Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents, Purification, and Instrumentation
2.2. Synthesis of 2,3-Dibromo-4-hydroxy-5-methoxybenzaldehyde (1c)
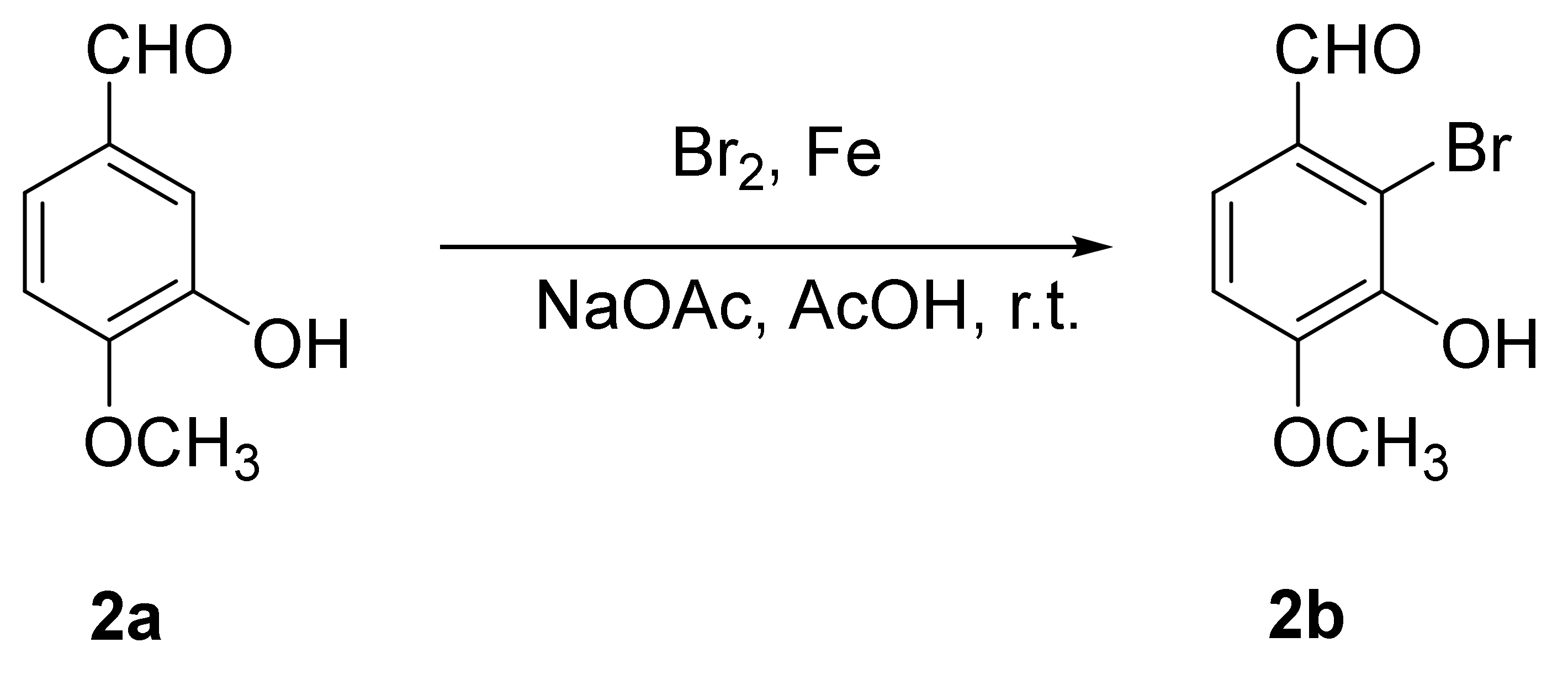
2.3. Synthesis of 2-Bromo-3-hydroxy-4-methoxybenzaldehyde (2b)
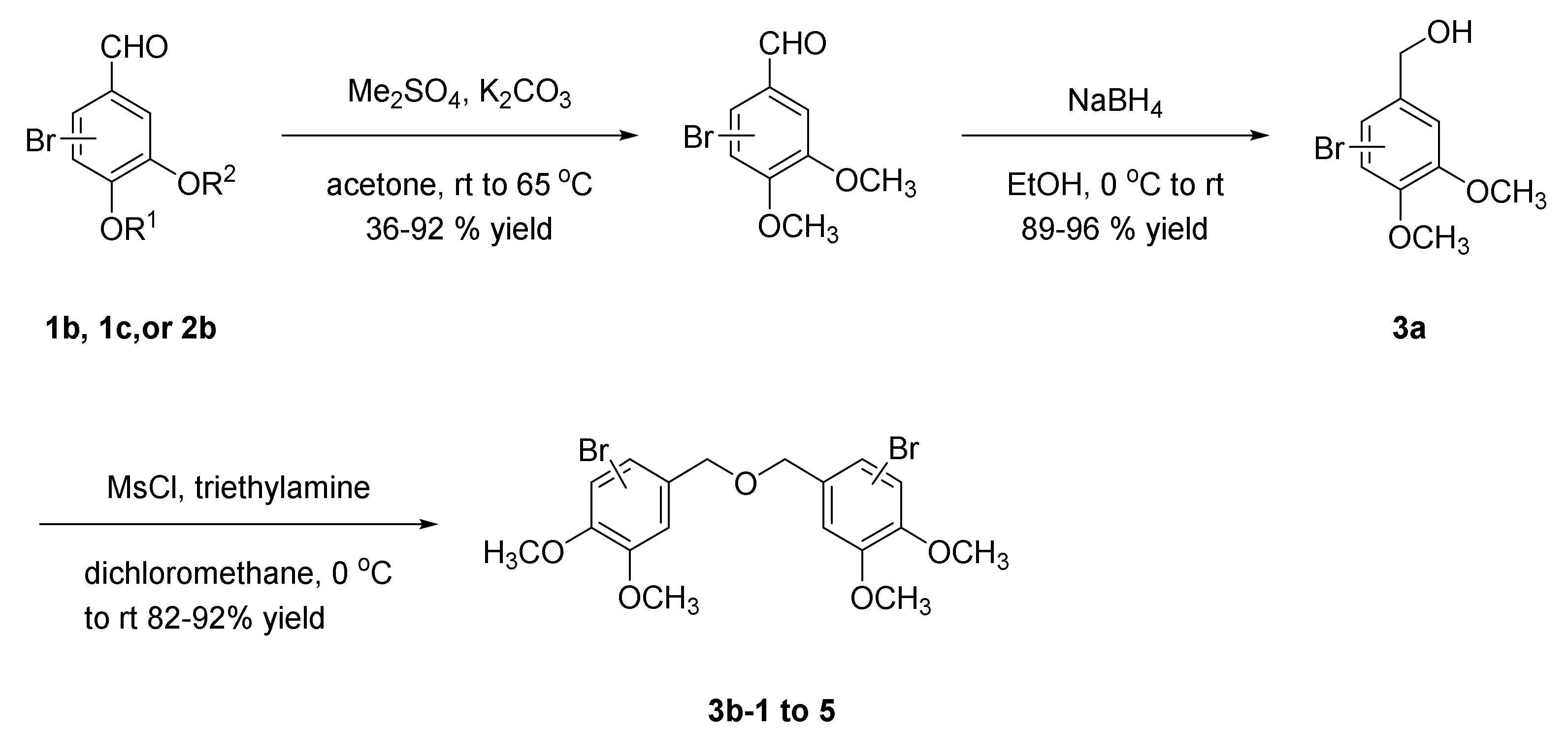
2.4. General Procedure of Methyl Bromophenol Derivatives
2.4.1. Bis(3,4-dimethoxybenzyl)ether (3b-1)

2.4.2. Bis(2,3-dibromo-4,5-dimethoxybenzene)ether (3b-2)
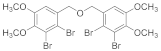
2.4.3. Bis(2-Bromo-4,5-dimethoxybenzene)ether (3b-3)
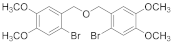
2.4.4. Bis(3-Bromo-4,5-dimethoxybenzene)ether (3b-4)
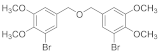
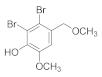
2.4.5. Bis(2,3,6-Tribromo-4,5-dihydroxybenzyl)ether (3b-5)
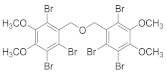
2.4.6. 2,3-Dibromo-1-(((3-bromo-4,5-dimethoxybenzyl)oxy)methyl)-4,5-dimethoxybenzene (3b-6)
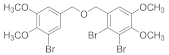
2.4.7. 1-Bromo-5-(((2-bromo-4,5-dimethoxybenzyl)oxy)methyl)-2,3-dimethoxybenzene (3b-7)
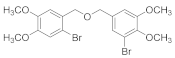
