Creeping Wood Sorrel and Chromium Picolinate Effect on the Nutritional Composition and Lipid Oxidative Stability of Broiler Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds and Experimental Design
2.2. Meat Samples Collection
2.3. Extraction Technique for Total Phenol Content (TPC) and Total Antioxidant Capacity (TAC) Analysis of Samples
2.4. Extraction Technique for Liposoluble Compounds Analysis of Samples
2.5. Chemical Composition Analysis
(Σ n − 3/Σ n − 6)]
C22:6)/(C14:0 + C16:0)]
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of Creeping Wood Sorrel (CWS)
3.2. Proximate Composition of Meat
3.3. Fatty Acid Profile of Broiler Meat
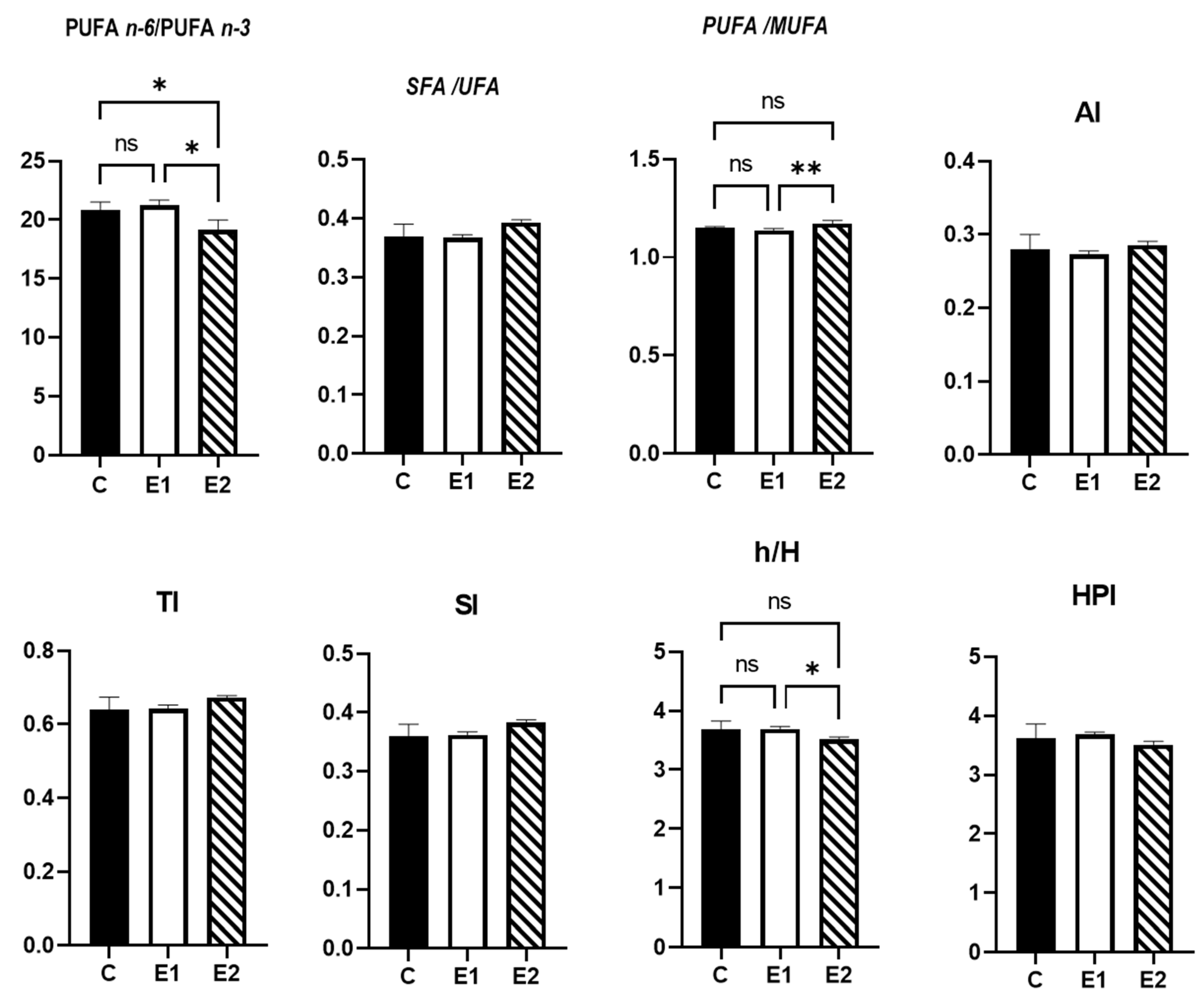
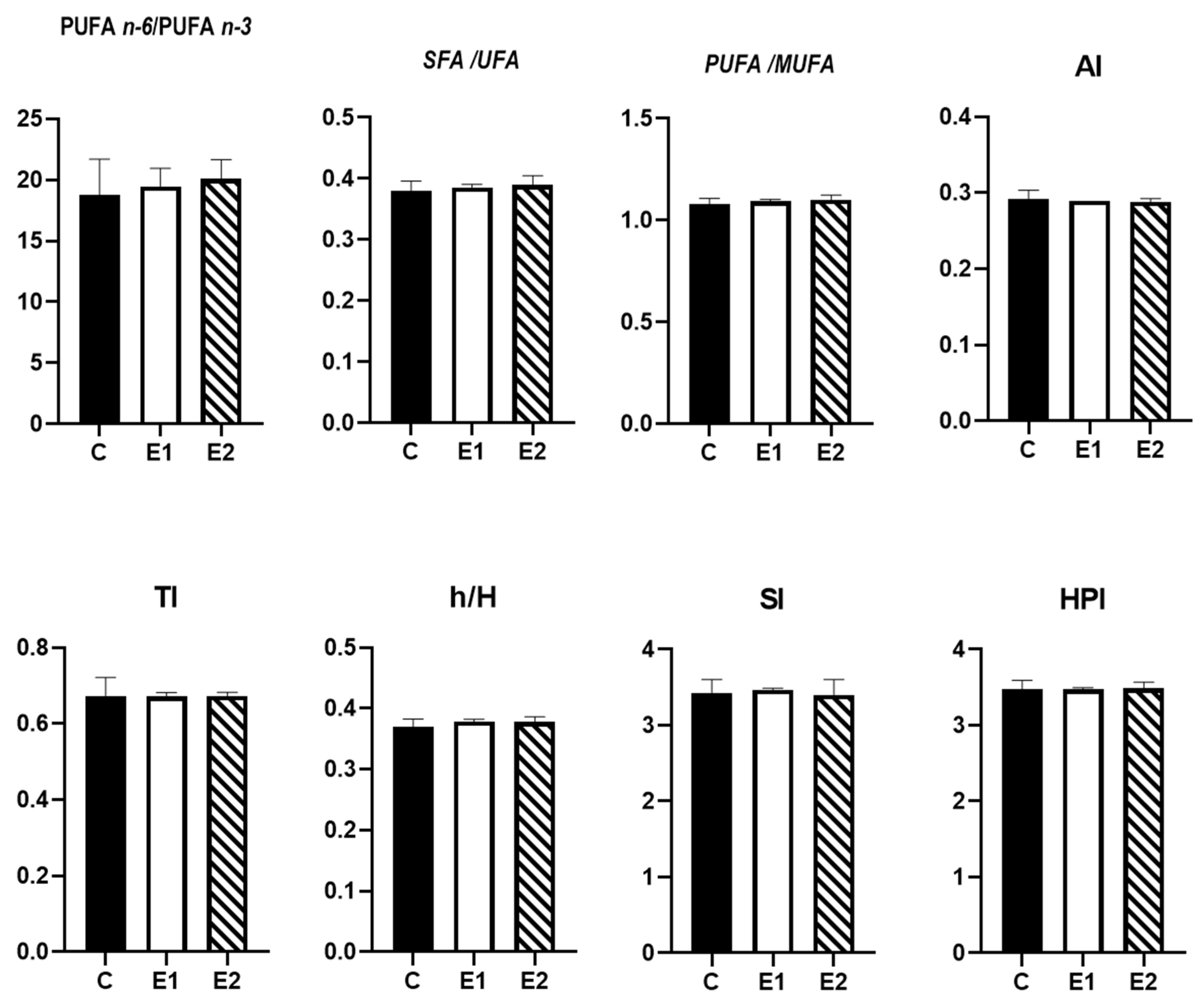
3.4. Nutritional Quality Indices of Meat Lipids
3.5. Bioactive Nutrient Content of Breast and Thigh Meat
3.6. Oxidative Stability of Broiler Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrell, D. The Role of Poultry in Human Nutrition. In Poultry Development Review; FAO: Rome, Italy, 2013; ISBN 978-92-5-108067-2. [Google Scholar]
- Van Hoeck, V.; Sonawane, M.; Gonzalez Sanchez, A.L.; Van Dosselaer, I.; Buyens, C.; Morisset, D. Chromium propionate improves performance and carcass traits in broilers. Anim. Nutr. 2020, 6, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Saracila, M.; Panaite, T.D.; Mironeasa, S.; Untea, A.E. Dietary supplementation of some antioxidants as attenuators of heat stress on chicken meat characteristics. Agriculture 2021, 11, 638. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Turcu, R.P.; Panaite, T.D.; Saracila, M. The use of dietary chromium associated with vitamins and minerals (synthetic and natural source) to improve some quality aspects of broiler thigh meat reared under heat stress condition. Ital. J. Anim. Sci. 2021, 20, 1491–1499. [Google Scholar] [CrossRef]
- Khan, R.U.; Shabana, N.; Kuldeep, D. Chromium: Pharmacological applications in heat-stressed poultry. Int. J. Pharmacol. 2014, 10, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Saracila, M.; Panaite, T.D.; Tabuc, C.; Soica, C.; Untea, A.; Varzaru, I.; Criste, R.D. Maintaining intestinal microflora balance in heat-stressed broilers using dietary creeping wood sorrel (Oxalis corniculata) powder and chromium (chromium picolinate). Span. J. Agric. Res. 2020, 18, 19. [Google Scholar] [CrossRef]
