Nutraceutical Profile of “Carosello” (Cucumis melo L.) Grown in an Out-of-Season Cycle under LEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Light-Emitting Diodes (LEDs) Application
2.3. Plant Material and Growing Conditions
2.4. Chemicals
2.5. Extraction and Analysis of Phenolic Compounds
2.6. Extraction and Quantification of Isoprenoids
2.7. Glucose and Fructose Assay and Sweetness Index
2.8. Macro and Micronutrients Analysis
2.9. Experimental Design and Statistical Analysis
3. Results
3.1. Supplementary Light Management and Its Effects on Fruits Yield and Morphology
3.2. Poliphenols Profile of “Carosello leccese” Fruits
3.3. Tocopherols and Chlorophylls Content of ‘Carosello leccese’ Fruits
3.4. Carotenoids Content of “Carosello leccese” Fruits
3.5. Sugars Content and Sweetness Index of “Carosello leccese” Fruits
3.6. Mineral Content of Carosello leccese Fruits
4. Discussion
4.1. LEDs as a Tool to Enhance Landraces of Cucumis melo L.
4.2. Polyphenol Fruit Profiles
4.3. Tochopherols and Clorophylls Fruits Profile
4.4. Carotenoids Fruits Profile
4.5. Sweetness Index, Sugars and Mineral Elements Fruits Profile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Renna, M.; Montesano, F.F.; Signore, A.; Gonnella, M.; Santamaria, P. Biodiverso: A case study of integrated project to preserve the biodiversity of vegetable crops in puglia (southern Italy). Agriculture 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Signore, A.; Renna, M.; Santamaria, P. Agrobiodiversity of vegetables crops: Aspect, Needs, and Future Perspectives. Annu. Plant Rev. 2019, 2, 1–24. [Google Scholar] [CrossRef]
- Somma, A.; Palmitessa, O.D.; Leoni, B.; Signore, A.; Renna, M.; Santamaria, P. Extraseasonal Production in a Soilless System and Characterisation of Landraces of Carosello and Barattiere (Cucumis melo L.). Sustainability 2021, 13, 11425. [Google Scholar] [CrossRef]
- Pavan, S.; Marcotrigiano, A.R.; Ciani, E.; Mazzeo, R.; Zonno, V.; Ruggieri, V.; Lotti, C.; Ricciardi, L. Genotyping-by-sequencing of a melon (Cucumis melo L.) germplasm collection from a secondary center of diversity highlights patterns of genetic variation and genomic features of different gene pools. BMC Genomics 2017, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Perrino, P.; Hammer, K.; Hanelt, P. Carosello and the taxonomy of Cucumis melo L., especially of its vegetables races. Acta Hortic. 1986, 182, 95–100. [Google Scholar] [CrossRef]
- Buttaro, D.; Bonasia, A.; Minuto, A.; Serio, F.; Santamaria, P. Effect of silicon in the nutrient solution on the incidence of powdery mildew and quality traits in carosello and barattiere (Cucumis melo L.) grown in a soilless system. J. Hortic. Sci. Biotechnol. 2009, 84, 300–304. [Google Scholar] [CrossRef]
- Elia, A.; Santamaria, P. Biodiversity in vegetable crops: A heritage to save. The case of the Puglia region. Ital. J. Agron. 2013, 8, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Palmitessa, O.D.; Durante, M.; Leoni, B.; Montesano, F.; Renna, M.; Serio, F.; Somma, A.; Santamaria, P. Enhancement of a Landrace of Carosello (Unripe Melon) through the Use of Light-Emitting Diodes (LED) and Nutritional Characterization of the Fruit Placenta. Sustainability 2021, 13, 11464. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PLoS ONE 2015, 10, e0138930. [Google Scholar] [CrossRef]
- van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Leoni, B.; Palmitessa, O.D.; Serio, F.; Signore, A.; Santamaria, P. Blue LED light irradiation enhances yield in green beans. Acta Hortic. 2021, 1321, 9–14. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Montesano, F.F.; Serio, F.; Signore, A.; Santamaria, P. Supplemental lighting with LED for efficient year-round production of soilless tomato in a Mediterranean greenhouse. Acta Hortic. 2021, 1311, 367–374. [Google Scholar] [CrossRef]