2.4.8. 1-Bromo-5-(((3,4-dimethoxybenzyl)oxy)methyl)-2,3-dimethoxybenzene (3b-8)
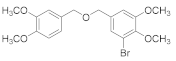
2.4.9. 2.,3-Dibromo-1-(((2-bromo-4,5-dimethoxybenzyl)oxy)methyl)-4,5-dimethoxybenzene (3b-9)
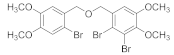
2.5. General Procedure of Acetylation of Bromophenol Derivatives
2.5.1. (Oxybis(methylene))bis(2-bromo-6-methoxy-3,1-phenylene) diacetate (4b-1)
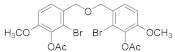
2.5.2. (Oxybis(methylene))bis(2,3-dibromo-6-methoxy-4,1-phenylene)diacetate (4b-2)
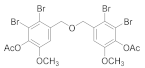
2.5.3. (Oxybis(methylene))bis(4-bromo-6-methoxy-3,1-phenylene) diacetate (4b-3)
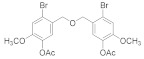
2.5.4. (Oxybis(methylene))bis(2-bromo-6-methoxy-4,1-phenylene) diacetate (4b-4)
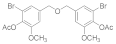
2.5.5. (Oxybis(methylene))bis(5-bromo-2-methoxy-4,1-phenylene) diacetate (4b-5)
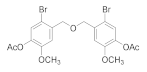
2.5.6. 2,3-Dibromo-6-methoxy-4-(methoxymethyl) phenol (4b-6)
2.6. Cell Lines and Cell Culture
2.7. Cell Viability Assessment
2.8. Intracellular ROS Assay
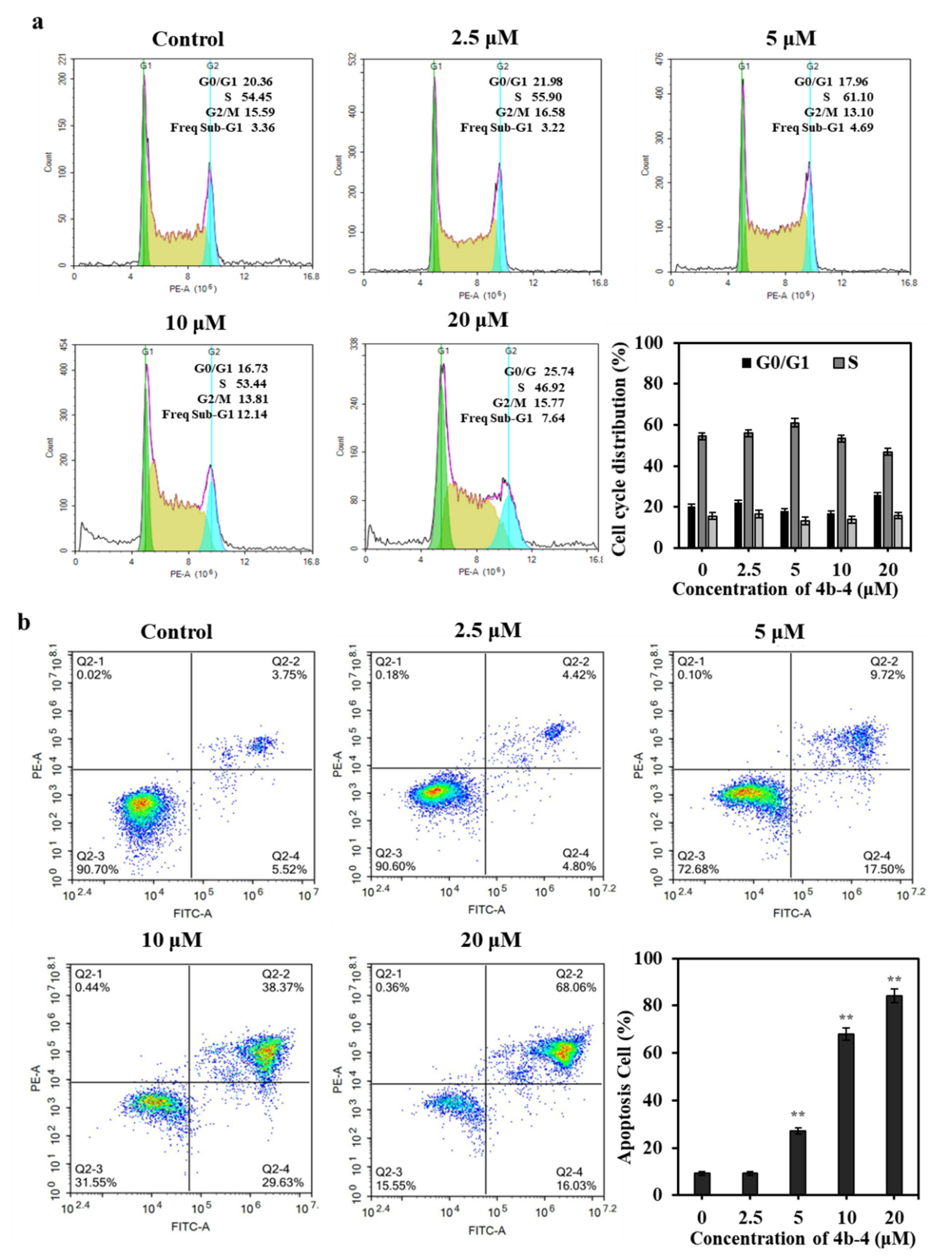
2.9. Cell Apoptosis Analysis
