Preliminary Findings on the Effect of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles and Acute Stress on Selected Markers of Oxidative Stress in Normotensive and Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Animals and Study Design
2.3. Sample Preparation
2.4. Measurement of Antioxidant Capacity
2.5. Measurements of Antioxidant Enzymes
2.6. Measurements of Parameters of Oxidative Damage to Proteins
2.7. Measurements of Parameters of Oxidative Damage to Lipids
2.8. Statistical Analysis
3. Results
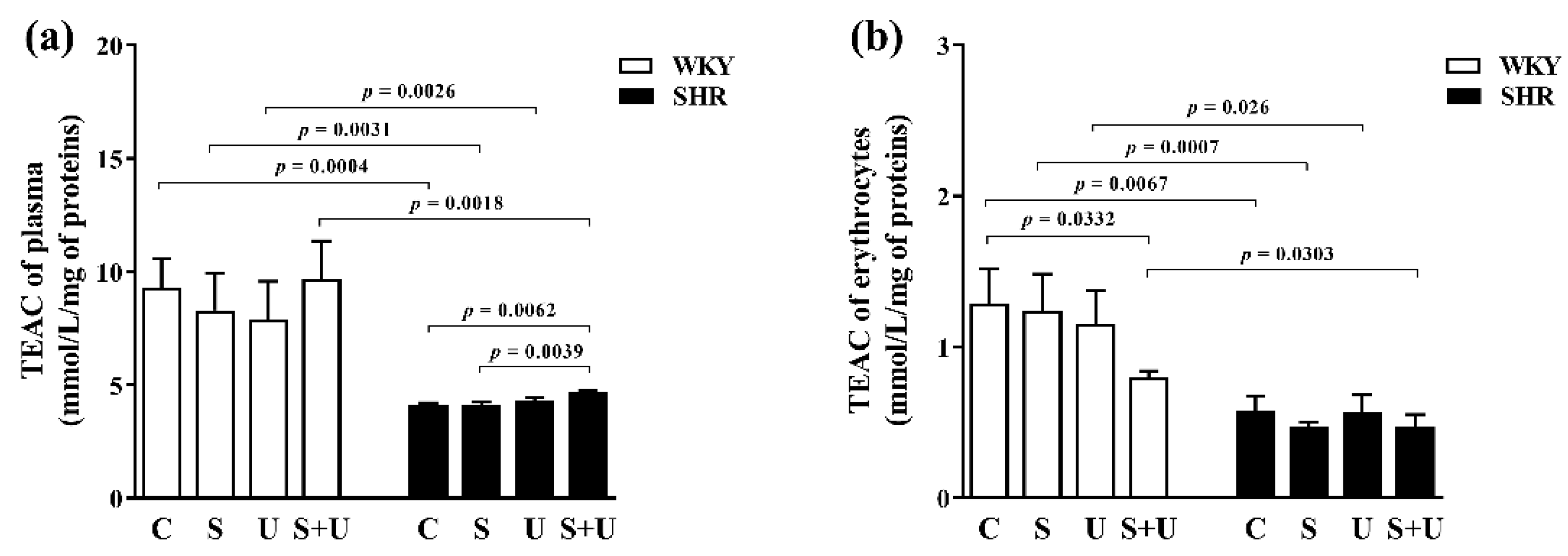
3.1. The Antioxidant Capacity of Plasma
3.2. The Antioxidant Capacity of Erythrocytes
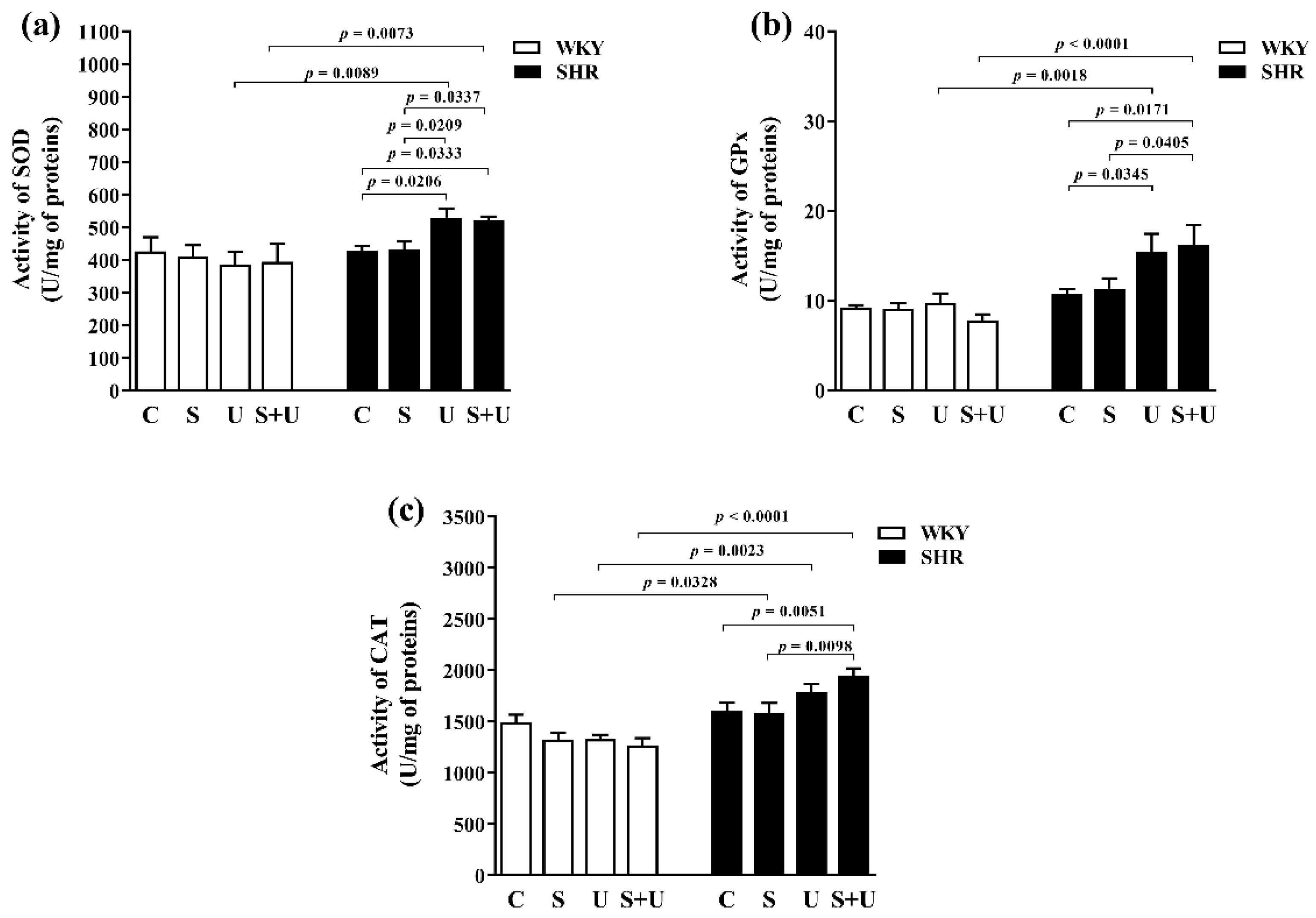
3.3. Superoxide Dismutase Activity
3.4. Glutathione Peroxidase Activity
3.5. Catalase Peroxidase Activity
3.6. Advanced Oxidation Protein Products
3.7. Protein Carbonyls
3.8. Lipoperoxides
3.9. 8-Isoprostanes
3.10. Correlations
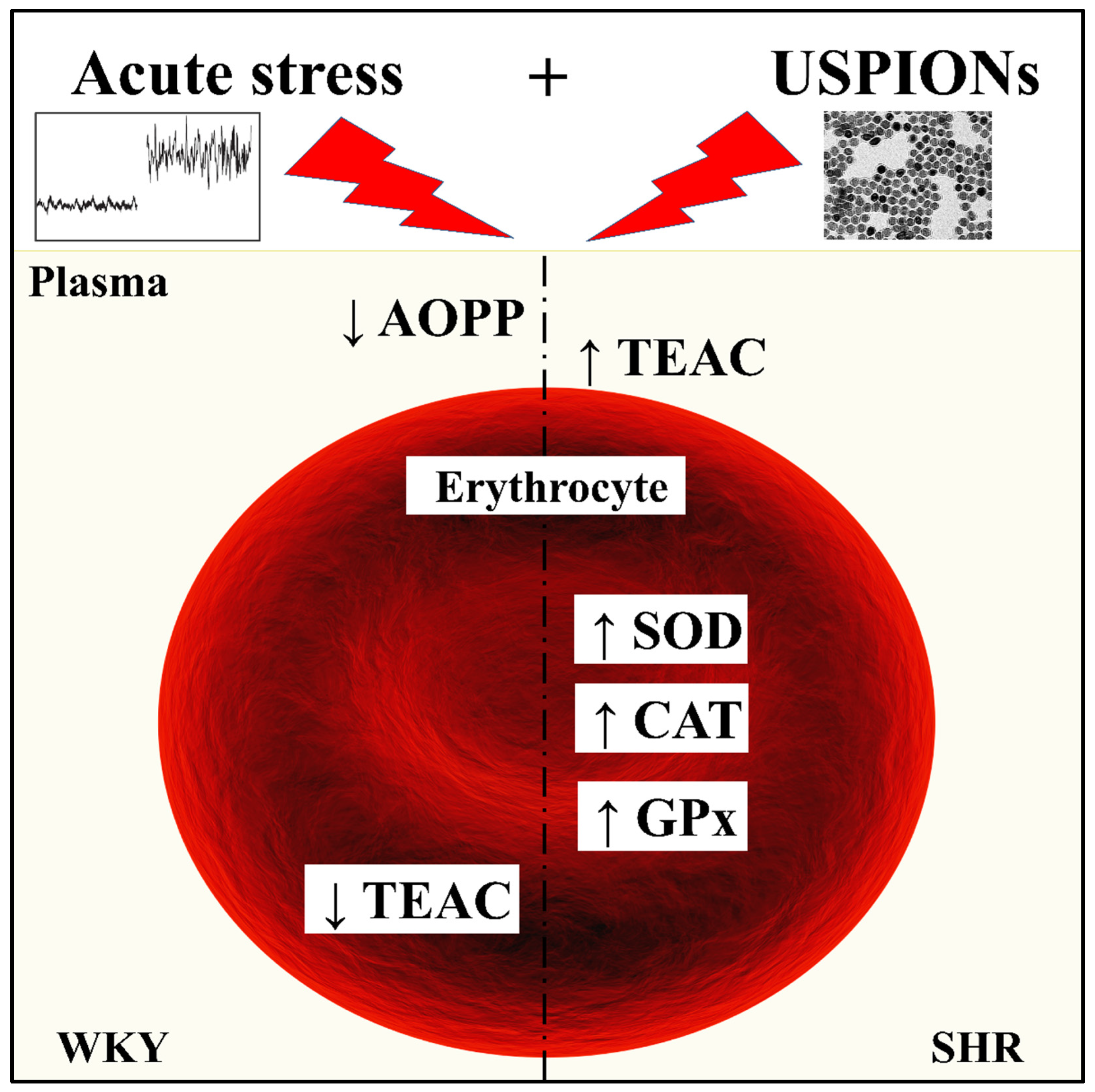
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape Anisotropic Iron Oxide-Based Magnetic Nanoparticles: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The Effect of Surface Charge of Functionalized Fe3O4 Nanoparticles on Protein Adsorption and Cell Uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.W.; Ehsan, S.M.; Mast, D.; Pauletti, G.M.; Xu, H.; Zhang, J.; Ewing, R.C.; Shi, D. Photothermal Effects and Toxicity of Fe3O4 Nanoparticles via near Infrared Laser Irradiation for Cancer Therapy. Mater Sci. Eng. C Mater. Biol. Appl. 2015, 46, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Smith, J.B.; Pham, T.; Whitaker, R.D.; Sucato, C.A.; Hamilton, J.A.; Bartolak-Suki, E.; Wong, J.Y. Effect of PEG Molecular Weight on Stability, T₂ Contrast, Cytotoxicity, and Cellular Uptake of Superparamagnetic Iron Oxide Nanoparticles (SPIONs). Colloids Surf. B Biointerfaces 2014, 119, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional Nanoparticles for Drug Delivery and Molecular Imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.