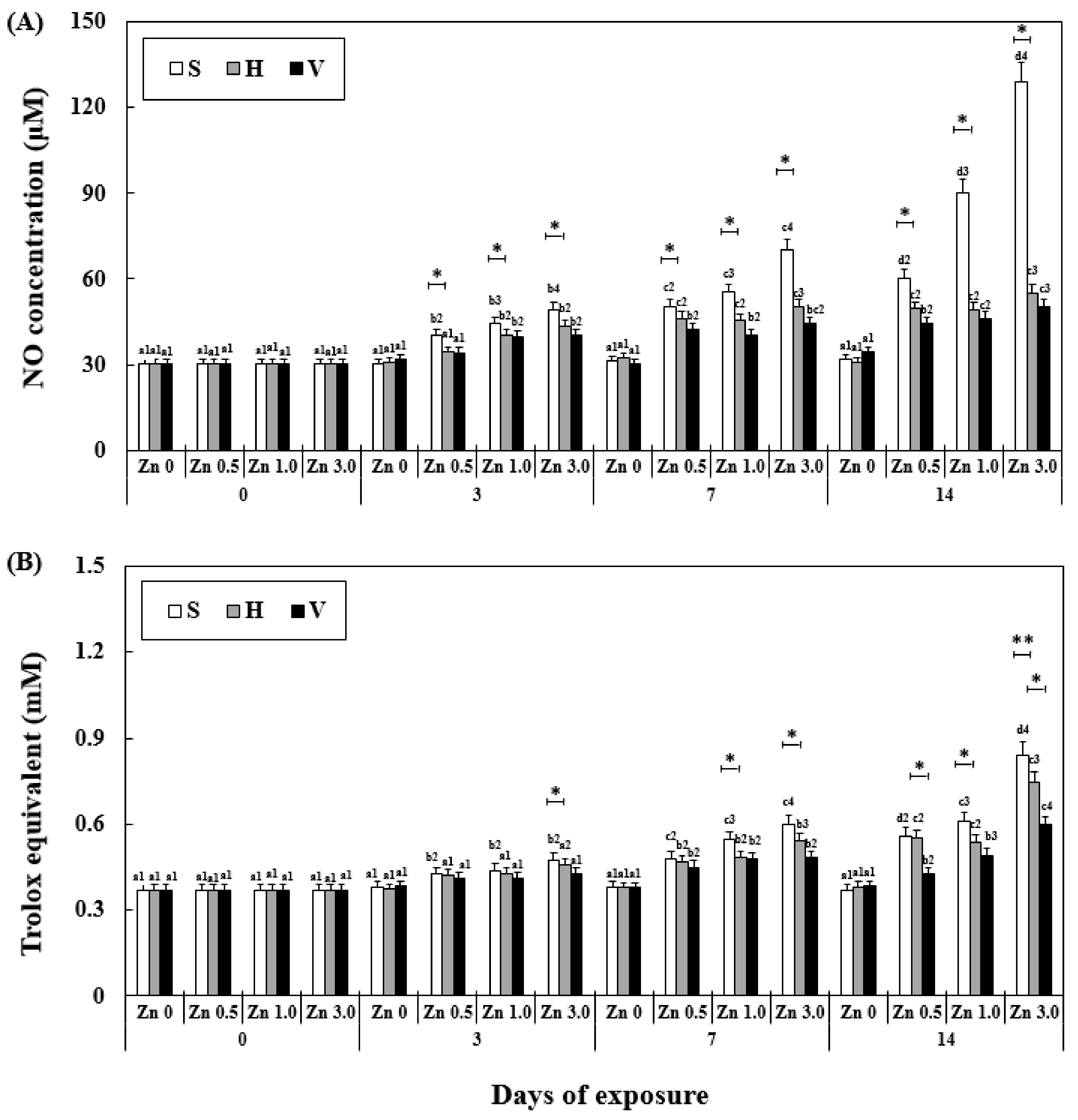
3.6. Changes NO Activity and TAC in Plasma
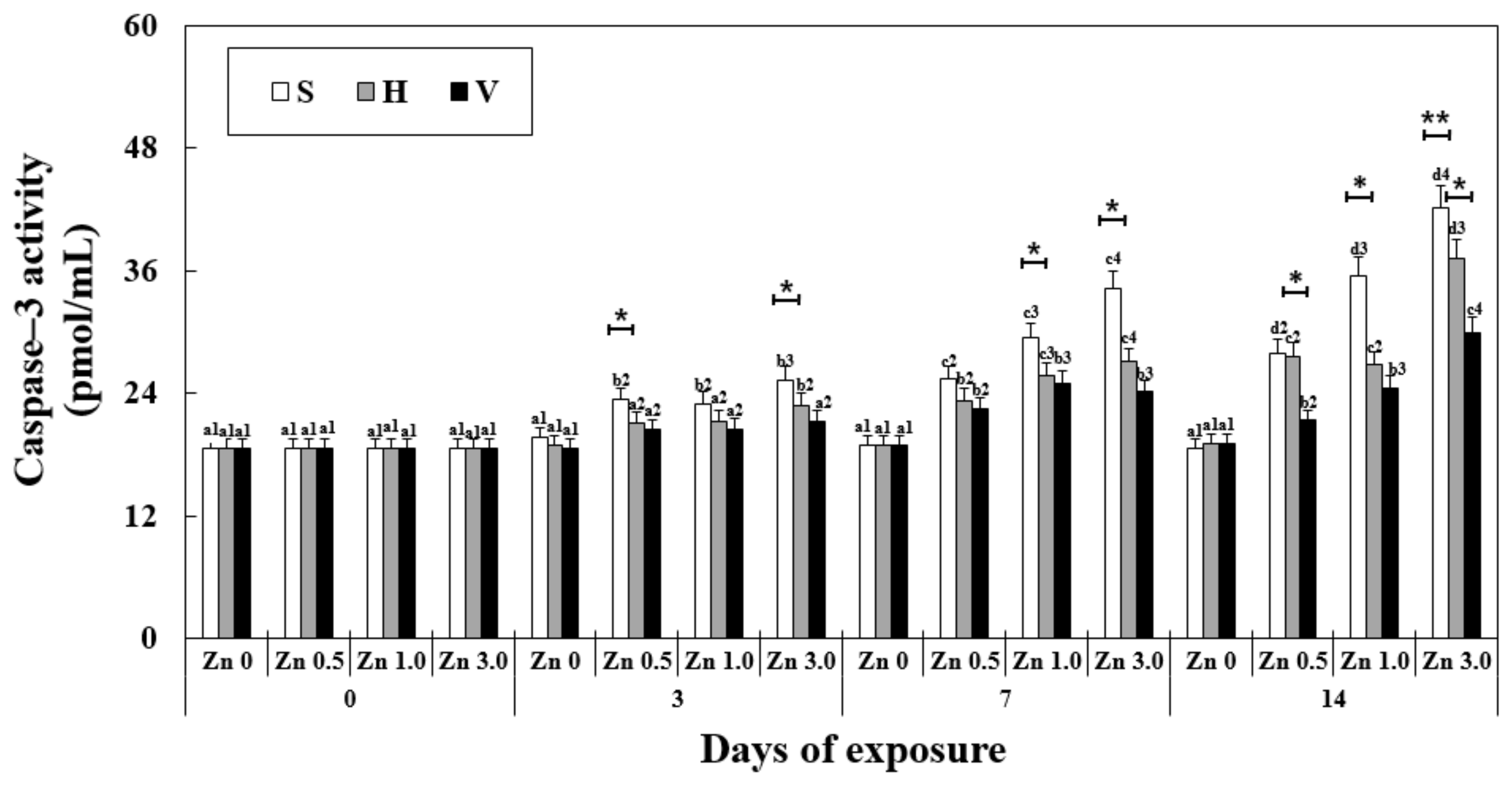
3.7. Changes in Caspase-3 Activity in the Liver
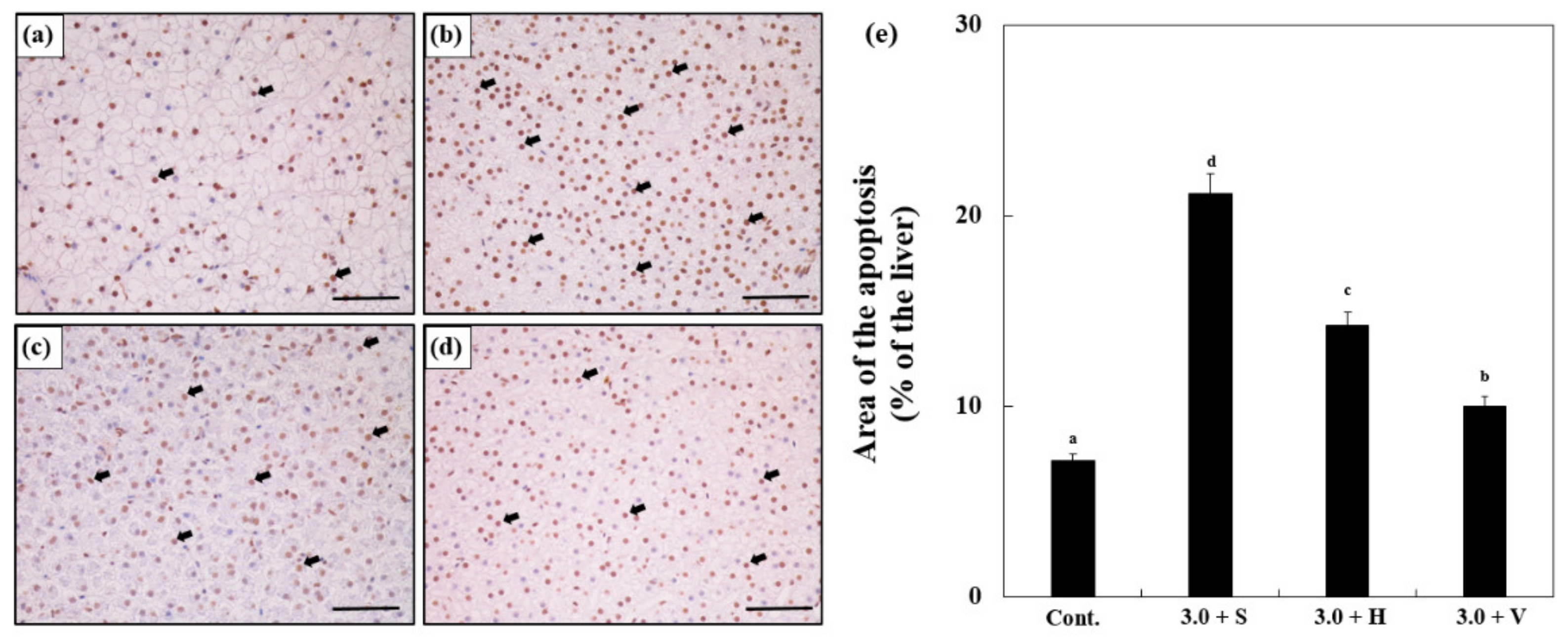
3.8. TUNEL Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luoma, S.N. Bioavailability of trace metals to aquatic organisms—A review. Sci. Total. Environ. 1983, 28, 1–22. [Google Scholar] [CrossRef]
- Dane, H.; Sisman, T. A morpho-histopathological study in the digestive tract of three fish species influenced with heavy metal pollution. Chemosphere 2020, 242, 125212. [Google Scholar] [CrossRef]
- Gillis, P.L.; Mitchell, R.J.; Schwalb, A.N.; McNichols, K.A.; Mackie, G.L.; Wood, C.M. Sensitivity of the glochidia (larvae) of freshwater mussels to copper: Assessing the effect of water hardness and dissolved organic carbon on the sensitivity of endangered species. Aquat. Toxicol. 2008, 88, 137–145. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Lee, S. Differentiation of the toxicities of silver nanoparticles and silver ions to the Japanese medaka (Oryzias latipes) and the cladoceran Daphnia Magna. Nanotoxicology 2008, 5, 208–214. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Warith, A.A.; Younis, E.M.; Al-Asgah, N.A.; Wahbi, O.M. Effect of zinc toxicity on liver histology of Nile tilapia, Oreochromis niloticus. J. Lit. Sci. 2011, 6, 3760–3769. [Google Scholar] [CrossRef]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, iron and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Qu, R.; Feng, M.; Wang, X.; Qin, L.; Wang, C.; Wang, Z.; Wang, L. Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat. Toxicol. 2014, 150, 9–16. [Google Scholar] [CrossRef]