2.10. Cell Cycle Analysis
2.11. Western Blotting Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Chemical Synthesis
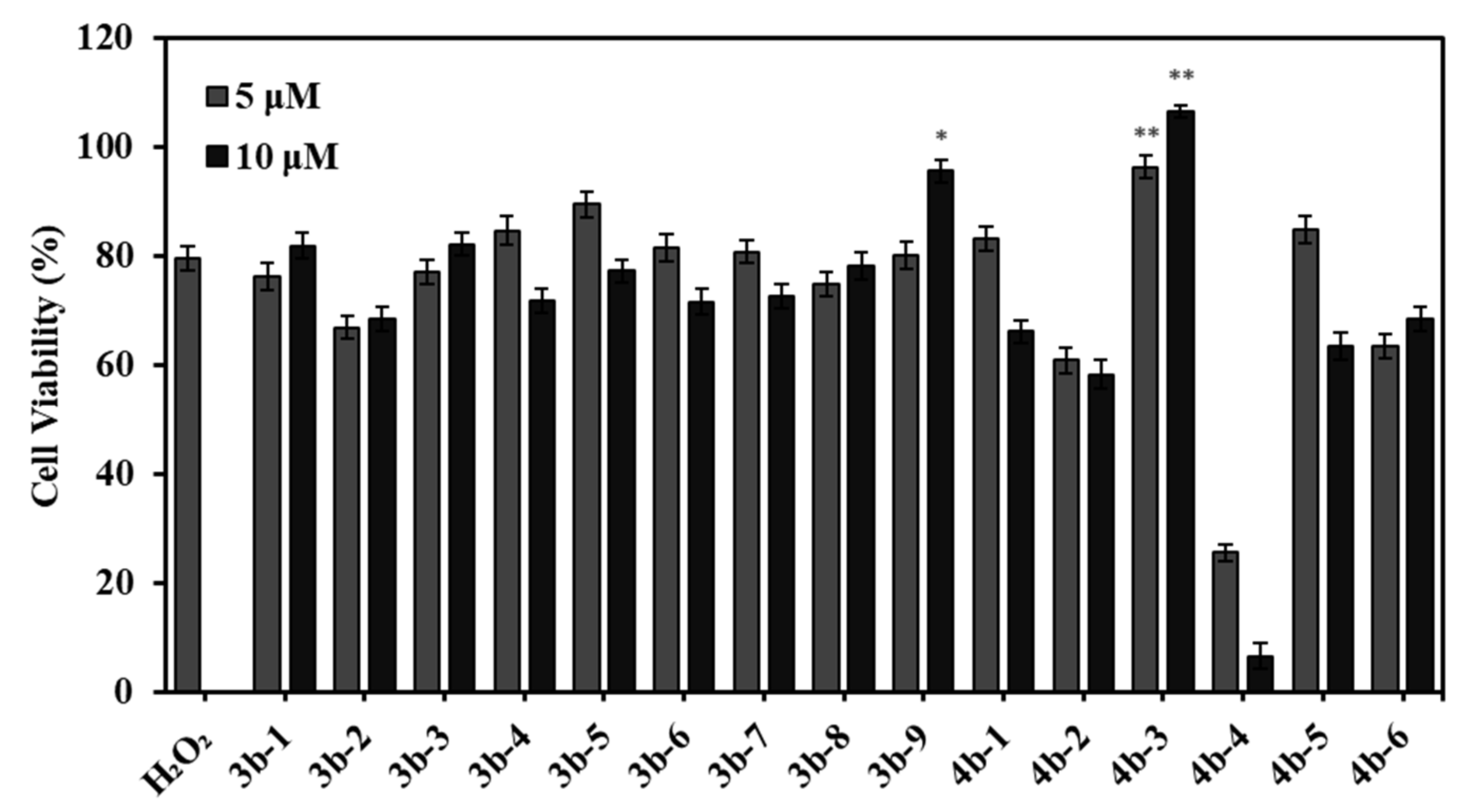
3.2. The Cellular Antioxidant Activity of 3b-1 to 9 and 4b-1 to 6
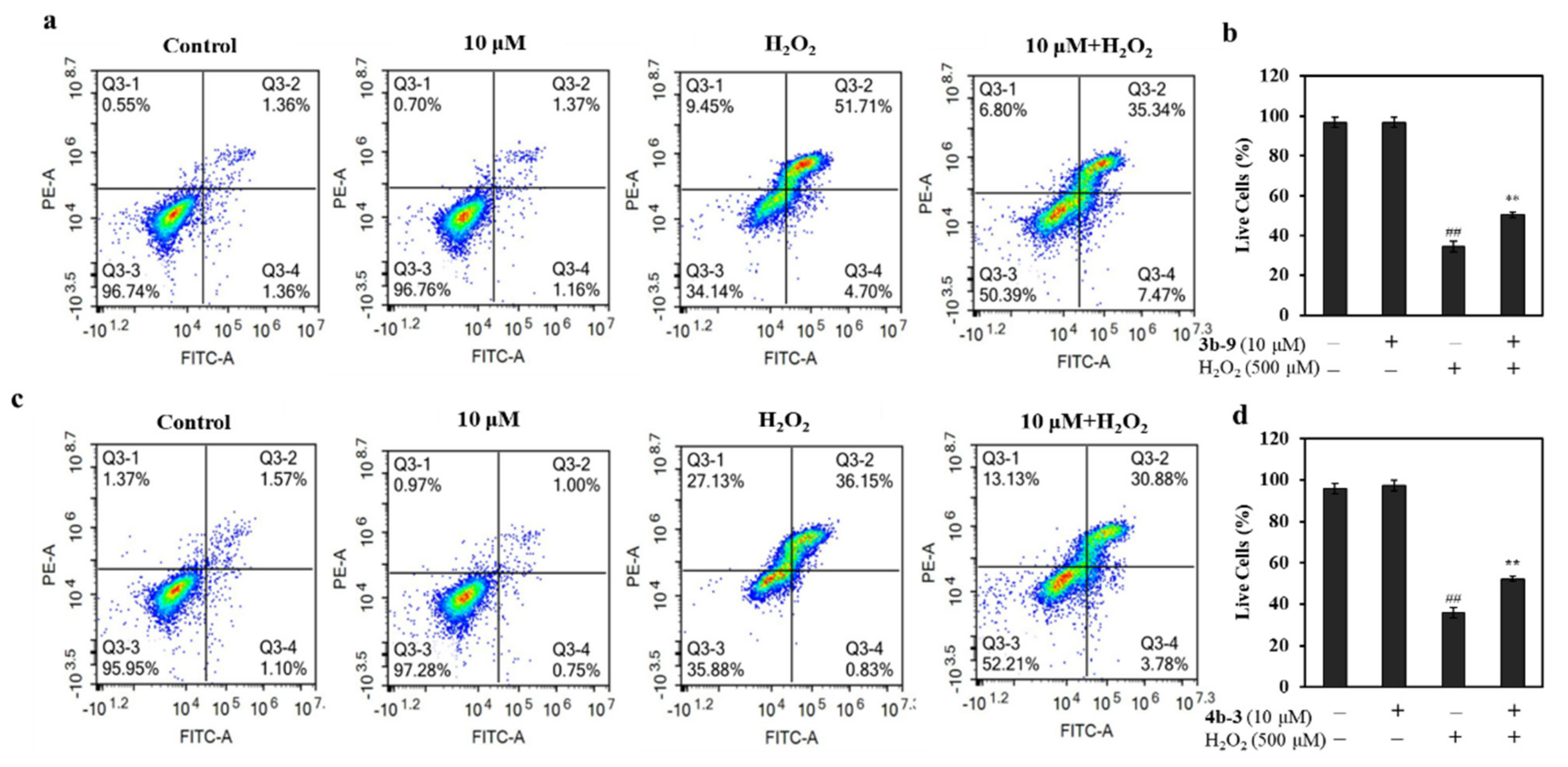
3.2.1. 3b-9 and 4b-3 Ameliorates H2O2-Induced Oxidative Cell Damage
3.2.2. 3b-9and 4b-3 Decreases the H2O2-Induced ROS Generation in HaCaT Cells
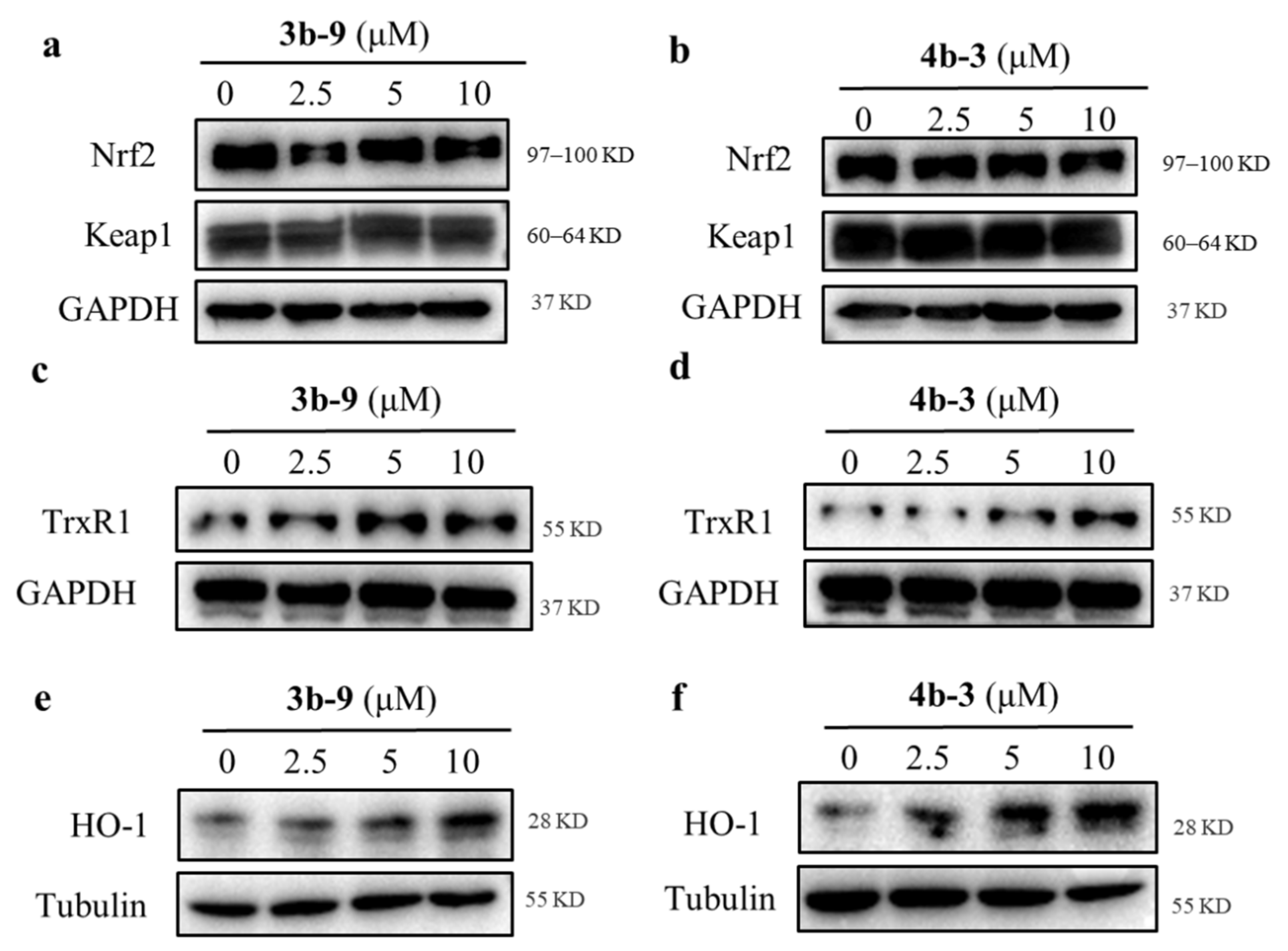
3.2.3. 3b-9and 4b-3 Promotes the Expression of the Antioxidant Protein TrxR1 and HO-1
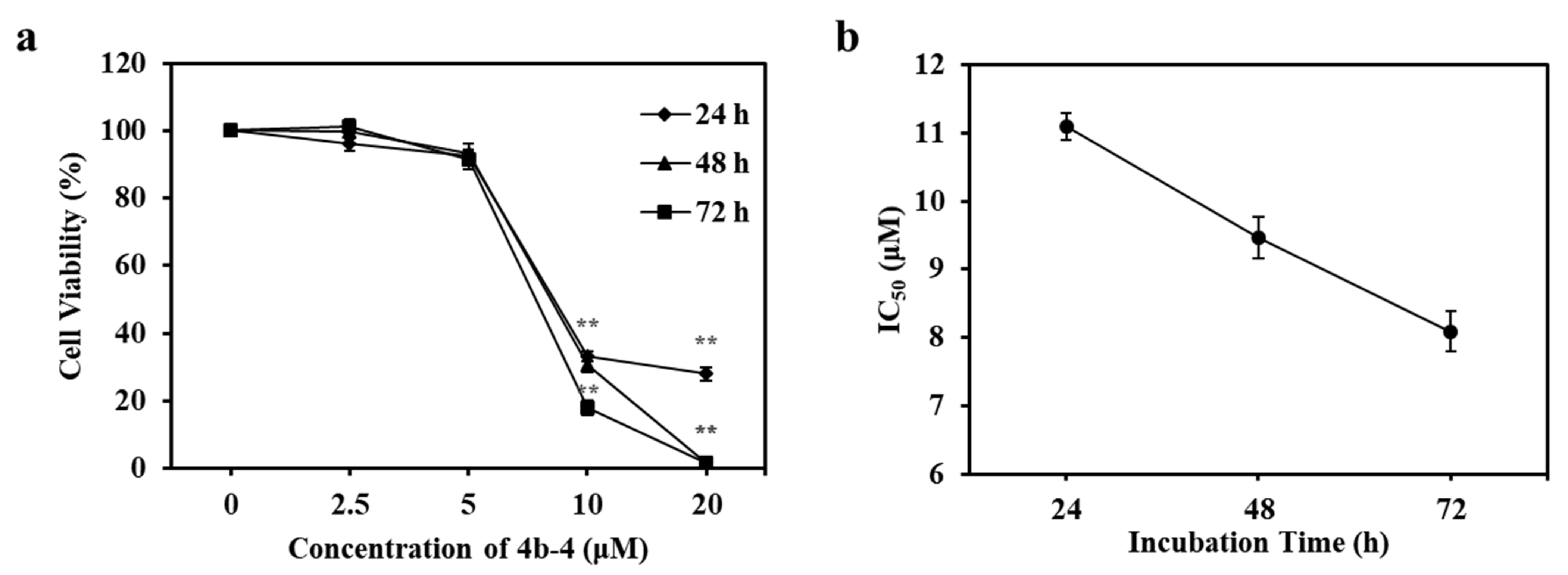
3.3. Cytotoxicity of 3b-1 to 9 and 4b-1 to 6 against Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mittler, R. ROS are good. Trends. Plant. Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romo González, M.; Moreno Paz, S.; García Hernández, V.; Sánchez Guijo, F.; Hernández Hernández, Á. Inhibition of xanthine oxidoreductase enhances the potential of tyrosine kinase inhibitors against chronic myeloid leukemia. Antioxidants 2020, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox. Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Liu, M.; Wang, L.; Liu, Y.; Lu, X.; Stagos, D.; Lin, X.; Liu, M. Bromophenol bis (2,3,6-Tribromo-4,5-dihydroxybenzyl) ether protects HaCaT skin cells from oxidative damage via Nrf2-mediated pathways. Antioxidants 2021, 10, 1436. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Hansen, P.E.; Lin, X.K. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Dong, S.T.; Hansen, P.E.; Stagos, D.; Lin, X.K.; Liu, M. Progress of bromophenols in marine algae from 2011 to 2020: Structure, bioactivities, and applications. Mar. Drugs 2020, 18, 411. [Google Scholar] [CrossRef]
- Kurata, K.; Amiya, T. Bis (2,3,6-tribromo-4,5-dihydroxybenzyl) ether from the red alga, Symphyocladia latiuscula. Phytochemistry 1980, 19, 141–142. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Z.; Wang, L.; Liu, Y.; Stagos, D.; Lin, X.; Liu, M. Marine bromophenol bis (2,3,6-Tribromo-4,5-dihydroxybenzyl) ether inhibits angiogenesis in human umbilical vein endothelial cells and reduces vasculogenic mimicry in human lung cancer A549 cells. Mar. Drugs 2021, 19, 641. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Devine, S.M.; Yong, C.; Amenuvegbe, D.; Aurelio, L.; Muthiah, D.; Pouton, C.; Callaghan, R.; Capuano, B.; Scammells, P.J. Synthesis and pharmacological evaluation of noscapine-inspired 5-substituted tetrahydroisoquinolines as cytotoxic agents. J. Med. Chem. 2018, 61, 8444–8456. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Li, C.; Luo, J.; Li, X.; Wang, L.; Jiang, B.; Shi, D. Toward a treatment of diabesity: In vitro and in vivo evaluation of uncharged bromophenol derivatives as a new series of PTP1B inhibitors. Eur. J. Med. Chem. 2019, 166, 178–185. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Shan, Y.; Li, N.; Ma, W.; He, L. Synthesis and preliminary biological evaluation of novel taspine derivatives as anticancer agents. Eur. J. Med. Chem. 2010, 45, 2798–2805. [Google Scholar] [CrossRef]
- Kim, K.C.; Hyun, Y.J.; Hewage, S.; Piao, M.J.; Kang, K.A.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Hyun, J.W. 3-bromo-4,5-dihydroxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2017, 15, 291. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.S.; Fernando, P.