- Raghavendra, M.P.; Satish, S.; Raveesha, K.A. Phytochemical analysis and antibacterialactivity of Oxalis corniculata; a known medicinalplant. Myscience 2006, 1, 72–78. [Google Scholar]
- Karimzadeh, K.; Bakhshi, N.; Ramzanpoor, M. Biogenic silver nanoparticles using Oxalis corniculata characterization and their clinical implications. J. Drug Deliv. Sci. Technol. 2019, 54, 101263. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Van, I. Distribution of nutrients with benefic potential for the eyes in several medicinal plants. Rom. Biotechnol. Lett. 2015, 20, 10773–10783. [Google Scholar]
- Untea, A.; Lupu, A.; Saracila, M.; Panaite, T. Comparison of ABTS, DPPH, phosphomolybdenum assays for estimating antioxidant activity and phenolic compounds in five different plant extracts. Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Untea, A.E.; Criste, R.C.; Vladescu, L. Development and validation of a microwave digestion–FAAS procedure for Cu, Mn and Zn determination in liver. Rev. Chim 2012, 63, 341–346. [Google Scholar]
- AOAC. International Official Method; AOAC International: Arlington, TX, USA, 1996. [Google Scholar]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The effects of dietary inclusion of bilberry and walnut leaves in laying hens’ diets on the antioxidant properties of eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panaite, T.D.; Nour, V.; Saracila, M.; Turcu, R.P.; Untea, A.E.; Vlaicu, P.A. Effects of linseed meal and carotenoids from different sources on egg characteristics, yolk fatty acid and carotenoid profile and lipid peroxidation. Foods 2021, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Król, B.; Słupczyńska, M.; Kinal, S.; Bodarski, R.; Tronina, W.; Mońka, M. Bioavailability of organic and inorganic sources of chromium in broiler chicken feeds. J. Elem. 2017, 22, 283–294. [Google Scholar]
- Untea, A.E.; Panaite, T.D.; Dragomir, C.; Ropota, M.; Olteanu, M.; Varzaru, I. Effect of dietary chromium supplementation on meat nutritional quality and antioxidant status from broilers fed with Camelina-meal-supplemented diets. Animal 2019, 13, 2939–2947. [Google Scholar] [CrossRef]
- Haq, Z.; Jain, R.K.; Khan, N.; Dar, M.Y.; Ali, S.; Gupta, M.; Varun, T.K. Recent advances in role of chromium and its antioxidant combinations in poultry nutrition: A review. Vet. World 2016, 9, 1392. [Google Scholar] [CrossRef]
