Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
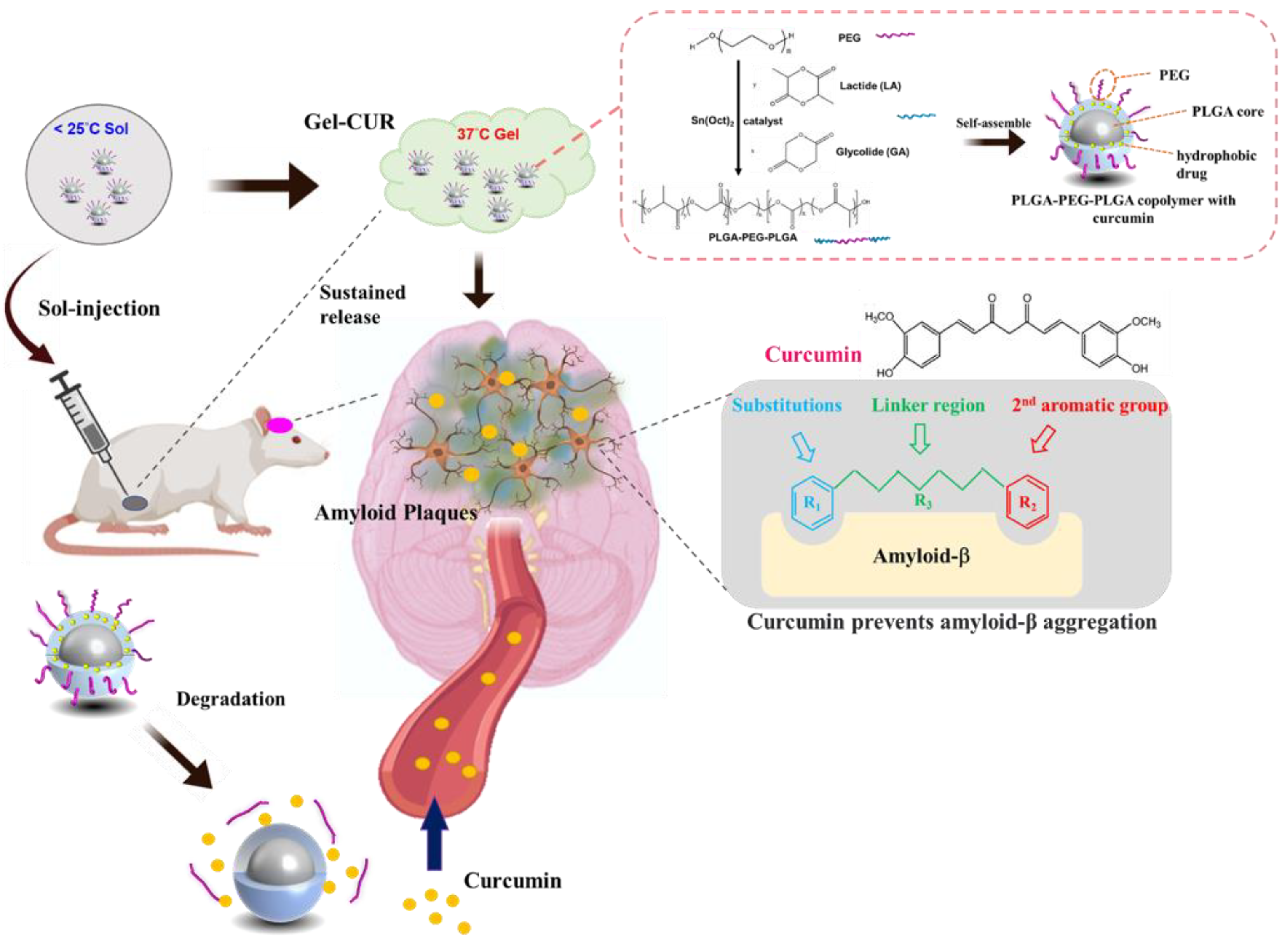
2.2. Synthesis of PLGA-PEG-PLGA Triblock Copolymer
2.3. Preparation of PLGA-PEG-PLGA Micelles
2.4. Preparation of Aβ Fibrils
2.5. Characterization of Triblock Copolymer
2.5.1. 1H Nuclear Magnetic Resonance Spectrum of Triblock Copolymer
2.5.2. Fourier Transform Infrared Analysis of Triblock Copolymer
2.5.3. Identification of Molecular Weight
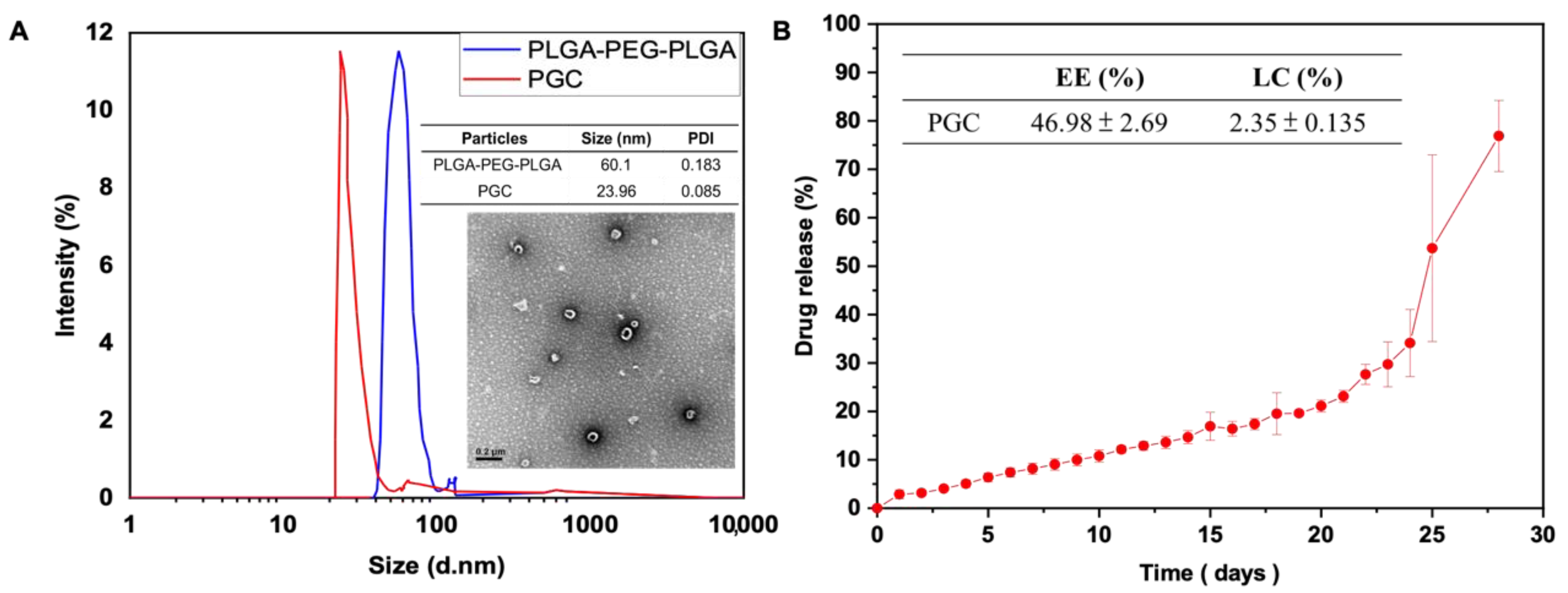
2.5.4. Dynamic Light Scattering Analysis
2.5.5. Gelation Test and Rheological Analysis
2.5.6. Encapsulation Efficiency and Drug Loading Efficiency
2.5.7. Drug Release Profile
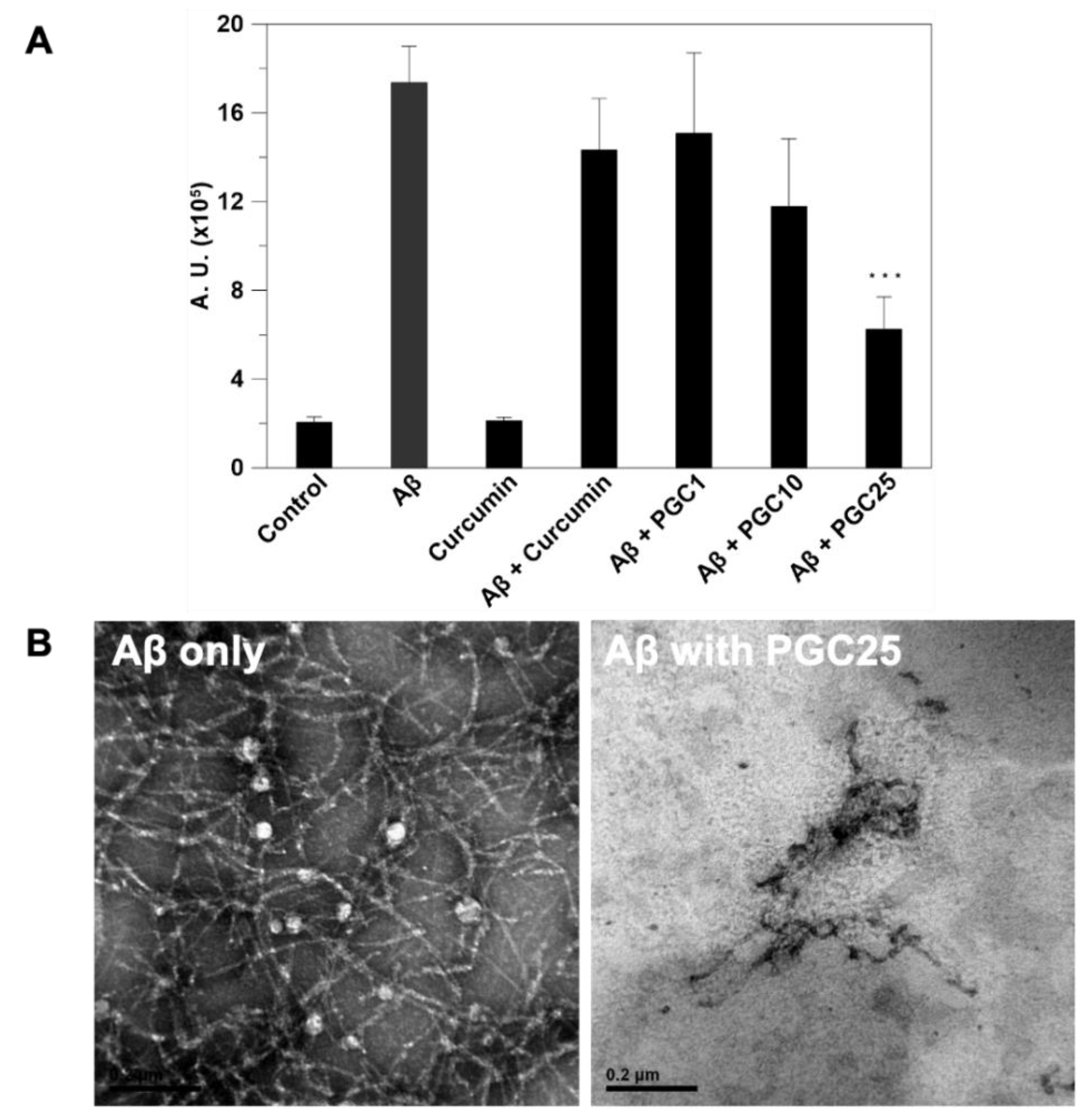
2.5.8. Morphology of Aβ Aggregation and Triblock Copolymer Micelle
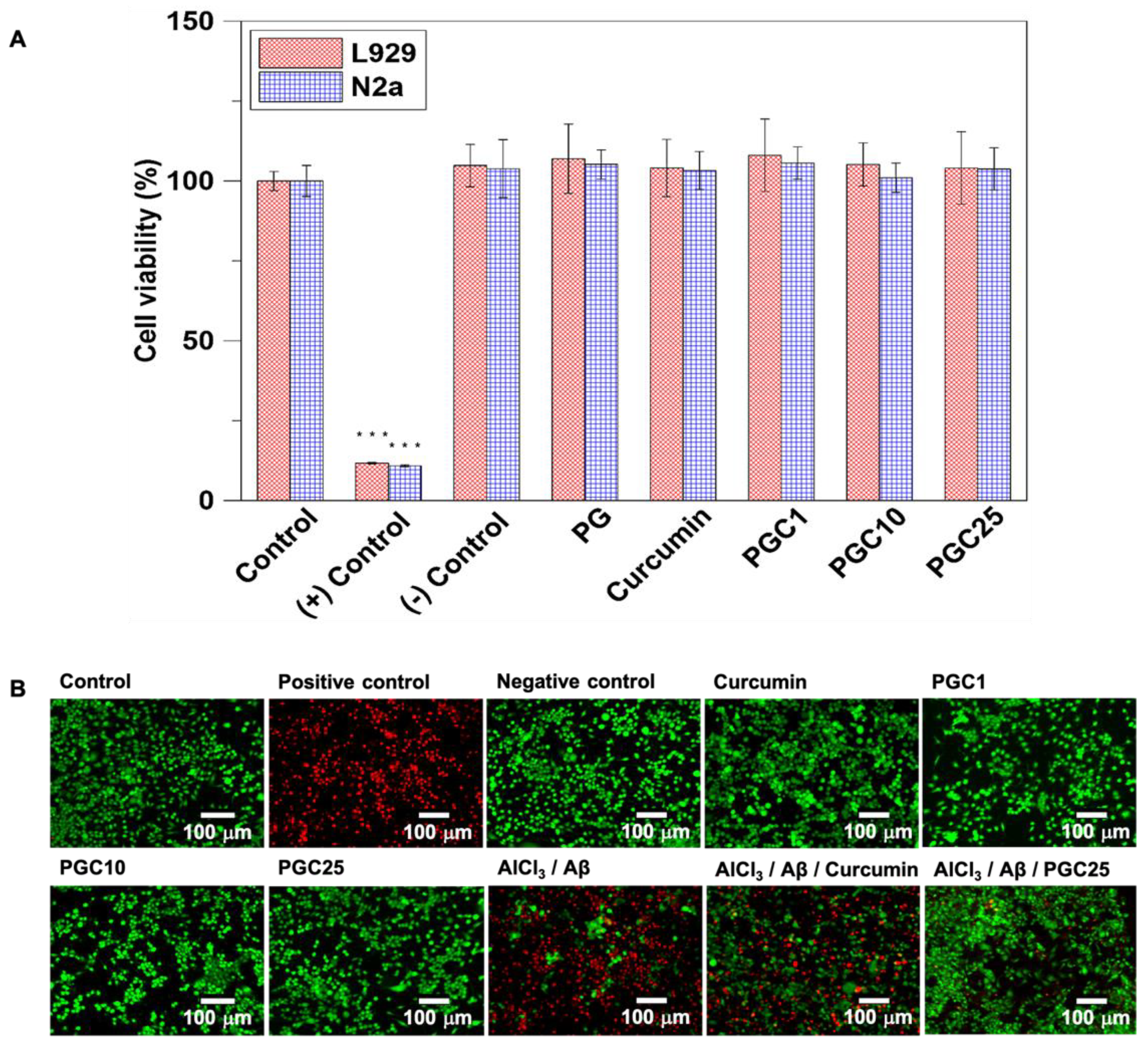
2.5.9. In Vitro Study
Biocompatibility of Thermo-Gel
Thioflavin T Fluorescence Assay
Western Blot of β-Secretase Inhibition
Determination of Cellular Reactive Oxygen Species Generation
PGC Inhibition of Aβ-Induced Inflammation
PGC Inhibition of Aβ-Induced Cytotoxicity
2.5.10. In Vivo Study
AD Animal Model
2.5.11. Morris Water Maze
Functional Magnetic Resonance Imaging
Histological Analysis and Immunohistochemical Staining
Statistical Analysis
3. Results
3.1. Morphology of Micelles, Particle Size Identification, Drug Loading Efficiency, and Drug Release Profile
3.2. The Evaluation of Cell Viability of Thermo-Gel and Inhibition of Aβ-Induced Cytotoxicity
3.3. ThT Stain for Inhibition of Aβ Aggregation