- Kim, H.; Lin, M.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Buono, D.D.; Ballerini, E.; Benincasa, P.; Falcinelli, B.; Guiducci, M. Effect of Light Spectrum on Gas Exchange, Growth and Biochemical Characteristics of Einkorn Seedlings. Agronomy 2020, 10, 1042. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mul, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidant 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Brazaityte, A.; Sakalauskiene, S.; Samuoliene, G.; Jankauskiene, J.; Viršile, A.; Novičkovas, A.; Sirtautas, R.; Miliauskiene, J.; Vaštakaite, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Palmitessa, O.D.; Durante, M.; Caretto, S.; Milano, F.; D’imperio, M.; Serio, F.; Santamaria, P. Supplementary light differently influences physico-chemical parameters and antioxidant compounds of tomato fruits hybrids. Antioxidants 2021, 10, 687. [Google Scholar] [CrossRef]
- Liu, W.; Zha, L.; Zhang, Y. Growth and nutrient element content of hydroponic lettuce are modified by LED continuous lighting of different intensities and spectral qualities. Agronomy 2020, 10, 1678. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Vella, F.M.; Cautela, D.; Laratta, B. Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Renna, M.; D’Imperio, M.; Gonnella, M.; Parente, A.; Santamaria, P.; Serio, F. Barattiere: An Italian Local Variety of Cucumis melo L. with Quality Traits between Melon and Cucumber. Plants 2020, 9, 578. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical Carbon Dioxide Extraction of Carotenoids from Pumpkin (Cucurbita spp.): A Review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, A.; Buttaro, D.; Conversa, G.; Serio, F.; Santamaria, P. Confronto tra sistemi di coltivazione di carosello e barattiere in serra II. Aspetti qualitativi. Colt. Protette 2005, 34, 28–35. [Google Scholar]
- Conversa, G.; Gonnella, M.; Santamaria, P.; Vincenzo, V. Caratterizzazione e valorizzazione di due tipici ortaggi pugliesi: Carosello e barattiere. Colt. Protette 2005, 34, 4–13. [Google Scholar]
- Palmitessa, O.D.; Paciello, P.; Santamaria, P. Supplemental LED Increases Tomato Yield in mediterranean Semi-Closed Greenhouse. Agronomy 2020, 10, 1353. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Quirantes-Piné, R.; Fernández-Arroyo, S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012, 46, 108–117. [Google Scholar] [CrossRef]
- Laddomada, B.; Durante, M.; Mangini, G.; D’Amico, L.; Lenucci, M.S.; Simeone, R.; Piarulli, L.; Mita, G.; Blanco, A. Genetic variation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet. Resour. Crop Evol. 2017, 64, 587–597. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid Extraction of Lycopene and P-Carotene from Reconstituted Tomato Paste and Pink Grapefruit Homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Perkins-veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 987, 983–987. [Google Scholar] [CrossRef]
- Durante, M.; Salvatore, M.; Paolo, P.; Rizzi, V.; Caroli, M.; De Piro, G.; Fini, P.; Luigi, G.; Mita, G. A -Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Imperio, M.D.; Gonnella, M.; Durante, M.; Parente, A.; Mita, G.; Santamaria, P.; Serio, F. Morphological and Chemical Profile of Three Tomato (Solanum lycopersicum L.) Landraces of A Semi-Arid Mediterranean Environment. Plants 2019, 8, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Imperio, M.; Montesano, F.F.; Renna, M.; Leoni, B.; Buttaro, D.; Parente, A.; Serio, F. NaCl stress enhances silicon tissue enrichment of hydroponic “baby leaf” chicory under biofortification process. Sci. Hortic. (Amsterdam) 2018, 235, 258–263. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Gómez, C.; Mitchell, C.A. Tomatoes grown with light-emitting diodes or high-pressure sodium supplemental lights have similar fruit-quality attributes. HortScience 2015, 50, 1498–1502. [Google Scholar] [CrossRef]