- Toghyani, M.; Khodami, A.; Gheisari, A.A. Effect of organic and inorganic chromium supplementation on meat quality of heat-stressed broiler chicks. Am. J. Anim. Vet. Sci. 2008, 3, 62–67. [Google Scholar] [CrossRef]
- Chen, G.; Gao, Z.; Chu, W.; Cao, Z.; Li, C.; Zhao, H. Effects of chromium picolinate on fat deposition, activity and genetic expression of lipid metabolism-related enzymes in 21-day old Ross broilers. Asian Australas. J. Anim. Sci. 2018, 31, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Paszkiewicz, W.; Muszyński, S.; Kwiecień, M.; Zhyla, M.; Świątkiewicz, S.; Arczewska-Włosek, A.; Tomaszewska, E. Effect of soybean meal substitution by raw chickpea seeds on thermal properties and fatty acid composition of subcutaneous fat tissue of broiler chickens. Animals 2020, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Chen, Y.; Li, J.; Dong, Y.; Miao, Z.; Li, J.; Zhang, L. Effects of organic chromium sources on growth performance, lipid metabolism, antioxidant status, breast amino acid and fatty acid profiles in broilers. J. Sci. Food Agric. 2021, 101, 3917–3926. [Google Scholar] [CrossRef]
- Kalakuntla, S.; Nagireddy, N.K.; Panda, A.K.; Jatoth, N.; Thirunahari, R.; Vangoor, R.R. Effect of dietary incorporation of n-3 polyunsaturated fatty acids rich oil sources on fatty acid profile, keeping quality and sensory attributes of broiler chicken meat. Anim. Nutr. 2017, 3, 386–391. [Google Scholar] [CrossRef]
- El-Samee, A.; Laila, D.; El-Wardany, I.; Abdel-Fattah, S.A.; El-Azeem, A.; Nafisa, A.; Elsharkawy, M.S. Dietary omega-3 and antioxidants improve long-chain omega-3 and lipid oxidation of broiler meat. Bull. Natl. Res. Cent. 2019, 43, 45. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect ofomega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinica 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.C.; Stark, K.D. Direct determinations of the fatty acid composition of daily dietary intakes incorporating nutraceuticals and functional food strategies to increase n-3 highly unsaturated fatty acids. J. Am. Coll. Nutr. 2008, 27, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Voljč, M.; Levart, A.; Žgur, S.; Salobir, J. The effect of α-tocopherol, sweet chestnut wood extract and their combination on oxidative stress in vivo and the oxidative stability of meat in broilers. Br. Poult. Sci. 2013, 54, 144–156. [Google Scholar] [CrossRef]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Gong, L.M.; Xue, J.X. Effects of graded levels of chromium methionine on performance, carcass traits, meat quality, fatty acid profiles of fat, tissue chromium concentrations, and antioxidant status in growing-finishing pigs. Biol. Trace Elem. Res. 2015, 168, 110–121. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive compounds in functional meat products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The effect of lutein/zeaxanthin intake on human macular pigment optical density: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef]
- Carreras, I.; Guerrero, L.; Guàrdia, M.D.; Esteve-Garcia, E.; García Regueiro, J.A.; Sárraga, C. Vitamin E levels, thiobarbituric acid test and sensory evaluation of breast muscles from broilers fed α-tocopheryl acetate- and β-carotene- supplemented diets. J. Sci. Food Agric. 2004, 84, 313–317. [Google Scholar] [CrossRef]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Saeed, A.A.; Sandhu, M.A.; Khilji, M.S.; Yousaf, M.S.; Rehman, H.U.; Tanvir, Z.I.; Ahmad, T. Effects of dietary chromium supplementation on muscle and bone mineral interaction in broiler chicken. J. Trace Elem. Med. Biol. 2017, 42, 25–29. [Google Scholar] [CrossRef]
- Tufft, L.; Nockels, C. The effects of stress, Escherichia coli, dietary ethylenediaminetetraacetic acid, and their interaction on tissue trace elements in chicks. Poult. Sci. 1991, 70, 2439–2449. [Google Scholar] [CrossRef]
- Pechova, A.; Pavlata, L. Chromium as an essential nutrient: A review. Vet. Med. 2007, 52, 1. [Google Scholar] [CrossRef] [Green Version]
- Aziza, A.E.; Quezada, N.; Cherian, G. Antioxidative effect of dietary Camelina meal in fresh, stored, or cooked broiler chicken meat. Poult. Sci. 2010, 89, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Toghyani, M.; Toghyani, M.; Shivazad, M. Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents. Biol. Trace Elem. Res. 2012, 146, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Tyagi, P.K.; Biswas, A.K.; Tyagi, P.K.; Mandal, A.B.; Sheikh, S.A.; Deo, C.; Sharma, D.; Verma, A.K. Impact of feeding chromium supplemented flaxseed based diet on fatty acid profile, oxidative stability and other functional properties of broiler chicken meat. J. Food Sci. Technol. 2017, 54, 3899–3907. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Englmaierová, M.; Bubancová, I.; Vít, T.; Skrivan, M. The effect of lycopene and vitamin E on growth performance, quality and oxidative stability of chicken leg meat. Czech J. Anim. Sci. 2011, 56, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Carrier, J.; Aghdassi, E.; Cullen, J.; Allard, J.P. Iron supplementation increases disease activity and vitamin e ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J. Nutr. 2002, 132, 3146–3150. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.Z.; Wang, M.; Li, Z.T.; Li, X.J.; Chen, J.F.; Yin, Q. Study on the correlations between mineral contents in musculus longissimus dorsi and meat quality for five breeds of pigs. Am. J. Anim. Vet. Sci. 2008, 3, 18–22. [Google Scholar]


| Ingredient | Grower (14–28 d) | Finisher (28–42 d) | ||||
|---|---|---|---|---|---|---|
| C | E1 | E2 | C | E1 | E2 | |
| % | ||||||
| Corn | 40.18 | 40.18 | 39.28 | 44.70 | 44.70 | 43.70 |
| Soybean meal | 26.33 | 26.33 | 26.20 | 21.32 | 21.32 | 21.32 |
| Wheat | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Corn gluten | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Creeping wood sorrel (CWS) | - | - | 1.00 | - | - | 1.00 |
| Oil | 3.78 | 3.78 | 3.80 | 4.62 | 4.62 | 4.62 |
| Monocalcium phosphate | 1.36 | 1.36 | 1.36 | 1.19 | 1.19 | 1.19 |
| Calcium carbonate | 1.25 | 1.25 | 1.24 | 1.13 | 1.13 | 1.13 |
| Salt | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| Methionine | 0.30 | 0.30 | 0.3 | 0.26 | 0.26 | 0.26 |
| Lysine | 0.30 | 0.30 | 0.31 | 0.30 | 0.30 | 0.30 |
| Treonine | 0.09 | 0.09 | 0.10 | 0.07 | 0.07 | 0.07 |
| Choline | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| A1 Premix | 1.00 1 | 1.00 2 | 1.00 3 | 1.00 1 | 1.00 2 | 1.00 3 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Chemical analysis—theoretical | ||||||
| Metabolisabe energy, Kcal/kg | 3128.99 | 3217.72 | ||||
| Crude protein, % | 21.50 | 20.00 | ||||
| Ether extract, % | 6.01 | 6.49 | ||||
| Crude fiber, % | 3.57 | 3.36 | ||||
| Ca., % | 0.87 | 0.81 | ||||
| P, % | 0.70 | 0.65 | ||||
| Available phosphorus., % | 0.43 | 0.41 | ||||
| Antioxidant profile—analysed | ||||||
| TPC, mg/g GAE | 1.70 | 1.75 | 1.99 | 1.83 | 1.84 | 1.97 |
| TAC, mmol Trolox equivalents/kg | 5.70 | 6.08 | 6.72 | 6.00 | 6.27 | 7.12 |
| Lutein and zeaxanthin, µg/g | 10.11 | 10.02 | 10.43 | 9.90 | 9.14 | 9.47 |
| Vitamin E, µg/g | 40.13 | 37.92 | 40.01 | 40.45 | 38.19 | 39.92 |
| Analysed Parameters | Creeping Wood Sorrel (CWS) |
|---|---|
| Proximate composition (%) | |