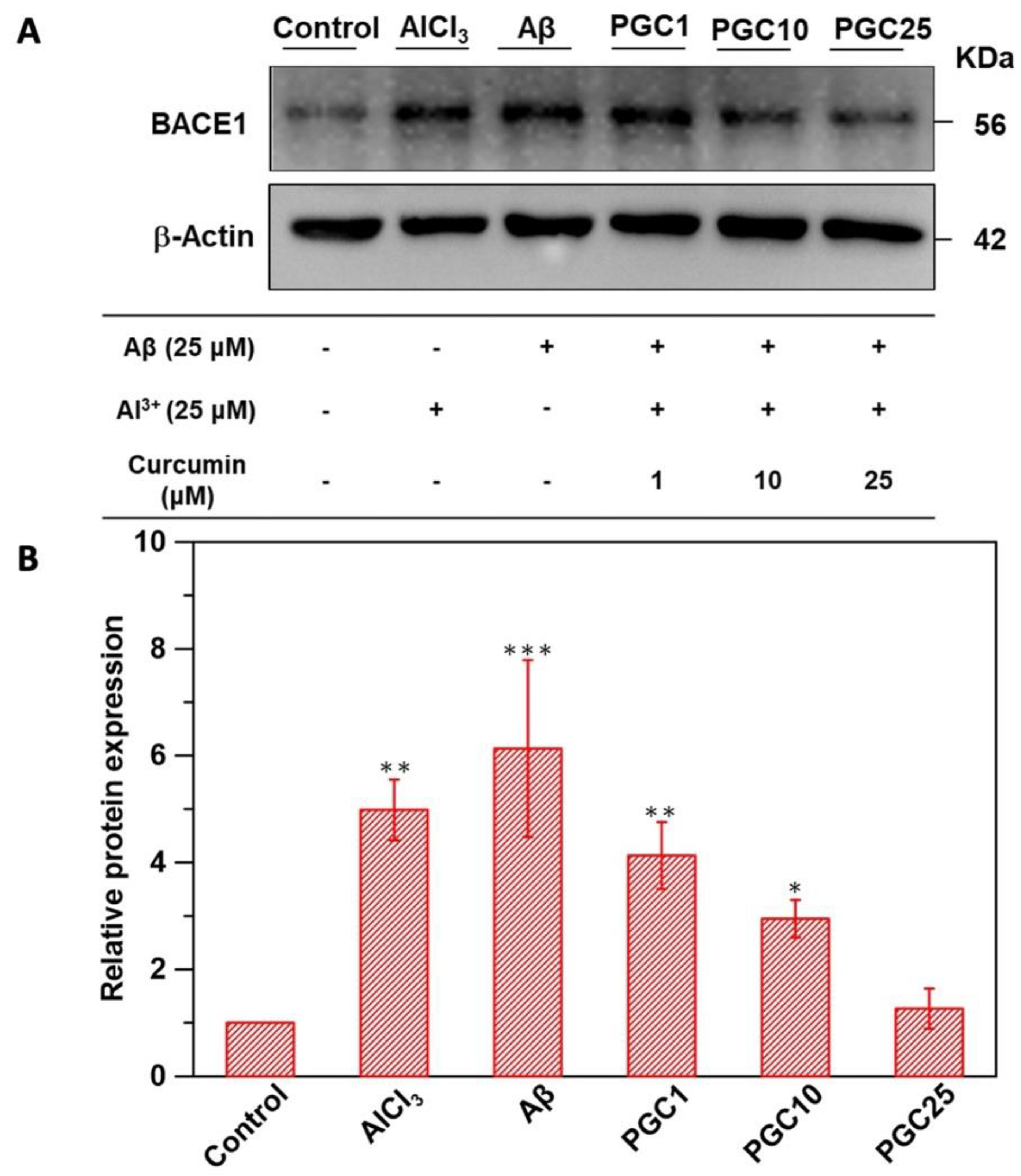
3.4. Western Blot Analysis of β-Secretase Inhibition
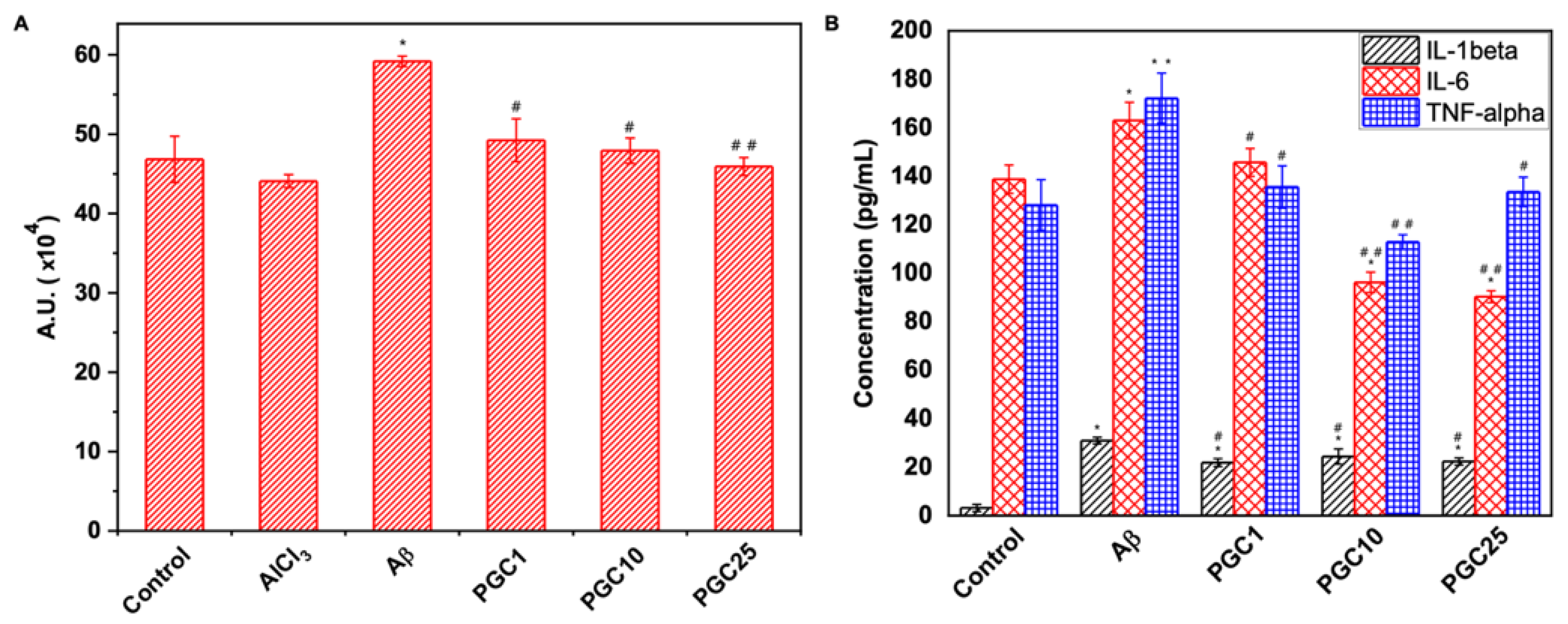
3.5. Antioxidant Effect and Anti-Inflammatory Effects of PGC
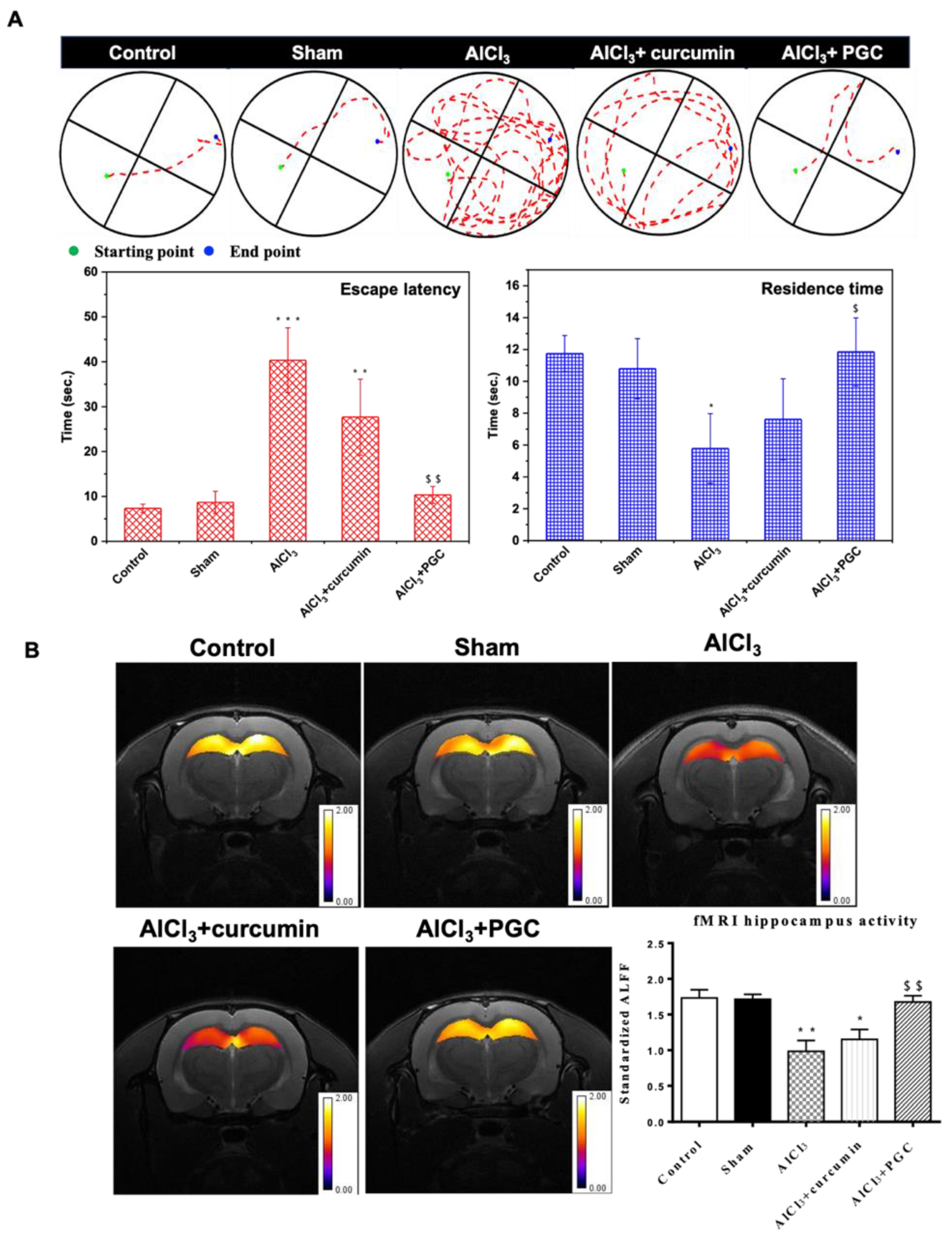
3.6. Morris WM Test and Determination of Hippocampal Activity by fMRI
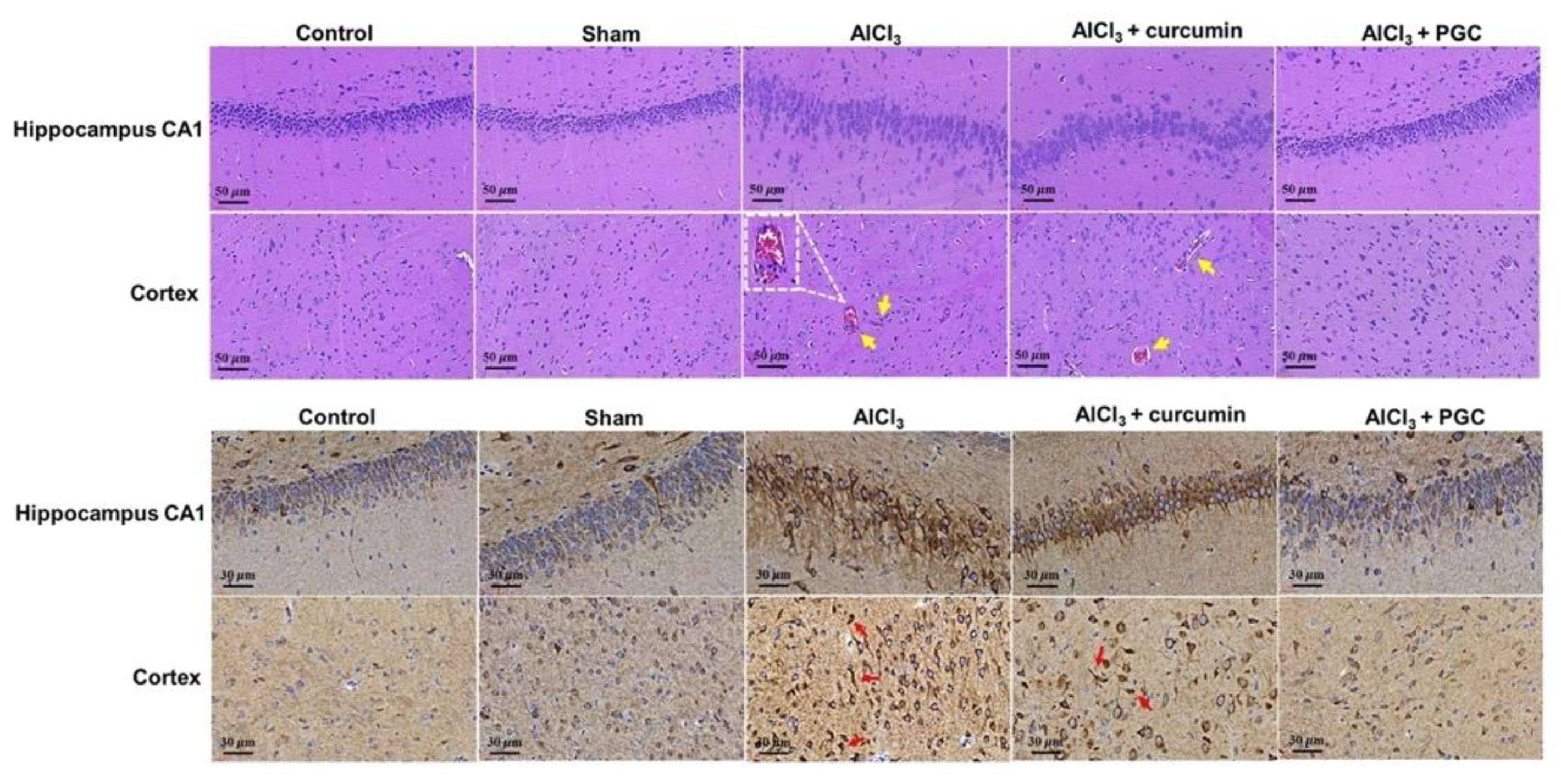
3.7. Histological Analysis and IHC Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chertkow, H.; Feldman, H.H.; Jacova, C.; Massoud, F. Definitions of dementia and predementia states in Alzheimer’s disease and vascular cognitive impairment: Consensus from the Canadian conference on diagnosis of dementia. Alzheimer’s Res. Ther. 2013, 5, S2–S8. [Google Scholar] [CrossRef]
- Ryan, N.S.; Rossor, M.N.; Fox, N.C. Alzheimer’s disease in the 100 years since Alzheimer’s death. Brain 2015, 138, 3816–3821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.G.; Salmon, D.P. The Centennial of Alzheimer’s disease and the publication of “Uber eine eigenartige Erkankung der Hirnrinde” by Alois Alzheimer. Cortex 2007, 43, 821–825. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Cantus, D.S.; Rochina, M.J.; Aguilar, M.A.; Yang, I.H. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimer’s Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2018, 21, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Zhang, Y.L.; Xu, H.; Luo, X.B.; Yu, J.; Liu, J.J.; Chang, R.C.C. Neuroprotection of Coenzyme Q10 in Neuro-degenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Marcason, W. What is the lowdown on Coral Calcium? J. Am. Diet. Assoc. 2003, 103, 1319. [Google Scholar] [PubMed]