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED Lighting Systems for Horticulture: Business Growth and Global Distribution. Sustainability 2020, 12, 7516. [Google Scholar] [CrossRef]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Signore, A.; Leoni, B.; Palmitessa, O.D.; Santamaria, P. Soilless System with Supplementary LED Light to Obtain a High-Quality Out-of-Season Production of Green Beans. Agronomy 2021, 11, 1999. [Google Scholar] [CrossRef]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F.; et al. Supplementary LED Interlighting Improves Yield and Precocity of Greenhouse Tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C. Plant Antioxidants for Food Safety and Quality: Exploring New Trends of Research. Antioxidants 2021, 10, 972. [Google Scholar] [CrossRef]
- Yunusa, A.K.; Rohin, M.A.K.; Bakar, C.A.A. Free radical scavenging activity of polyphenols Free radical scavenging activity of polyphenols. J. Chem. Pharm. Res. 2015, 7, 1975–1980. [Google Scholar]
- Girelli, C.R.; Accogli, R.; Del Coco, L.; Angilè, F.; De Bellis, L.; Fanizzi, F.P. H-NMR-based metabolomic profiles of different sweet melon (Cucumis melo L.) Salento varieties: Analysis and comparison. Food Res. Int. 2018, 114, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.S.; Tornese, R.; Mita, G. Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt). Antioxidants 2022, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Asci, H.; Ozmen, O.; Ellidag, H.Y.; Aydin, B.; Bas, E.; Yilmaz, N. The impact of gallic acid on the methotrexate-induced kidney damage in rats. J. Food Drug Anal. 2017, 25, 890–897. [Google Scholar] [CrossRef]
- Kamatham, S.; Kumar, N.; Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015, 2, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Jiang, F.; Jiang, H.; Wu, K.; Zheng, X.; Cai, Y.; Katakowski, M.; Chopp, M.; To, S.S.T. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur. J. Pharmacol. 2010, 641, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Nam, B.; Rho, J.K.; Shin, D.M.; Son, J. Gallic acid induces apoptosis in EGFR-mutant non-small cell lung cancers by accelerating EGFR turnover. Bioorg. Med. Chem. Lett. 2016, 26, 4571–4575. [Google Scholar] [CrossRef]
- Szwajgier, D.; Pielecki, J.; Targonski, Z. Antioxidant Activities of Cinnamic and Benzoic Acid Derivates. Acta Sci. Pol. 2005, 4, 129–142. [Google Scholar]
- Ghiasi, M.; Heravi, M.M. Quantum mechanical study of antioxidative ability and antioxidative mechanism of rutin (vitamin P) in solution. Carbohydr. Res. 2011, 346, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Damin, F.; Meinhart, A.; Caldeirão, L.; Filho, M.; da Silva, L.; Constant, L.; Teixeira-Filho, J.; Wagner, R.; Godoy, H. Determination of rutin in fruits and vegetables in natura. J. Food Nutr. Res. 2019, 58, 328–338. [Google Scholar]
- Barouh, N.; Durand, E.; Bourlieu-lacanal, C.; Villeneuve, P.; Figueroa-espinoza, M.C. Tocopherols as antioxidants in lipid-based systems: The combination of chemical and physicochemical interactions determines their efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. [Google Scholar] [CrossRef]
- Kamai-eldin, A.; Appelqvist, L. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Hovi-Pekkanen, T.; Tahvonen, R. Effects of interlighting on yield and external fruit quality in year-round cultivated cucumber. Sci. Hortic. (Amsterdam) 2008, 116, 152–161. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Little, C.; Zheng, J.M.; Cao, R. Far-red LEDs improve fruit production in greenhouse tomato grown under high-pressure sodium lighting. Acta Hortic. 2016, 1134, 95–102. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle-a mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Aksic, M.; Tosti, T.; Sredojevic, M.; Jasminka, M.; Meland, M.; Natic, M. Comparison of Sugar Profile between Leaves and Fruits of Blueberry and Strawberry Cultivars Grown. Plants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture Nutrients Composition of Cucumber. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169225/nutrients (accessed on 13 February 2022).
- United States Department of Agriculture Nutrients Composition of Cucumber. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169092/nutrients (accessed on 13 February 2022).