- Faheem, M.; Lone, K.P. Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish, Ctenopharyngodon idella. Braz. J. Pharm. Sci. 2018, 53, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, Q.; Ren, B.; Luo, J.; Yuan, J.; Ding, X.; Yao, X. Trends and health risks of dissolved heavy metal pollution in global river and lake water from 1970 to 2017. Rev. Environ. Contam. Toxicol. 2019, 251, 1–24. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Viarengo, A.; Burlando, B.; Cavaletto, M.; Marchi, B.; Ponzano, E.; Blasco, J. Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am. J. Physiol-Reg. I. 1999, 277, R1612–R1619. [Google Scholar] [CrossRef]
- Khodadoust, D.; Ahmad, I. Metallothionein-like protein levels in Java Medaka fish (Oryzias javanicus) exposed to different concentrations of cadmium. Walailak. J. Sci. Technol. 2014, 11, 883–893. [Google Scholar] [CrossRef]
- Sevcikova, M.; Modra, H.; Slaninova, A.; Svobodova, Z. Metals as a cause of oxidative stress in fish: A review. Vet. Med. Sci. 2011, 56, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Akarasereenont, P.; Bakhle, Y.S.; Thiemermann, C.; Vane, J.R. Cytokine-mediated induction of cyclo-oxygenase-2 by activation of tyrosine kinase in bovine endothelial cells stimulated by bacterial lipopolysaccharide. Brit. J. Pharmacol. 1995, 115, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Ramadori, G.; Armbrust, T. Cytokines in the liver. Eur. J. Gastroenterol. Hepatol. 2001, 13, 777–784. [Google Scholar] [CrossRef]