| DM | 92.18 |
| CP | 16.74 |
| EE | 3.18 |
| CF | 19.36 |
| Ash | 22.56 |
| Fatty acids (g/100 g FAME) | |
| C18:3n3 | 0.98 |
| SFA | 36.35 |
| MUFA | 43.54 |
| PUFA | 19.55 |
| UFA | 63.09 |
| Σ n − 3 | 2.98 |
| Σ n − 6 | 15.73 |
| Σ n − 6/n − 3 | 5.28 |
| Antioxidant profile | |
| TPC, mg/g GAE | 7.87 |
| TAC, µM Trolox | 170.70 |
| Lutein and zeaxanthin, µg/g | 267.41 |
| Vitamin E, µg/g | 210.93 |
| Mineral profile (mg/kg) | |
| Copper | 6.40 |
| Iron | 243.68 |
| Manganese | 41.00 |
| Zinc | 92.10 |
| Specification | C | E1 | E2 | SEM | p-Value |
|---|---|---|---|---|---|
| Breast | |||||
| DM, % | 25.33 | 25.71 | 25.36 | 0.214 | 0.743 |
| CP, % | 22.79 | 23.18 | 22.98 | 0.192 | 0.801 |
| EE, % | 1.47 a | 1.51 a | 1.32 b | 0.024 | <0.0001 |
| Ash, % | 1.05 | 1.07 | 1.05 | 0.010 | 0.820 |
| Thigh | |||||
| DM, % | 23.48 | 23.90 | 24.02 | 0.266 | 0.710 |
| CP, % | 18.85 | 19.22 | 18.96 | 0.206 | 0.778 |
| EE, % | 3.74 | 3.71 | 3.96 | 0.059 | 0.0515 |
| Ash, % | 0.86 | 0.89 | 0.90 | 0.012 | 0.364 |
| Fatty Acids (g/100 g Fatty Acids Methyl Esters) | C | E1 | E2 | SEM | p-Value |
|---|---|---|---|---|---|
| C14:0 | 0.61 | 0.43 | 0.45 | 0.047 | 0.251 |
| C15:0 | 0.14 a | 0.11 b | 0.11 a,b | 0.005 | 0.027 |
| C16:0 | 18.15 b | 18.08 b | 18.65 a | 0.091 | 0.008 |
| C17:0 | 0.21 a | 0.21 a | 0.18 b | 0.004 | <0.0001 |
| C18:0 | 7.57 b | 7.76 a,b | 8.30 a | 0.134 | 0.047 |
| C24:0 | 0.26 a | 0.22 b | 0.26 a | 0.006 | 0.008 |
| Σ SFA | 26.88 | 26.84 | 27.98 | 0.244 | 0.084 |
| C14:1 | 0.08 a | 0.07 a,b | 0.05 b | 0.005 | 0.046 |
| C15:1 | 0.56 | 0.64 | 0.74 | 0.041 | 0.202 |
| C16:1 | 2.53 a | 2.48 a | 2.17 b | 0.048 | <0.0001 |
| C17:1 | 0.13 b | 0.16 a,b | 0.20 a | 0.010 | 0.008 |
| C18:1 | 29.46 a | 29.63 a | 28.48 b | 0.175 | 0.004 |
| C22:1n9 | 0.09 a | 0.07 b | 0.10 a | 0.005 | 0.002 |
| C24:1n9 | 1.03 b | 1.08 b | 1.37 a | 0.047 | <0.0001 |
| Σ MUFA | 33.85 a | 34.11 a | 33.10 b | 0.161 | 0.009 |
| C18:2n6 | 32.42 a | 32.35 a | 31.31 b | 0.189 | 0.013 |
| C18:3n6 | 0.29 a | 0.30 a | 0.23 b | 0.010 | <0.0001 |
| C20:2n6 | 0.26 | 0.22 | 0.22 | 0.009 | 0.096 |
| C20:3n6 | 0.79 a | 0.70 b | 0.82 a | 0.017 | 0.002 |
| C20:4n6 | 2.64 b | 2.89 b | 3.43 a | 0.106 | <0.0001 |
| C22:2n6 | 0.16 b | 0.15 b | 0.22 a | 0.010 | 0.003 |
| C22:3n6 | 0.13 | 0.17 | 0.18 | 0.009 | 0.067 |
| C22:4n6 | 0.34 b | 0.30 b | 0.40 a | 0.012 | 0.001 |
| Σ n − 6 | 37.26 | 37.08 | 36.81 | 0.117 | 0.311 |