- Kopsell, D.A.; Sams, C.E. Increases in Shoot Tissue Pigments, Glucosinolates, and Mineral Elements in Sprouting Broccoli after Exposure to Short-duration Blue Light from Light Emitting Diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef] [Green Version]
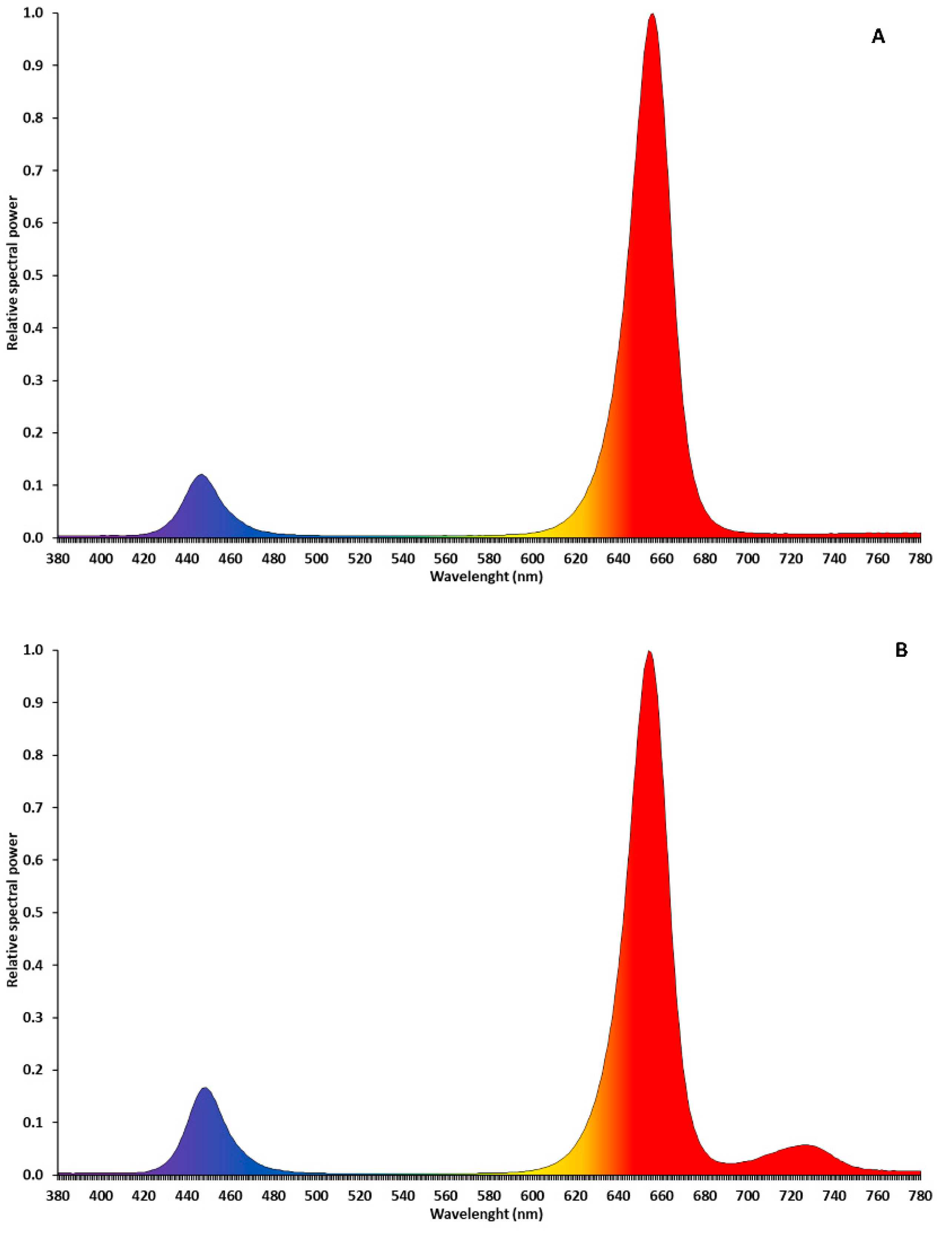



| Gallic Acid | 3,4-dihydroxybenzoic Acid | Methyl Gallate | p-Coumaric Acid | Chlorogenic Acid | Rutin | Total | |
|---|---|---|---|---|---|---|---|
| µg·g−1 DW | |||||||
| Day after transplant (DAT) | |||||||
| 32 | 12.1 ± 2.6 | 7.29 ± 0.99 | 35.0 ± 5.2 | 5.96 ± 0.78 | 5.81 ± 0.66 | 4.15 ± 0.50 | 70.7 ± 8.2 |
| 50 | 14.7 ± 2.8 | 8.69 ± 1.18 | 36.1 ± 5.5 | 5.63 ± 0.53 | 4.97 ± 0.67 | 3.41 ± 0.75 | 73.6 ± 6.7 |
| Light treatment | |||||||
| Control (NL) | 15.3 ± 2.5 | 8.00 ± 0.96 | 37.4 ± 3.4 | 5.59 ± 0.82 | 5.44 ± 1.16 | 3.47 ± 0.87 | 75.2 ± 6.0 |
| R + B + FR | 11.8 ± 2.9 | 8.13 ± 2.01 | 37.0 ± 6.7 | 5.49 ± 0.32 | 5.08 ± 0.52 | 3.62 ± 0.63 | 71.7 ± 9.9 |
| R + B | 13.2 ± 2.7 | 7.83 ± 0.73 | 32.4 ± 4.2 | 6.30 ± 0.55 | 5.65 ± 0.50 | 4.23 ±0.48 | 69.6 ± 5.8 |
| Significance 1 | |||||||
| DAT | ns | ns | ns | ns | ** | ns | ns |
| NL vs. LEDs | ns | ns | ns | ns | ns | ns | ns |
| R + B vs. R + B + FR | ns | ns | ns | ns | ns | ns | ns |
| Date * (NL vs. LEDs) 2 | * | ns | ns | ns | ns | ns | ns |