- Zhang, M.; Schmitt-Ulms, G.; Sato, C.; Xi, Z.R.; Zhang, Y.L.; Zhou, Y.; St George-Hyslop, P.; Rogaeva, E. Drug Repositioning for Alzheimer’s Disease Based on Systematic ’omics’ Data Mining. PLoS ONE 2016, 11, e0168812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lleó, A. Current Therapeutic Options for Alzheimer’s Disease. Curr. Genom. 2007, 8, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.X.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science & Business Media: New York, NY, USA, 2007; Volume 595. [Google Scholar]
- Reinke, A.A.; Gestwicki, J.E. Structure-activity Relationships of Amyloid Beta-aggregation Inhibitors Based on Curcumin: Influence of Linker Length and Flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yugay, D.; Goronzy, D.P.; Kawakami, L.M.; Claridge, S.A.; Song, T.-B.; Yan, Z.; Xie, Y.-H.; Gilles, J.; Yang, Y.; Weiss, P.S. Copper Ion Binding Site in beta;-Amyloid Peptide. Nano Lett. 2016, 16, 6282–6289. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Bush, A.I.; Cho, H.-H.; Smith, D.H.; Thomson, A.M.; Friedlich, A.L.; Lahiri, D.K.; Leedman, P.J.; Huang, X.; Cahill, C.M. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: Riboregulation against neural oxidative damage in Alzheimer’s disease. Biochem. Soc. Trans. 2008, 36, 1282–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin—In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer-Am. Cancer Soc. 