-C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric Oxide Synthase Inhibition and Oxidative Stress in Cardiovascular Diseases: Possible Therapeutic Targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Líšková, S.; Bališ, P.; Mičurová, A.; Kluknavský, M.; Okuliarová, M.; Puzserová, A.; Škrátek, M.; Sekaj, I.; Maňka, J.; Valovič, P.; et al. Effect of Iron Oxide Nanoparticles on Vascular Function and Nitric Oxide Production in Acute Stress-Exposed Rats. Physiol. Res. 2020, 69, 1067–1083. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [Green Version]
- Dvořáková, M.; Rollerová, E.; Scsuková, S.; Bujňáková Mlynarčíková, A.; Laubertová, L.; Žitňanová, I. Effect of Neonatal Exposure to Poly(Ethylene Glycol)-Block-Poly(Lactic Acid) Nanoparticles on Oxidative State in Infantile and Adult Female Rats. Oxid. Med. Cell Longev. 2017, 2017, 7430435. [Google Scholar] [CrossRef] [Green Version]
- Wahajuddin, S.A. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Oleksa, V.; Bernátová, I.; Patsula, V.; Líšková, S.; Bališ, P.; Radošinská, J.; Mičurová, A.; Kluknavský, M.; Jasenovec, T.; Radošinská, D.; et al. Poly(Ethylene Glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats. Nanomaterials 2021, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Ekstrand-Hammarström, B.; Ahlinder, L.; Guldevall, K.; Pazik, R.; Kępiński, L.; Kvashnina, K.O.; Butorin, S.M.; Brismar, H.; Önfelt, B.; et al. Visualization of Custom-Tailored Iron Oxide Nanoparticles Chemistry, Uptake, and Toxicity. Nanoscale 2012, 4, 7383–7393. [Google Scholar] [CrossRef] [PubMed]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryszczyńska, B.; Formanowicz, D.; Budzyń, M.; Wanic-Kossowska, M.; Pawliczak, E.; Formanowicz, P.; Majewski, W.; Strzyżewski, K.W.; Kasprzak, M.P.; Iskra, M. Advanced Oxidation Protein Products and Carbonylated Proteins as Biomarkers of Oxidative Stress in Selected Atherosclerosis-Mediated Diseases. Biomed. Res. Int. 2017, 2017, 4975264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of Se-Glutathione Peroxidase, Catalase, and Cu/Zn-SOD for Cell Survival against Oxidative Stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Kedziora-Kornatowska, K.; Czuczejko, J.; Pawluk, H.; Kornatowski, T.; Motyl, J.; Szadujkis-Szadurski, L.; Szewczyk-Golec, K.; Kedziora, J. The Markers of Oxidative Stress and Activity of the Antioxidant System in the Blood of Elderly Patients with Essential Arterial Hypertension. Cell Mol. Biol. Lett. 2004, 9, 635–641. [Google Scholar]