- Lirk, P.; Hoffmann, G.; Rieder, J. Inducible nitric oxide synthase-time for reappraisal. Curr. Drug. Targets. Inflamm. Allergy. 2002, 1, 89–108. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell. Death. Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Tyagi, S.; Sharma, B.; Singh, P.; Dobhal, R. Water quality assessment in terms of water quality index. J. Am. Water. Resour. Assoc. 2013, 1, 34–38. [Google Scholar] [CrossRef]
- Baldisserotto, B. Water pH and hardness affect growth of freshwater teleosts. Braz. J. Vet. Res. Anim. Sci. 2011, 40, 138–144. [Google Scholar]
- Golombieski, J.I.; Koakoski, G.; Becker, A.J.; Almeida, A.P.G.; Toni, C.; Finamor, I.A.; Baldisserotto, B. Nitrogenous and phosphorus excretions in juvenile silver catfish (Rhamdia quelen) exposed to different water hardness, humic acid, and pH levels. Fish. Physiol. Biochem. 2013, 39, 837–849. [Google Scholar] [CrossRef]
- Michelotti, B.T.; Passini, G.; Carvalho, C.; Salbego, J.; Mori, N.C.; Rodrigues, R.V.; Cerqueira, V.R. Growth and metabolic parameters of common snook juveniles raised in freshwater with different water hardness. Aquaculture. 2018, 482, 31–35. [Google Scholar] [CrossRef]
- Kiyani, V.; Hosynzadeh, M.; Ebrahimpour, M. Investigation acute toxicity some of heavy metals at different water hardness. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 134–142. [Google Scholar]
- Alsop, D.; Wood, C.M. Metal uptake and acute toxicity in zebrafish: Common mechanisms across multiple metals. Aquat. Toxicol. 2011, 105, 385–393. [Google Scholar] [CrossRef]
- Rani, S.; Gupta, R.K.; Rani, M. Heavy metal induced toxicity in fish with special reference to zinc and cadmium. Int. J. Fish. Aquat. Stud. 2015, 3, 118–123. [Google Scholar]