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Gao, J.M.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.X.; Chu, W.; Zhuo, X.Z.; Zhang, Y.; Gou, J.X.; Ren, T.Y.; He, H.B.; Yin, T.; Tang, X. Modified PLGA–PEG–PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J. Mater. Chem. B 2017, 5, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, R.A.; Assi, A.-A.A.; Kostandy, B.B. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur. J. Pharmacol. 2011, 659, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Singh, N.A.; Bhardwaj, V.; Ravi, C.; Ramesh, N.; Mandal, A.K.A.; Khan, Z.A. EGCG Nanoparticles Attenuate Aluminum Chloride Induced Neurobehavioral Deficits, Beta Amyloid and Tau Pathology in a Rat Model of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Arumana, J.M.; Li, D.; Dharmakumar, R. Deriving blood-oxygen-level-dependent contrast in MRI withT2*-weighted, T2-prepared and phase-cycled SSFP methods: Theory and experiment. Magn. Reson. Med. 2008, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.K.; Sanabria-DeLong, N.; Coburn, J.M.; Tew, G.N.; Bhatia, S.R. Novel drug release profiles from micellar solutions of PLA–PEO–PLA triblock copolymers. J. Control. Release 2006, 112, 64–71. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). Evid.-Based Complement. Altern. Med. 2015, 2015, 678218. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, T.N.S.; Larasati, D.; Nugroho, A.K.; Choiri, S. Assessment of the Effect of PLGA Co-polymers and PEG on the Formation and Characteristics of PLGA-PEG-PLGA Co-block Polymer Using Statistical Approach. Adv. Pharm. Bull. 2019, 9, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Zhang, Z.; Ding, J. Influence of LA and GA Sequence in the PLGA Block on the Properties of Thermogelling PLGA-PEG-PLGA Block Copolymers. Biomacromolecules 2011, 12, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cui, F.; Xing, Y.; Mei, Q.; Zhang, Z. Investigation of a new injectable thermosensitive hydrogel loading solid lipid nanoparticles. Pharmazie 2011, 66, 948–952. [Google Scholar] [PubMed]
- Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Naylor, A.; Whitaker, M.; Palmieri, G.F.; Giorgioni, G.; Casettari, L. Evaluation of P(L)LA-PEG-P(L)LA as processing aid for biodegradable particles from gas saturated solutions (PGSS) process. Int. J. Pharm. 2014, 468, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-Y.; Gresser, J.D.; Stewart, R.R.; Trantolo, D.J.; Lyons, C.M.; Simons, G.A.; Gangadharam, P.R.J.; Wise, D.L. Mechanisms of Isoniazid Release from Poly(d,l-lactide-co-glycolide) Matrices Prepared by Dry-Mixing and Low Density Polymeric Foam Methods. J. Pharm. Sci. 1996, 85, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Uhr, M.; Grauer, M.T.; Holsboer, F. Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-Glycoprotein gene disruption. Biol. Psychiatry 2003, 54, 840–846. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Hu, Q.; Zhu, L.; Huang, Z.; Yi, J.; Yang, X.; Hu, J.; Feng, X. Preparation and Drug Release Study of Novel Nanopharmaceuticals with Polysorbate 80 Surface Adsorption. J. Nanomater. 