N-Acetylcysteine Enhances the Recovery of Ischemic Limb in Type-2 Diabetic Mice
Abstract
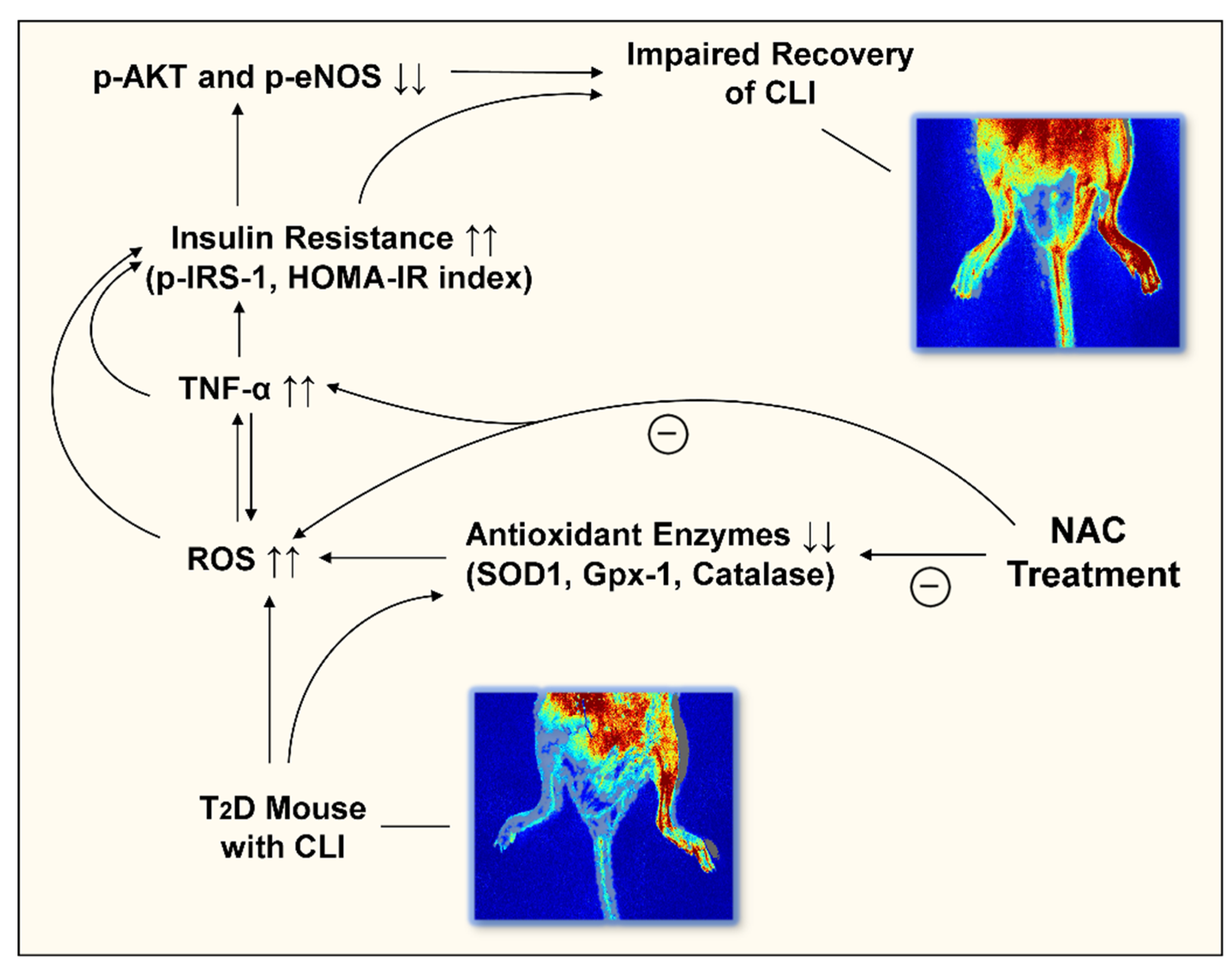
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Body Weight, Blood Glucose, Plasma AGEs, and Insulin
2.3. Creation of CLI Model and Assessment of Blood Perfusion and Function
2.4. CD31 Immunofluorescent Staining and DHE Staining for ROS Production
2.5. Histological Analysis for Muscle Structure and Tissue Fibrosis
2.6. Western Blot Assay and Measurement of Plasma TNF-α
2.7. Statistical Analysis
3. Results
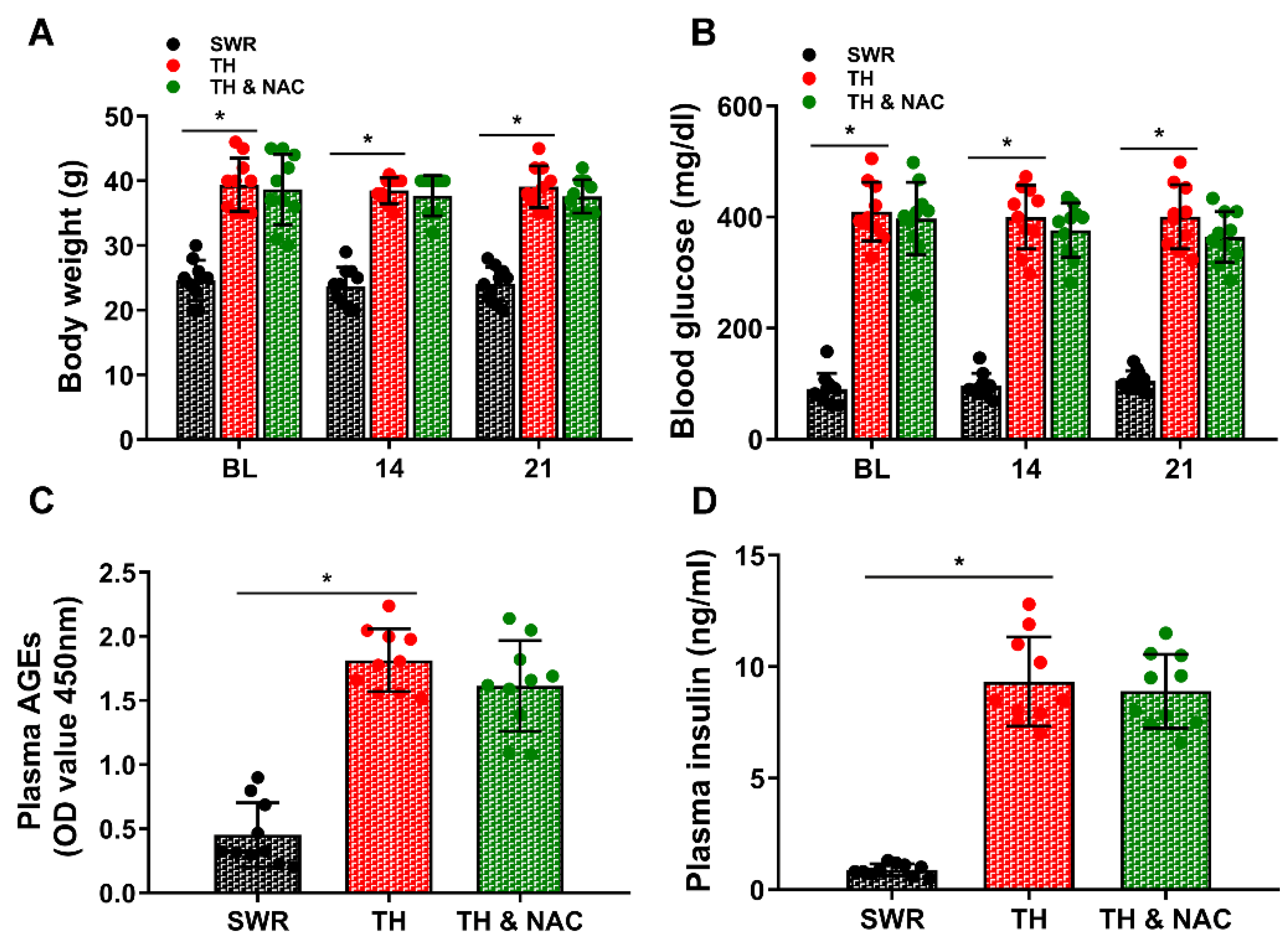
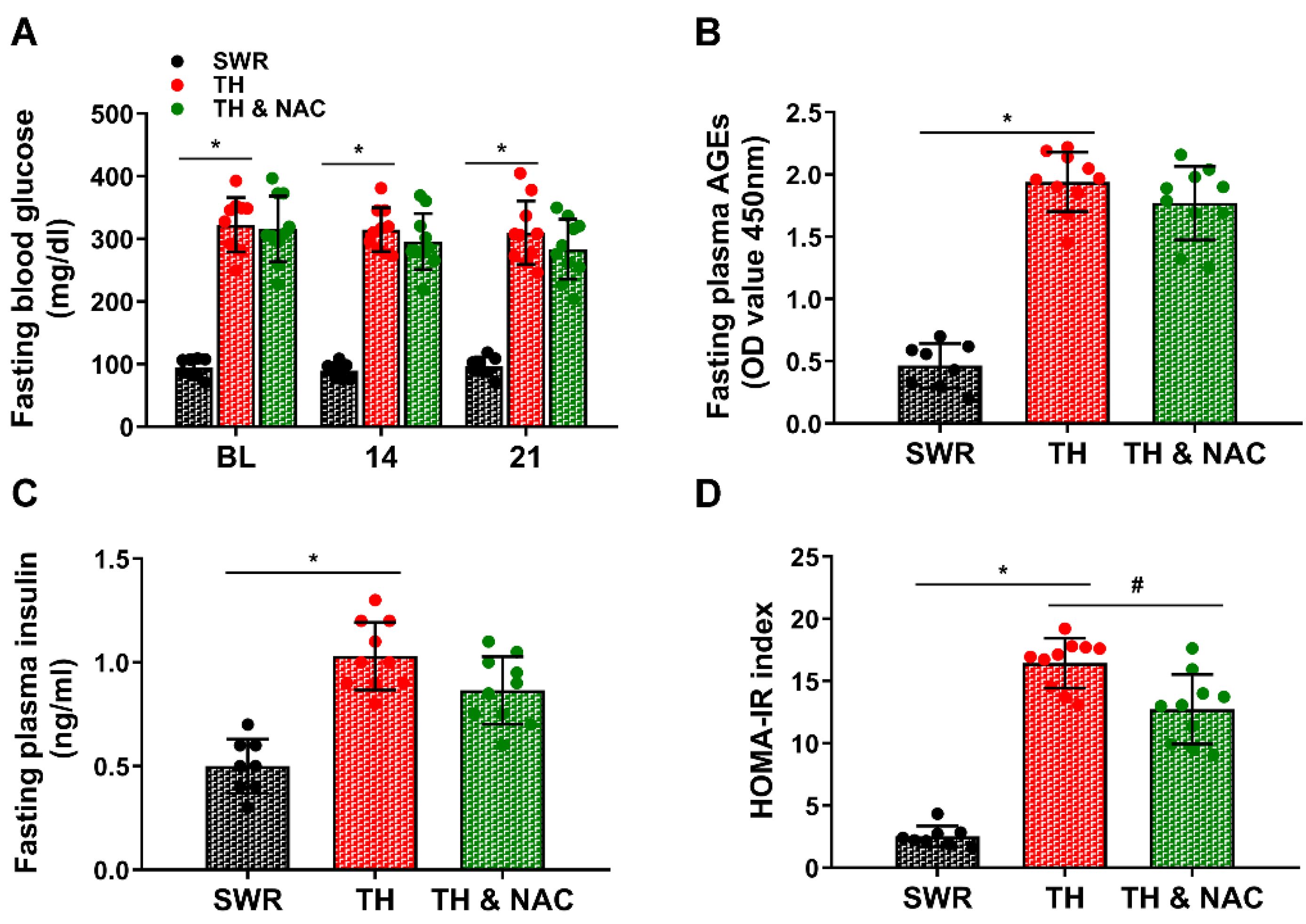
3.1. NAC Treatment Improved Insulin Resistance without Significant Changes in Plasma Insulin Levels