- Houri, D.; Koo, C.M. Water quality evaluation of PET bottled water by mineral balance in the Northeast Asian region: A case study of South Korea. Yonago Acta Med. 2015, 58, 115. [Google Scholar]
- Huo, J. Acute toxicity of zinc to goldfish and its accumulation and distribution in the body. Shanghai. Environ. Sci. 1996, 15, 42–43. [Google Scholar] [CrossRef]
- Hollis, L.; Hogstrand, C.; Wood, C.M. Tissue-specific cadmium accumulation, metallothionein induction, and tissue zinc and copper levels during chronic sublethal cadmium exposure in juvenile rainbow trout. Arch. Environ. Contam. Toxicol. 2001, 41, 468–474. [Google Scholar] [CrossRef]
- Hogstrand, C.; Verbost, P.M.; Bonga, S.E.; Wood, C.M. Mechanisms of zinc uptake in gills of freshwater rainbow trout: Interplay with calcium transport. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1996, 270, R1141–R1147. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Javed, M.; Rehman, M.T.; Urooj, M.; Ahmad, M.I. Heavy metal pollution and risk assessment by the battery of toxicity tests. Sci. Rep. 2020, 10, 16593. [Google Scholar] [CrossRef] [PubMed]
- Macirella, R.; Guardia, A.; Pellegrino, D.; Bernabò, I.; Tronci, V.; Ebbesson, L.O.; Brunelli, E. Effects of two sublethal concentrations of mercury chloride on the morphology and metallothionein activity in the liver of zebrafish (Danio rerio). Int. J. Mol. Sci. 2016, 17, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, N.; Egnew, N.; Quintero, H.; Kelly, A.; Sinha, A.K. The effects of water hardness on the growth, metabolic indicators and stress resistance of largemouth bass Micropterus salmoides. Aquaculture 2020, 527, 735469. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, X.; Zhu, R.; Luo, Z.; Ren, B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 2016, 180, 56–70. [Google Scholar] [CrossRef]