- Pena, E.; El Alam, S.; Siques, P.; Brito, J. Oxidative Stress and Diseases Associated with High-Altitude Exposure. Antioxidants 2022, 11, 267. [Google Scholar] [CrossRef]
- Majzunova, M.; Dovinova, I.; Barancik, M.; Chan, J.Y.H. Redox Signaling in Pathophysiology of Hypertension. J. Biomed. Sci. 2013, 20, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madamanchi, N.R.; Runge, M.S. Redox Signaling in Cardiovascular Health and Disease. Free Radic. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M. Reactive Oxygen Species, Vascular Oxidative Stress, and Redox Signaling in Hypertension: What Is the Clinical Significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechánová, O.; Zicha, J.; Paulis, L.; Zenebe, W.; Dobesová, Z.; Kojsová, S.; Jendeková, L.; Sládková, M.; Dovinová, I.; Simko, F.; et al. The Effect of N-Acetylcysteine and Melatonin in Adult Spontaneously Hypertensive Rats with Established Hypertension. Eur. J. Pharmacol. 2007, 561, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Covas, M.I.; Martín, S.; Rubiés-Prat, J. Decreased Endogenous Antioxidant Enzymatic Status in Essential Hypertension. J. Hum. Hypertens. 2000, 14, 343–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluknavsky, M.; Balis, P.; Skratek, M.; Manka, J.; Bernatova, I. (-)-Epicatechin Reduces the Blood Pressure of Young Borderline Hypertensive Rats During the Post-Treatment Period. Antioxidants 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Amirkhizi, F.; Siassi, F.; Djalali, M.; Foroushani, A.R. Assessment of Antioxidant Enzyme Activities in Erythrocytes of Pre-Hypertensive and Hypertensive Women. J. Res. Med. Sci. 2010, 15, 270–278. [Google Scholar]
- Yan, S.; Resta, T.C.; Jernigan, N.L. Vasoconstrictor Mechanisms in Chronic Hypoxia-Induced Pulmonary Hypertension: Role of Oxidant Signaling. Antioxidants 2020, 9, 999. [Google Scholar] [CrossRef]
- Yamazato, M.; Ohya, Y.; Nakamoto, M.; Sakima, A.; Tagawa, T.; Harada, Y.; Nabika, T.; Takishita, S. Sympathetic Hyperreactivity to Air-Jet Stress in the Chromosome 1 Blood Pressure Quantitative Trait Locus Congenic Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R709–R714. [Google Scholar] [CrossRef]