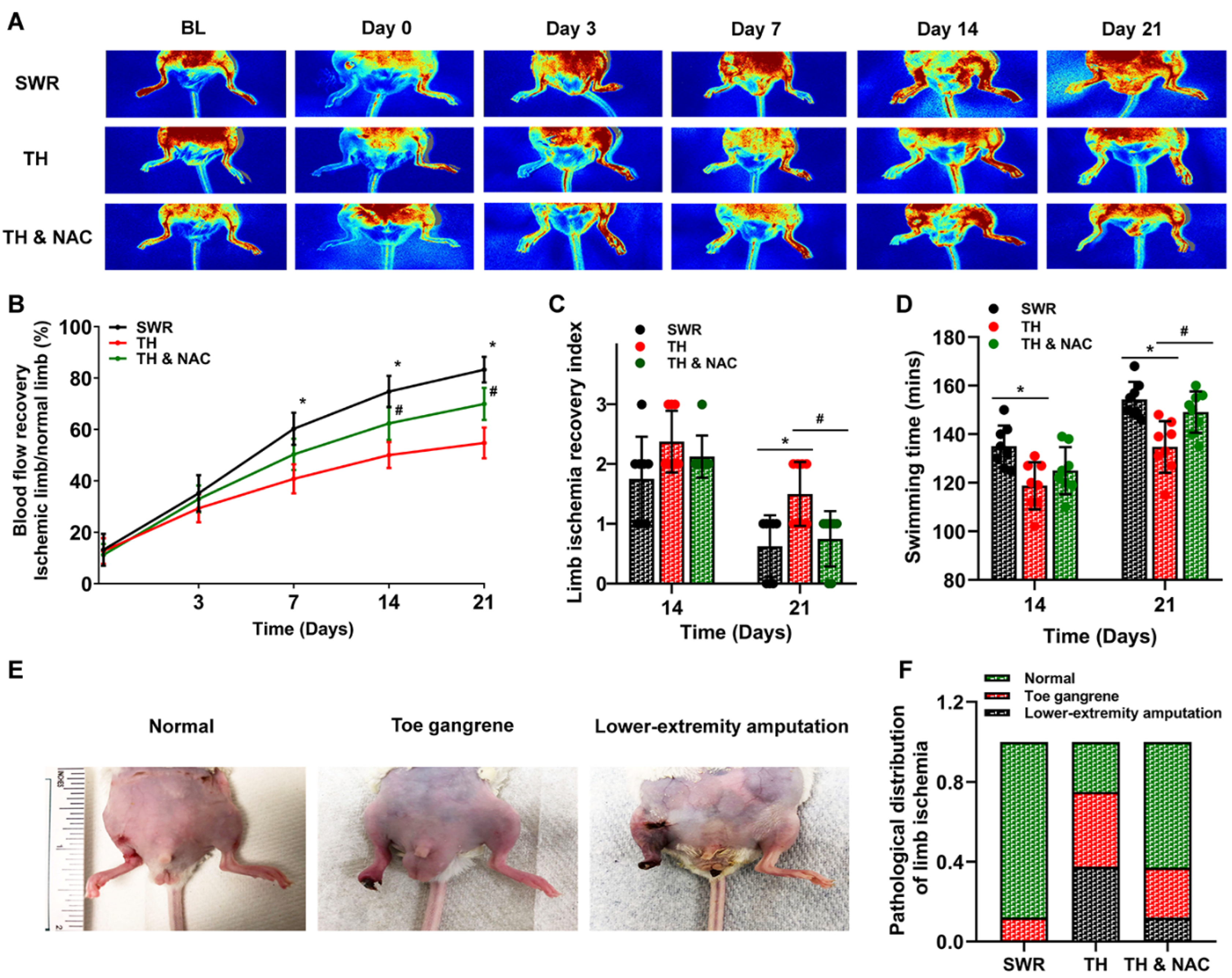
3.2. NAC Treatment Enhanced the Recovery of Blood Flow and Function of Diabetic Ischemic Limb
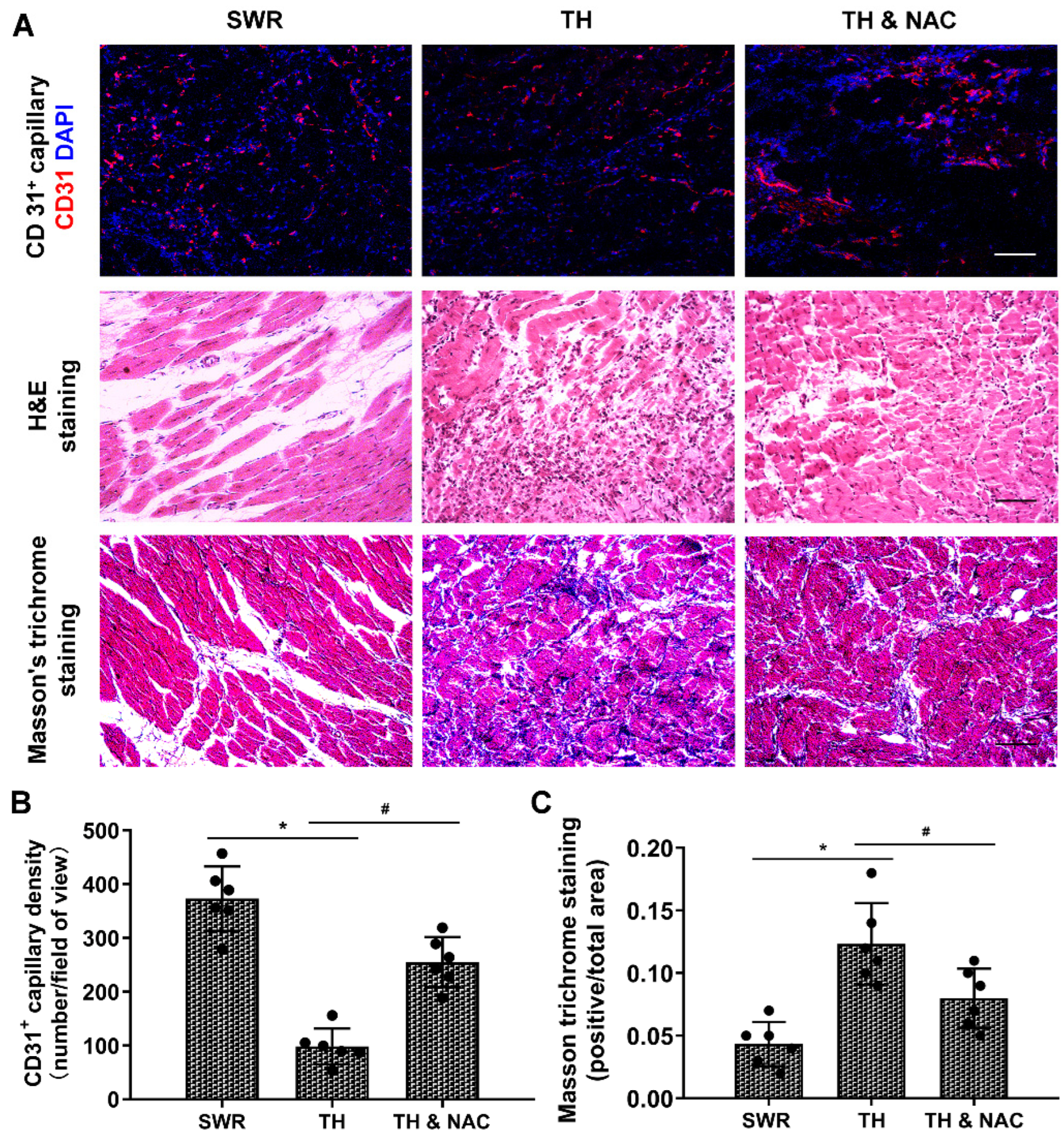
3.3. NAC Treatment Improved Capillary Density and Muscular Structure, While Decreasing Inflammation and Fibrosis in Diabetic Ischemic Limbs
3.4. NAC Effectively Prevented ROS Production, While Increasing the Expression of Antioxidant Enzymes in Diabetic Ischemic Limbs
3.5. NAC Treatment Increased the Phosphorylation of Akt and eNOS Expression, While Decreasing Plasma TNF-a and Phosphorylated IRS-1 in Diabetic Ischemic