- Mo, J.; Lin, D.; Wang, J.; Li, P.; Liu, W. Apoptosis in HepG2 cells induced by zinc pyrithione via mitochondrial dysfunction pathway: Involvement of zinc accumulation and oxidative stress. Ecotoxicol. Environ. Saf. 2018, 161, 515–525. [Google Scholar] [CrossRef]

| Genes (Accession No.) | Forward Primer | Reverse Primer |
|---|---|---|
| Metallothionein (X97271.1) | 5′-TTA ACT GTG CCA CCT GC-3′ | 5′-AGG AAT TGC CCT TAC ACA CG-3′ |
| iNOS (AY904362.1) | 5′-AAG TCG TTT GCA TGG AGG AC-3′ | 5′-GGT GTC TAA GGT TGT TCA GG-3′ |
| β-actin (LC382464) | 5′-TTC CCT TGC TCC TTC CAC CA-3′ | 5′-TGG AGC CAC CAA TCC AGA CA-3′ |
| Time | Accumulation | MT | iNOS | NO | TAC | Caspase-3 | |
|---|---|---|---|---|---|---|---|
| Day 0 | F | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| p | 0.998 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |
| Day 3 | F | 4.256 | 2.658 | 9.661 | 3.225 | 1.985 | 2.065 |
| p | 0.045 * | 0.084 | 0.010 * | 0.052 | 0.152 | 0.149 | |
| Day 7 | F | 5.226 | 15.68 | 16.52 | 2.226 | 2.336 | 4.658 |
| p | 0.032 * | 0.002* | 0.002 * | 0.068 | 0.114 | 0.041 * | |
| Day 14 | F | 29.28 | 28.10 | 5.662 | 9.658 | 3.521 | 7.632 |
| p | <0.001 * | <0.001 * | 0.030 * | 0.010 * | 0.050 * | 0.024 * |
| Time | Hardness | Zn 0 | Zn 0.5 | Zn 1.0 | Zn 3.0 |
|---|---|---|---|---|---|
| Day 0 | Soft | 22.10 ± 1.54 a1 | 21.91 ± 0.98 a1 | 21.54 ± 1.66 a1 | 22.10 ± 1.55 a1 |
| Hard | 22.00 ± 1.01 a1 | 23.05 ± 1.39 a1 | 21.80 ±1.26 a1 | 21.75 ± 1.65 a1 | |