2018, 2018, 4718045. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Poshina, D.N.; Raik, S.V.; Urtti, A.; Skorik, Y.A. Polysaccharides in Ocular Drug Delivery. Pharmaceutics 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Gu, L.; Ren, L.; Chen, J.; Li, T.; Wang, X.; Yang, J.; Chen, C.; Sun, L. Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression. Am. J. Transl. Res. 2019, 11, 6775–6789. [Google Scholar]
- Bolognin, S.; Zatta, P.; Lorenzetto, E.; Valenti, M.T.; Buffelli, M. β-Amyloid-aluminum complex alters cytoskeletal stability and increases ROS production in cortical neurons. Neurochem. Int. 2013, 62, 566–574. [Google Scholar] [CrossRef]
- Hickman, S.E.; El Khoury, J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 495–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.M.; Bhattacharjee, S.; Dua, P.; Hill, J.M.; Zhao, Y.; Lukiw, W.J. Regulating amyloidogenesis through the natural triggering receptor expressed in myeloid/microglial cells 2 (TREM2). Front. Cell. Neurosci. 2014, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lukiw, W.J. TREM2 signaling, miRNA-34a and the extinction of phagocytosis. Front. Cell. Neurosci. 2013, 7, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, E.; Bryant-Thomas, T.; Pacheco Quinto, J.; Henry, T.L.; Poeggeler, B.; Herbert, D.; Cruz-Sanchez, F.; Chyan, Y.-J.; Smith, M.A.; Perry, G.; et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J. Neurochem. 