| Date * (R + B vs. R + B + FR)2 | ns | ns | ns | ns | ns | ns | ns |
| Tocopherols | Chlorophylls | |||
|---|---|---|---|---|
| α-T | β-T | Total | Chl a + b | |
| µg·g−1 DW | µg·g−1 DW | |||
| Day after transplant (DAT) | ||||
| 32 | 7.7 ± 1.4 | 2.66 ± 1.05 | 10.4 ± 2.1 | 54 ± 12 |
| 50 | 9.0 ± 2.1 | 4.40 ± 1.37 | 13.4 ± 2.0 | 74 ± 22 |
| Light treatment | ||||
| Control (NL) | 10.1 ± 1.4 | 3.16 ± 1.36 | 13.3 ± 2.4 | 42 ± 6 |
| R + B + FR | 7.4 ± 1.4 | 3.97 ± 1.11 | 11.4 ± 1.9 | 78 ± 12 |
| R + B | 7.5 ± 1.4 | 3.46 ± 2.01 | 10.9 ± 3.0 | 72 ± 22 |
| Significance 1 | ||||
| DAT | * | ** | * | ns |
| NL vs. LEDs | * | ns | ns | ** |
| R + B vs. R + B + FR | ns | ns | ns | ns |
| Date * (NL vs. LEDs) | ns | ns | ns | ns |
| Date * (R + B vs. R + B + FR) | ns | ns | ns | ns |
| Violaxanthin | Antheraxanthin | Lutein | Zeaxanthin | β-cryptoxanthin | β-carotene | 9 cis-β-carotene | Total | |
|---|---|---|---|---|---|---|---|---|
| µg·g−1 DW | ||||||||
| Day after transplant (DAT) | ||||||||
| 32 | 2.84 ± 0.33 | 19.8 ± 4.5 | 13.3 ± 4.0 | 2.00 ± 3.01 | 6.50 ± 1.02 | 5.31 ± 0.89 | 3.37 ± 0.39 | 53 ± 10 |
| 50 | 3.49 ± 0.38 | 27.8 ± 8.8 | 17.7 ± 4.5 | 2.71 ± 3.86 | 6.93 ± 0.86 | 8.07 ± 1.12 | 3.50 ± 0.44 | 70 ± 14 |
| Light treatment | ||||||||