- Bernátová, I.; Bališ, P.; Goga, R.; Behuliak, M.; Zicha, J.; Sekaj, I. Lack of Reactive Oxygen Species Deteriorates Blood Pressure Regulation in Acute Stress. Physiol. Res. 2016, 65, S381–S390. [Google Scholar] [CrossRef]
- Kim, H.-G.; Lee, J.-S.; Choi, M.-K.; Han, J.-M.; Son, C.-G. Ethanolic Extract of Astragali Radix and Salviae Radix Prohibits Oxidative Brain Injury by Psycho-Emotional Stress in Whisker Removal Rat Model. PLoS ONE 2014, 9, e98329. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxid. Med. Cell Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial Function and Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Pennathur, S.; Heinecke, J.W. Oxidative Stress and Endothelial Dysfunction in Vascular Disease. Curr. Diab. Rep. 2007, 7, 257–264. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of Inflammation, Oxidative Stress, and Vascular Dysfunction in Hypertension. Biomed. Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puzserova, A.; Bernatova, I. Blood Pressure Regulation in Stress: Focus on Nitric Oxide-Dependent Mechanisms. Physiol. Res. 2016, 65, S309–S342. [Google Scholar] [CrossRef] [PubMed]
- Katrenčíková, B.; Vaváková, M.; Paduchová, Z.; Nagyová, Z.; Garaiova, I.; Muchová, J.; Ďuračková, Z.; Trebatická, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced Glycation End-Products and Advanced Oxidation Protein Products in Patients with Diabetes Mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar]
- el-Saadani, M.; Esterbauer, H.; el-Sayed, M.; Goher, M.; Nassar, A.Y.; Jürgens, G. A Spectrophotometric Assay for Lipid Peroxides in Serum Lipoproteins Using a Commercially Available Reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, R.; González, J.; Paoletto, F. The Role of Oxidative Stress in the Pathophysiology of Hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Stefánsson, B.V.; Haraldsson, B.; Nilsson, U. Acute Oxidative Stress Following Intravenous Iron Injection in Patients on Chronic Hemodialysis: A Comparison of Iron-Sucrose and Iron-Dextran. Nephron. Clin. Pract. 