2003, 85, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Conte, V.; Uryu, K.; Fujimoto, S.; Yao, Y.; Rokach, J.; Longhi, L.; Trojanowski, J.Q.; Lee, V.M.-Y.; McIntosh, T.K.; Praticò, D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J. Neurochem. 2004, 90, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Ishihara, T.; Yokota, O.; Terada, S.; Trojanowski, J.Q.; Lee, V.M.; Kuroda, S. Effects of alpha-tocopherol on an animal model of tauopathies. Free Radic. Biol. Med. 2004, 37, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kim, J.-R.; Lee, S.-B.; Kim, Y.-J.; Jung, M.Y.; Kwon, H.-W.; Ahn, Y.-J. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer’s disease Drosophila models. BMC Complement. Altern. Med. 2014, 14, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathologyin vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.S.H.L. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Narlawar, R.; Pickhardt, M.; Leuchtenberger, S.; Baumann, K.; Krause, S.; Dyrks, T.; Weggen, S.; Mandelkow, E.; Schmidt, B. Curcumin-Derived Pyrazoles and Isoxazoles: Swiss Army Knives or Blunt Tools for Alzheimer’s Disease? ChemMedChem 2008, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Park, S.Y.; Kim, J.Y. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheo-chromocytoma and normal human umbilical vein endothelial cells from PA(1-42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.S.