| Control (NL) | 3.35 ± 0.22 | 25.3 ± 6.3 | 15.4 ± 3.0 | 6.74 ± 2.05 | 7.47 ± 0.45 | 3.23 ± 0.74 | 3.67 ± 0.53 | 65 ± 5 |
| R + B + FR | 3.21 ± 0.65 | 27.3 ± 3.1 | 18.3 ± 6.5 | 0.20 ± 0.05 | 5.94 ± 0.76 | 9.29 ± 1.89 | 3.13 ± 0.61 | 67 ± 11 |
| R + B | 2.93 ± 0.46 | 18.8 ± 10.9 | 12.9 ± 2.3 | 0.13 ± 0.06 | 6.73 ± 0.91 | 8.15 ± 1.44 | 3.32 ± 0.65 | 53 ± 6 |
| Significance 1 | ||||||||
| DAT | ns | ns | ns | ns | ns | ns | ns | ns |
| NL vs. LEDs | ns | ns | ns | *** | ns | * | ns | ns |
| R + B vs. R + B + FR | ns | * | * | ns | ns | ns | ns | ns |
| Date * (NL vs. LEDs) 2 | ns | ns | ns | ns | ns | * | ns | ns |
| Date * (R + B vs. R + B + FR) 2 | ns | ns | ns | ns | ns | * | ns | ns |
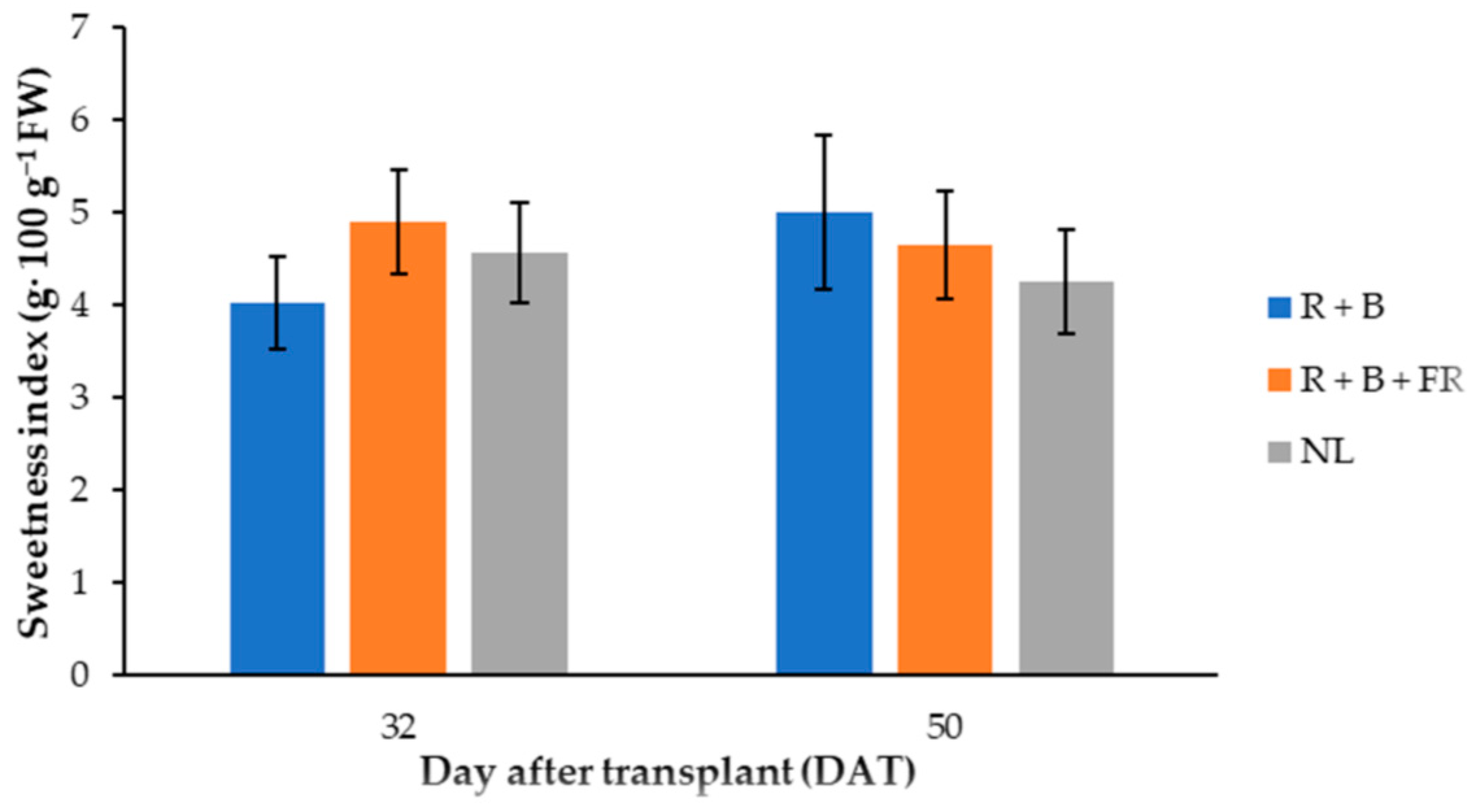
| Glucose | Fructose | Total Sugars 3 | Sweetness Index | |
|---|---|---|---|---|
| mg·g−1 DW | g·100 g−1 FW | |||
| Day after transplant (DAT) | ||||
| 32 | 215 ± 19 | 313 ± 29 | 528 ± 42 | 4.61 ± 0.22 |
| 50 | 221 ± 22 | 274 ±27 | 495 ± 43 | 4.76 ± 0.34 |
| Light treatment | ||||
| Control (NL) | 205 ± 13 | 281 ± 18 | 487 ± 31 | 4.53 ± 0.24 |
| R + B + FR | 219 ± 16 | 310 ± 18 | 529 ± 29 | 4.89 ± 0.34 |
| R + B | 229 ± 14 | 290 ± 53 | 519 ± 62 | 4.63 ± 0.20 |
| Significance 1 | ||||
| DAT | ns | * | ns | ns |
| NL vs. LEDs | * | ns | * | ns |
| R + B vs. R + B + FR | ns | ns | ns | ns |