2011, 118, c249–c256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newaz, M.A.; Nawal, N.N. Effect of Gamma-Tocotrienol on Blood Pressure, Lipid Peroxidation and Total Antioxidant Status in Spontaneously Hypertensive Rats (SHR). Clin. Exp. Hypertens. 1999, 21, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Moreno, K.; Ayala, M.; Vazquez-Duhalt, R. Antioxidant Capacity of Poly(Ethylene Glycol) (PEG) as Protection Mechanism Against Hydrogen Peroxide Inactivation of Peroxidases. Appl. Biochem. Biotechnol. 2015, 177, 1364–1373. [Google Scholar] [CrossRef]
- Gupta, H.; Paul, P.; Kumar, N.; Baxi, S.; Das, D.P. One Pot Synthesis of Water-Dispersible Dehydroascorbic Acid Coated Fe3O4 Nanoparticles under Atmospheric Air: Blood Cell Compatibility and Enhanced Magnetic Resonance Imaging. J. Colloid Interface Sci. 2014, 430, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Radosinska, J.; Jasenovec, T.; Radosinska, D.; Balis, P.; Puzserova, A.; Skratek, M.; Manka, J.; Bernatova, I. Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats. Biomedicines 2021, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron Homeostasis and Iron-Regulated ROS in Cell Death, Senescence and Human Diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Wang, H.; Feng, Y.; Li, Y.; Hua, X.; Pang, X.; Zhang, S.; Song, L.; Zhang, Y.; Gu, N. Cardioprotective Activity of Iron Oxide Nanoparticles. Sci. Rep. 2015, 5, 8579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, T.; Pinho, D.; Morato, M.; Marques-Lopes, J.; Fernandes, E.; Afonso, J.; Oliveira, S.; Carvalho, F.; Albino-Teixeira, A. Role of Superoxide and Hydrogen Peroxide in Hypertension Induced by an Antagonist of Adenosine Receptors. Eur. J. Pharmacol. 2008, 588, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sainz, J.; Wangensteen, R.; Rodríguez Gómez, I.; Moreno, J.M.; Chamorro, V.; Osuna, A.; Bueno, P.; Vargas, F. Antioxidant Enzymes and Effects of Tempol on the Development of Hypertension Induced by Nitric Oxide Inhibition. Am. J. Hypertens. 