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Liu, P.T.; Espinosa-Jeffrey, A.; Rosenthal, M.J.; Bernard, G.; Ringman, J.M.; Sayre, J.; Zhang, L.; Zaghi, J.; Dejbakhsh, S.; et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. USA 2007, 104, 12849–12854. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Boyle, K. Amyloid-beta peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef] [PubMed]
| Group | Treatment |
|---|---|
| 1 (control group) | Rats without any treatment |
| 2 (sham group) | Rats treated with PBS (i.p.) |
| 3 (AlCl3) | Rats induced with AlCl3 (100 mg/kg, i.p.) three times a week |
| 4 (AlCl3 + curcumin) | Rats induced with AlCl3 (100 mg/kg, i.p.) three times a week and curcumin-free drug (16.5 mg/kg, i.m.) every four weeks |
| 5 (AlCl3 + PGC25) | Rats induced with AlCl3 (100 mg/kg, i.p.) three times a week and curcumin (16.5 mg/kg, i.m.)-loaded with micelle solution every four weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-W.; Fang, C.-H.; Yang, C.-Y.; Liang, Y.-J.; Lin, F.-H. Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease. Antioxidants 2022, 11, 727. https://doi.org/10.3390/antiox11040727
Lin Y-W, Fang C-H, Yang C-Y, Liang Y-J, Lin F-H. Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease. Antioxidants. 2022; 11(4):727. https://doi.org/10.3390/antiox11040727
Chicago/Turabian StyleLin, Yi-Wen, Chih-Hsiang Fang, Ching-Yun Yang, Ya-Jyun Liang, and Feng-Huei Lin. 2022. "Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease" Antioxidants 11, no. 4: 727. https://doi.org/10.3390/antiox11040727