| Date * (NL vs. LEDs) 2 | ns | ns | ns | * |
| Date * (R + B vs. R + B + FR) 2 | ns | ns | ns | ** |
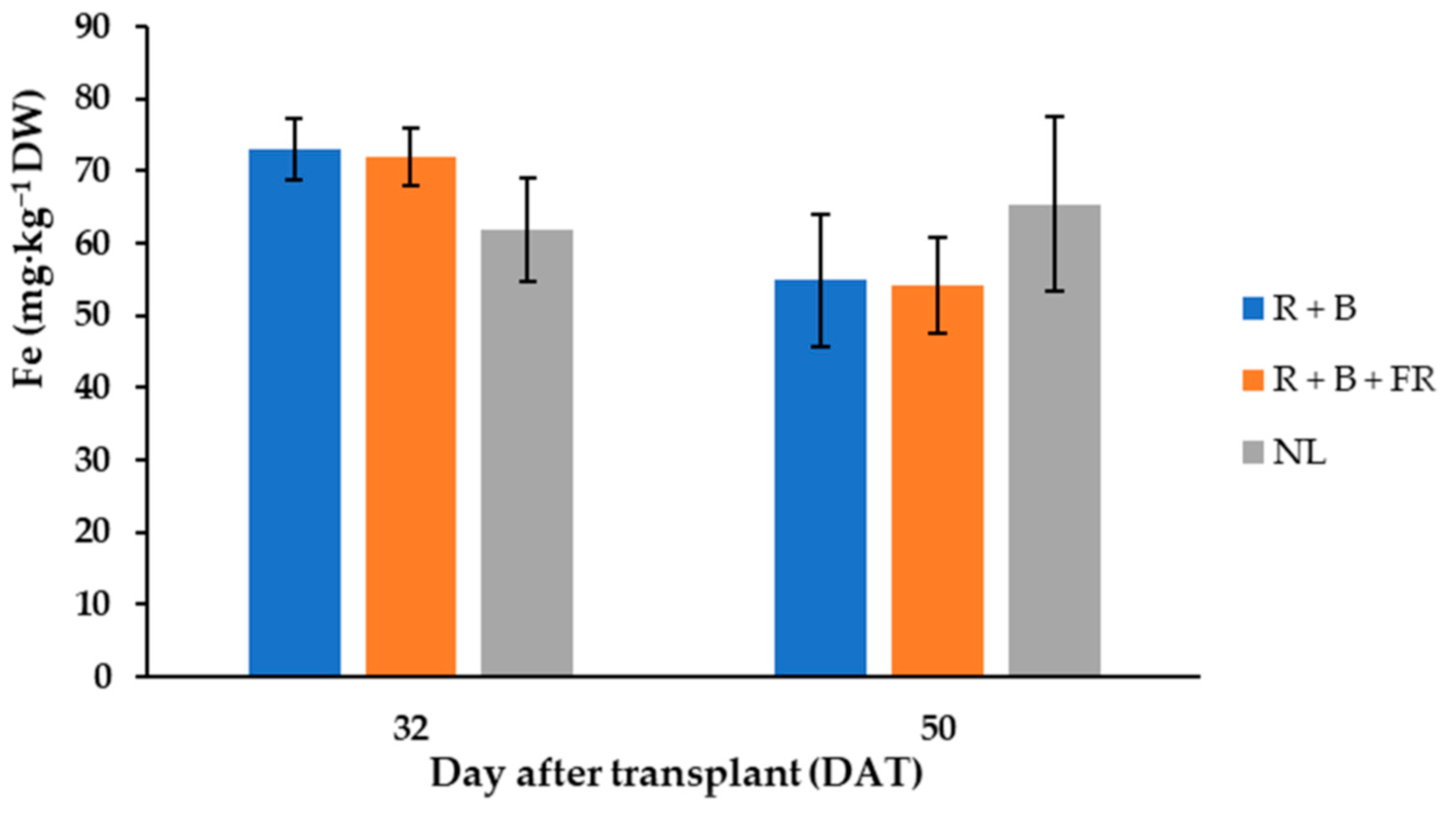
| Ca2+ | K+ | P5+ | Mg2+ | Na+ | Fe2+ | B3+ | Mn2+ | Ni2+ | Zn2+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| mg·g−1 DW | mg·kg−1 DW | |||||||||
| Day after transplant (DAT) | ||||||||||
| 32 | 8.47 ± 1.02 | 71.0 ± 6.0 | 13.06 ± 1.96 | 4.21 ± 0.54 | 1.18 ± 0.23 | 67.7 ± 7.1 | 22.7 ± 2.6 | 22.0 ± 3.2 | 1.09 ± 0.38 | 52.4 ± 7.7 |
| 50 | 6.21 ± 0.85 | 55.9 ± 5.9 | 9.61 ± 1.00 | 2.83 ± 0.39 | 1.28 ± 0.32 | 58.1 ± 9.8 | 22.8 ± 4.4 | 20.2 ± 3.1 | 1.11 ± 0.44 | 44.0 ± 7.9 |
| Light treatment | ||||||||||
| Control (NL) | 6.89 ± 0.69 | 65.8 ± 7.8 | 12.36 ± 3.02 | 3.45 ± 1.12 | 1.08 ± 0.08 | 63.7 ± 9.1 | 21.8 ± 3.6 | 22.2 ± 3.9 | 1.17 ± 0.31 | 49.8 ± 5.1 |
| R + B + FR | 7.24 ± 0.45 | 60.9 ± 6.7 | 11.50 ± 1.78 | 3.55 ± 0.53 | 1.54 ± 0.22 | 61.1 ± 9.6 | 24.3 ± 3.6 | 21.7 ± 3.8 | 0.80 ± 0.30 | 45.3 ± 7.6 |
| R + B | 7.90 ± 1.18 | 63.8 ± 6.8 | 10.14 ± 1.78 | 3.51 ± 0.92 | 1.08 ± 0.16 | 63.9 ± 9.8 | 22.2 ± 3.4 | 19.3 ± 3.4 | 1.33 ± 0.44 | 49.5 ± 7.4 |
| Significance 1 | ||||||||||
| DAT | * | * | * | ** | ns | * | ns | ns | ns | ns |
| NL vs. LEDs | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| R + B vs. R + B + FR | ns | ns | ns | ns | * | ns | ns | ns | * | ns |