2005, 18, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvathova, M.; Zitnanova, I.; Kralovicova, Z.; Balis, P.; Puzserova, A.; Muchova, J.; Kluknavsky, M.; Durackova, Z.; Bernatova, I. Sex Differences in the Blood Antioxidant Defense System in Juvenile Rats with Various Genetic Predispositions to Hypertension. Hypertens. Res. 2016, 39, 64–69. [Google Scholar] [CrossRef]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative Imbalance and Kidney Damage: New Study Perspectives from Animal Models to Hospitalized Patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.-D.; Sindhu, R.K.; Pang, J.; Ehdaie, A.; Vaziri, N.D. Superoxide Dismutase, Catalase and Glutathione Peroxidase in the Spontaneously Hypertensive Rat Kidney: Effect of Antioxidant-Rich Diet. J. Hypertens. 2004, 22, 2025–2033. [Google Scholar] [CrossRef]
- Petrulea, M.; Muresan, A.; Duncea, I. Oxidative Stress and Antioxidant Status in Hypo- and Hyperthyroidism; IntechOpen: London, UK, 2012; ISBN 978-953-51-0789-7. [Google Scholar]
- Sun, L.; Gao, Y.-H.; Tian, D.-K.; Zheng, J.-P.; Zhu, C.-Y.; Ke, Y.; Bian, K. Inflammation of Different Tissues in Spontaneously Hypertensive Rats. Sheng Li Xue Bao 2006, 58, 318–323. [Google Scholar]
- Tanito, M.; Nakamura, H.; Kwon, Y.-W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced Oxidative Stress and Impaired Thioredoxin Expression in Spontaneously Hypertensive Rats. Antioxid. Redox Signal 2004, 6, 89–97. [Google Scholar] [CrossRef]
- Tyther, R.; Ahmeda, A.; Johns, E.; Sheehan, D. Protein Carbonylation in Kidney Medulla of the Spontaneously Hypertensive Rat. Proteom. Clin. Appl. 2009, 3, 338–346. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Protection against Reactive Oxygen Species by Selenoproteins. Biochim Biophys Acta 2009, 1790, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Hozawa, A.; Ebihara, S.; Ohmori, K.; Kuriyama, S.; Ugajin, T.; Koizumi, Y.; Suzuki, Y.; Matsui, T.; Arai, H.; Tsubono, Y.; et al. Increased Plasma 8-Isoprostane Levels in Hypertensive Subjects: The Tsurugaya Project. Hypertens. Res. 2004, 27, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Faria, A.P.C.; Fontana, V.; Modolo, R.; Barbaro, N.R.; Sabbatini, A.R.; Pansani, I.F.; Ferreira-Melo, S.E.; Moreno, H. Plasma 8-Isoprostane Levels Are Associated with Endothelial Dysfunction in Resistant Hypertension. Clin. Chim. Acta 2014, 433, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Guichard, C.; Bächler, J.P. Relationship between Oxidative Stress and Essential Hypertension. Hypertens. Res. 2007, 30, 1159–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Genotype | Parameter | Parameter | n | p | r |