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine compound 3-bromo-4,5-dihydroxybenzaldehyde protects skin cells against oxidative damage via the Nrf2/HO-1 pathway. Mar. Drugs 2019, 17, 234. [Google Scholar] [CrossRef] [Green Version]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox. Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, H.R.; Kim, J.; Park, K.D.; Kim, D.J.; Hwang, O. The novel neuroprotective compound KMS99220 has an early anti-neuroinflammatory effect via AMPK and HO-1, independent of Nrf2. Exp. Neurobiol. 2018, 27, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Meussen-Elholm, E.T.; Holme, J.A.; Hongslo, J.K. Brominated phenols: Characterization of estrogen-like activity in the human breast cancer cell-line MCF-7. Toxicol. Lett. 2002, 129, 55–63. [Google Scholar] [CrossRef]
- Legler, J.; Brouwer, A. Are brominated flame retardants endocrine disruptors? Environ. Int. 2003, 29, 879–885. [Google Scholar] [CrossRef]



| Number | Concentration (μM) | K562 Cell Viability (%) | HaCaT Cell Viability (%) |
|---|---|---|---|
| 3b-1 | 5 | 91.35 ± 1.39 | 97.35 ± 3.48 |
| 10 | 88.12 ± 0.31 | 104.96 ± 1.89 | |
| 3b-2 | 5 | 101.46 ± 1.89 | 86.50 ± 3.23 |
| 10 | 101.87 ± 1.08 | 85.35 ± 3.42 | |
| 3b-3 | 5 | 92.67 ± 2.58 | 101.43 ± 2.83 |
| 10 | 96.28 ± 0.92 | 87.34 ± 4.68 | |
| 3b-4 | 5 | 102.63 ± 1.54 | 89.70 ± 4.90 |
| 10 | 102.83 ± 2.41 | 91.86 ± 3.82 | |
| 3b-5 | 5 | 91.32 ± 3.09 | 106.41 ± 4.15 |
| 10 | 85.75 ± 0.73 | 99.59 ± 3.69 | |
| 3b-6 | 5 | 86.91 ± 0.47 | 93.14 ± 7.07 |
| 10 | 74.34 ± 0.95 * | 96.67 ± 3.28 | |
| 3b-7 | 5 | 95.94 ± 2.35 | 122.03 ± 2.74 |
| 10 | 85.23 ± 0.20 | 106.34 ± 2.59 | |
| 3b-8 | 5 | 73.69 ± 0.91 * | 97.21 ± 4.24 |
| 10 | 49.43 ± 0.92 * | 96.50 ± 5.35 | |
| 3b-9 | 5 | 86.86 ± 2.47 | 93.63 ± 4.69 |
| 10 | 78.71 ± 1.22 * | 105.39 ± 4.16 | |
| 4b-1 | 5 | 102.16 ± 5.04 | 100.47 ± 0.91 |
| 10 | 99.10 ± 3.23 | 97.30 ± 3.27 | |
| 4b-2 | 5 | 98.45 ± 1.82 | 99.27 ± 0.81 |
| 10 | 93.38 ± 1.82 | 92.09 ± 1.75 | |
| 4b-3 | 5 | 96.43 ± 0.99 | 94.56 ± 6.84 |
| 10 | 95.62 ± 1.97 | 100.07 ± 3.25 | |
| 4b-4 | 5 | 40.96 ± 3.72 * | 100.42 ± 4.27 |
| 10 | 35.27 ± 0.65 * | 4.19 ± 0.21 * | |
| 4b-5 | 5 | 90.49 ± 2.17 | 91.44 ± 2.12 |
| 10 | 63.93 ± 4.12 * | 94.56 ± 6.84 | |