| C18:3n3 | 0.69 a | 0.59 b | 0.54 b | 0.023 | 0.006 |
| C18:4n3 | 0.19 c | 0.30 b | 0.38 a | 0.024 | <0.0001 |
| C20:3n3 | 0.50 b | 0.48 b | 0.57 a | 0.013 | 0.007 |
| C20:5n3 | 0.22 a | 0.17 b | 0.20 a,b | 0.007 | 0.005 |
| C22:5n3 | 0.11 | 0.12 | 0.13 | 0.004 | 0.063 |
| C22:6n3 | 0.06 a | 0.07 a,b | 0.10 b | 0.007 | 0.015 |
| Σ n − 3 | 1.79 b | 1.74 b | 1.93 a | 0.029 | 0.007 |
| Σ PUFA | 39.05 | 38.82 | 38.73 | 0.114 | 0.551 |
| Other fatty acids | 0.23 | 0.23 | 0.19 | 0.013 | 0.474 |
| Fatty Acids (g/100 g Fatty Acids Methyl Esters) | C | E1 | E2 | SEM | p-Value |
|---|---|---|---|---|---|
| C14:0 | 0.50 | 0.46 | 0.45 | 0.015 | 0.057 |
| C15:0 | 0.19 | 0.15 | 0.15 | 0.009 | 0.054 |
| C16:0 | 19.13 | 18.87 | 19.26 | 0.182 | 0.709 |
| C17:0 | 0.22 | 0.24 | 0.23 | 0.003 | 0.326 |
| C18:0 | 7.15 b | 7.96 a | 7.71 a | 0.096 | <0.0001 |
| C24:0 | 0.25 | 0.24 | 0.23 | 0.054 | 0.159 |
| Σ SFA | 27.44 | 27.69 | 27.92 | 0.152 | 0.473 |
| C14:1 | 0.10 | 0.08 | 0.08 | 0.004 | 0.180 |
| C15:1 | 0.91 | 0.83 | 0.70 | 0.045 | 0.159 |
| C16:1 | 3.33 a | 2.95 b | 2.94 b | 0.055 | <0.0001 |
| C17:1 | 0.16 | 0.13 | 0.17 | 0.014 | 0.496 |
| C18:1 | 29.59 | 29.42 | 29.33 | 0.084 | 0.444 |
| C22:1n9 | 0.07 | 0.06 | 0.05 | 0.006 | 0.277 |
| C24:1n9 | 0.59 b | 0.88 a | 0.81 a,b | 0.045 | 0.011 |
| Σ MUFA | 34.74 a | 34.36 a,b | 34.08 b | 0.099 | 0.012 |
| C18:2n6 | 31.27 | 31.16 | 31.30 | 0.067 | 0.676 |
| C18:3n6 | 0.21 | 0.23 | 0.20 | 0.006 | 0.097 |
| C20:2n6 | 0.28 a | 0.26 a,b | 0.18 b | 0.017 | 0.029 |
| C20:3n6 | 0.49 | 0.54 | 0.52 | 0.016 | 0.533 |
| C20:4n6 | 2.82 | 3.10 | 3.03 | 0.061 | 0.151 |
| C22:2n6 | 0.19 | 0.18 | 0.18 | 0.008 | 0.796 |
| C22:3n6 | 0.15 b | 0.54 a | 0.14 b | 0.046 | <0.0001 |
| C22:4n6 | 0.19 | 0.20 | 0.19 | 0.008 | 0.880 |
| Σ n − 6 | 35.61 | 35.75 | 35.74 | 0.101 | 0.834 |
| C18:3n3 | 0.53 a | 0.50 b | 0.51 b | 0.004 | 0.003 |
| C18:4n3 | 0.38 a | 0.32 a,b | 0.30 b | 0.013 | 0.038 |
| C20:3n3 | 0.34 | 0.37 | 0.35 | 0.023 | 0.929 |
| C20:5n3 | 0.44 | 0.51 | 0.45 | 0.035 | 0.723 |
| C22:5n3 | 0.12 | 0.12 | 0.10 | 0.005 | 0.571 |
| C22:6n3 | 0.08 | 0.05 | 0.06 | 0.005 | 0.094 |
| Σ n − 3 | 1.85 | 1.85 | 1.83 | 0.042 | 0.981 |
| Σ PUFA | 37.55 | 37.60 | 37.53 | 0.131 | 0.979 |
| Other fatty acids | 0.36 | 0.48 | 0.21 | 0.048 | 0.073 |
| Bioactive Nutrient | C | E1 | E2 | SEM | p-Value |
|---|---|---|---|---|---|
| Antioxidant profile | |||||