|---|---|---|---|---|---|
| WKY | AOPP | TEAC-PL | 23 | 0.005 | −0.568 |
| AOPP | LPx | 23 | 0.038 | 0.435 | |
| AOPP | Carb-P | 23 | 0.047 | 0.418 | |
| AOPP | GPx | 23 | 0.018 | 0.488 | |
| Carb-P | TEAC-PL | 23 | 0.010 | −0.525 | |
| Carb-P | GPx | 23 | 0.010 | 0.525 | |
| GPx | TEAC-PL | 23 | 0.0001 | −0.716 | |
| SHR | AOPP | LPx | 28 | 0.001 | 0.602 |
| AOPP | Iso-P | 28 | 0.011 | 0.474 | |
| SOD | GPx | 28 | 0.0001 | 0.742 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laubertova, L.; Dvorakova, M.; Balis, P.; Puzserova, A.; Zitnanova, I.; Bernatova, I. Preliminary Findings on the Effect of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles and Acute Stress on Selected Markers of Oxidative Stress in Normotensive and Hypertensive Rats. Antioxidants 2022, 11, 751. https://doi.org/10.3390/antiox11040751
Laubertova L, Dvorakova M, Balis P, Puzserova A, Zitnanova I, Bernatova I. Preliminary Findings on the Effect of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles and Acute Stress on Selected Markers of Oxidative Stress in Normotensive and Hypertensive Rats. Antioxidants. 2022; 11(4):751. https://doi.org/10.3390/antiox11040751
Chicago/Turabian StyleLaubertova, Lucia, Monika Dvorakova, Peter Balis, Angelika Puzserova, Ingrid Zitnanova, and Iveta Bernatova. 2022. "Preliminary Findings on the Effect of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles and Acute Stress on Selected Markers of Oxidative Stress in Normotensive and Hypertensive Rats" Antioxidants 11, no. 4: 751. https://doi.org/10.3390/antiox11040751
APA StyleLaubertova, L., Dvorakova, M., Balis, P., Puzserova, A., Zitnanova, I., & Bernatova, I. (2022). Preliminary Findings on the Effect of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles and Acute Stress on Selected Markers of Oxidative Stress in Normotensive and Hypertensive Rats. Antioxidants, 11(4), 751. https://doi.org/10.3390/antiox11040751