| 4b-6 | 5 | 98.45 ± 1.82 | 88.65 ± 4.01 |
| 10 | 93.38 ± 1.32 | 84.45 ± 3.25 | |
| Adriamycin | 1 | 11.24 ± 0.67 * | 3.39 ± 0.08 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Wang, L.; Guo, M.; Stagos, D.; Giakountis, A.; Trachana, V.; Lin, X.; Liu, Y.; Liu, M. Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. Antioxidants 2022, 11, 786. https://doi.org/10.3390/antiox11040786
Dong H, Wang L, Guo M, Stagos D, Giakountis A, Trachana V, Lin X, Liu Y, Liu M. Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. Antioxidants. 2022; 11(4):786. https://doi.org/10.3390/antiox11040786
Chicago/Turabian StyleDong, Hui, Li Wang, Meng Guo, Dimitrios Stagos, Antonis Giakountis, Varvara Trachana, Xiukun Lin, Yankai Liu, and Ming Liu. 2022. "Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols" Antioxidants 11, no. 4: 786. https://doi.org/10.3390/antiox11040786
APA StyleDong, H., Wang, L., Guo, M., Stagos, D., Giakountis, A., Trachana, V., Lin, X., Liu, Y., & Liu, M. (2022). Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. Antioxidants, 11(4), 786. https://doi.org/10.3390/antiox11040786