| Very hard | 23.10 ± 1.18 a1 | 22.65 ± 1.25 a1 | 22.56 ± 0.99 a1 | 22.25 ± 1.54 a1 | |
| Day 3 | Soft | 22.18 ± 1.49 a1 | 32.22 ± 2.22 b2 | 37.18 ± 2.21 b2** | 40.22 ± 2.69 b2** |
| Hard | 23.28 ± 1.95 a1 | 29.09 ± 1.96 b2 | 30.87 ± 1.96 b2 | 34.45 ± 2.26 b3* | |
| Very hard | 23.79 ± 1.89 a1 | 28.58 ± 2.01 b2 | 28.91 ± 1.55 b2 | 29.09 ± 1.14 b2 | |
| Day 7 | Soft | 24.18 ± 1.22 a1 | 41.27 ± 2.14 c2** | 48.57 ± 2.15 c3* | 57.39 ± 3.00 c4** |
| Hard | 23.99 ± 1.36 a1 | 36.15 ± 1.54 c2* | 48.48 ± 2.44 c3* | 49.60 ± 2.54 c3* | |
| Very hard | 23.14 ± 0.78 a1 | 33.12 ± 1.33 c2α | 41.88 ± 2.01 c3 | 40.33 ± 1.96 c3 | |
| Day 14 | Soft | 23.47 ± 0.95 a1 | 45.33 ± 1.92 d2** | 69.05 ± 3.24 d3** | 113.53 ± 5.94 d4** |
| Hard | 30.12 ± 1.12 b1* | 40.97 ± 2.01 d2* | 54.54 ± 2.94 d3* | 90.07 ± 5.69 d4* | |
| Very hard | 24.48 ± 1.77 a1 | 35.96 ± 1.87 c2 | 47.59 ± 2.11 d3 | 60.96 ± 4.36 d4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, C.Y.; Li, Z.; Song, J.A.; Park, Y.-S. Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus. Antioxidants 2022, 11, 715. https://doi.org/10.3390/antiox11040715
Choi CY, Li Z, Song JA, Park Y-S. Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus. Antioxidants. 2022; 11(4):715. https://doi.org/10.3390/antiox11040715
Chicago/Turabian StyleChoi, Cheol Young, Zhongze Li, Jin Ah Song, and Young-Su Park. 2022. "Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus" Antioxidants 11, no. 4: 715. https://doi.org/10.3390/antiox11040715
APA StyleChoi, C. Y., Li, Z., Song, J. A., & Park, Y.-S. (2022). Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus. Antioxidants, 11(4), 715. https://doi.org/10.3390/antiox11040715