| Date * (NL vs. LEDs) 2 | ns | ns | ns | ns | ns | * | ns | ns | ns | ns |
| Date * (R + B vs. R + B + FR) 2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmitessa, O.D.; Durante, M.; Somma, A.; Mita, G.; D’Imperio, M.; Serio, F.; Santamaria, P. Nutraceutical Profile of “Carosello” (Cucumis melo L.) Grown in an Out-of-Season Cycle under LEDs. Antioxidants 2022, 11, 777. https://doi.org/10.3390/antiox11040777
Palmitessa OD, Durante M, Somma A, Mita G, D’Imperio M, Serio F, Santamaria P. Nutraceutical Profile of “Carosello” (Cucumis melo L.) Grown in an Out-of-Season Cycle under LEDs. Antioxidants. 2022; 11(4):777. https://doi.org/10.3390/antiox11040777
Chicago/Turabian StylePalmitessa, Onofrio Davide, Miriana Durante, Annalisa Somma, Giovanni Mita, Massimiliano D’Imperio, Francesco Serio, and Pietro Santamaria. 2022. "Nutraceutical Profile of “Carosello” (Cucumis melo L.) Grown in an Out-of-Season Cycle under LEDs" Antioxidants 11, no. 4: 777. https://doi.org/10.3390/antiox11040777
APA StylePalmitessa, O. D., Durante, M., Somma, A., Mita, G., D’Imperio, M., Serio, F., & Santamaria, P. (2022). Nutraceutical Profile of “Carosello” (Cucumis melo L.) Grown in an Out-of-Season Cycle under LEDs. Antioxidants, 11(4), 777. https://doi.org/10.3390/antiox11040777