| TPC, mg/g GAE | 1.64 | 1.61 | 1.67 | 0.062 | 0.943 |
| TAC, µM Trolox | 1.81 | 1.99 | 1.98 | 0.044 | 0.177 |
| Lutein and zeaxanthin, µg/g | 2.73 b | 3.16 a,b | 3.64 a | 0.140 | 0.010 |
| Vitamin E, µg/g | 56.62 a | 47.56 b | 50.25 b | 1.352 | 0.003 |
| Mineral profile (mg/kg) | |||||
| Copper | nd | nd | nd | - | - |
| Iron | 28.60 a | 26.01 b | 25.03 b | 0.342 | 0.0001 |
| Manganese | nd | nd | nd | - | - |
| Zinc | 28.42 a | 26.18 b | 27.76 a | 0.329 | 0.003 |
| Bioactive Nutrient | C | E1 | E2 | SEM | p-Value |
|---|---|---|---|---|---|
| Antioxidant profile | |||||
| TPC, mg/g GAE | 1.20 | 1.23 | 1.29 | 0.023 | 0.284 |
| TAC, µM Trolox | 1.98 | 2.00 | 2.05 | 0.062 | 0.897 |
| Lutein and zeaxanthin, µg/g | 1.45 b | 1.69 a,b | 1.87 a | 0.068 | 0.027 |
| Vitamin E, µg/g | 161.11 a | 146.48 a,b | 138.79 b | 3.878 | 0.037 |
| Mineral profile (mg/kg) | |||||
| Copper | 2.31 | 2.04 | 2.07 | 0.047 | 0.0761 |
| Iron | 36.18 b | 38.14 a | 35.69 b | 0.298 | 0.0135 |
| Manganese | 0.38 | 0.58 | 0.36 | 0.034 | 0.1161 |
| Zinc | 69.96 b | 68.58 c | 73.35 a | 0.412 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saracila, M.; Untea, A.E.; Panaite, T.D.; Varzaru, I.; Oancea, A.; Turcu, R.P.; Vlaicu, P.A. Creeping Wood Sorrel and Chromium Picolinate Effect on the Nutritional Composition and Lipid Oxidative Stability of Broiler Meat. Antioxidants 2022, 11, 780. https://doi.org/10.3390/antiox11040780
Saracila M, Untea AE, Panaite TD, Varzaru I, Oancea A, Turcu RP, Vlaicu PA. Creeping Wood Sorrel and Chromium Picolinate Effect on the Nutritional Composition and Lipid Oxidative Stability of Broiler Meat. Antioxidants. 2022; 11(4):780. https://doi.org/10.3390/antiox11040780
Chicago/Turabian StyleSaracila, Mihaela, Arabela Elena Untea, Tatiana Dumitra Panaite, Iulia Varzaru, Alexandra Oancea, Raluca Paula Turcu, and Petru Alexandru Vlaicu. 2022. "Creeping Wood Sorrel and Chromium Picolinate Effect on the Nutritional Composition and Lipid Oxidative Stability of Broiler Meat" Antioxidants 11, no. 4: 780. https://doi.org/10.3390/antiox11040780
APA StyleSaracila, M., Untea, A. E., Panaite, T. D., Varzaru, I., Oancea, A., Turcu, R. P., & Vlaicu, P. A. (2022). Creeping Wood Sorrel and Chromium Picolinate Effect on the Nutritional Composition and Lipid Oxidative Stability of Broiler Meat. Antioxidants, 11(4), 780. https://doi.org/10.3390/antiox11040780