Limbs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, J.L.; Halperin, J.L.; Albert, N.M.; Bozkurt, B.; Brindis, R.G.; Curtis, L.H.; DeMets, D.; Guyton, R.A.; Hochman, J.S.; Kovacs, R.J.; et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 1425–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffstad, O.; Mitra, N.; Walsh, J.; Margolis, D.J. Diabetes, lower-extremity amputation, and death. Diabetes Care 2015, 38, 1852–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiss, L.S.; Li, Y.; Hora, I.; Albright, A.; Rolka, D.; Gregg, E.W. Resurgence of Diabetes-Related Nontraumatic Lower-Extremity Amputation in the Young and Middle-Aged Adult U.S. Population. Diabetes Care 2019, 42, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, R.A.; Tesfaye, S.; Ziegler, D. Medical strategies to reduce amputation in patients with type 2 diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2013, 30, 893–900. [Google Scholar] [CrossRef]
- Howangyin, K.Y.; Silvestre, J.S. Diabetes mellitus and ischemic diseases: Molecular mechanisms of vascular repair dysfunction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1126–1135. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Winocour, P.; Farrington, K. Oxidative stress in early diabetic nephropathy: Fueling the fire. Nat. Rev. Endocrinol. 2011, 7, 176–184. [Google Scholar] [CrossRef]
- Arden, G.B.; Sivaprasad, S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr. Diabetes Rev. 2011, 7, 291–304. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxidative Med. Cell. Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.; Neuhofer, W.; Bartels, H.; Fraek, M.L.; Beck, F.X. Role of focal adhesion kinase (FAK) in renal ischaemia and reperfusion. Pflug. Arch. Eur. J. Physiol. 2007, 455, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Takhtfooladi, H.A.; Hesaraki, S.; Razmara, F.; Takhtfooladi, M.A.; Hajizadeh, H. Effects of N-acetylcysteine and pentoxifylline on remote lung injury in a rat model of hind-limb ischemia/reperfusion injury. J. Bras. Pneumol. Public. Soc. Bras. Pneumol. Tisilogia 2016, 42, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Cakir, O.; Erdem, K.; Oruc, A.; Kilinc, N.; Eren, N. Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovasc. Surg. 2003, 11, 375–379. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.; Zhang, Q.; Xu, J.; Zhan, L.; Zhu, Q.; Lian, Q.; Liu, H.; Xia, Z.Y.; Xia, Z.; et al. N-acetylcysteine attenuates myocardial dysfunction and postischemic injury by restoring caveolin-3/eNOS signaling in diabetic rats. Cardiovasc. Diabetol. 2016, 15, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Liu, L.; Xiao, Y.; Li, X.; Zhang, J.; Xie, X.; Tian, J.; Sen, C.K.; He, X.; Hao, H.; et al. N-acetylcysteine differentially regulates the populations of bone marrow and circulating endothelial progenitor cells in mice with limb ischemia. Eur. J. Pharmacol. 2020, 881, 173233. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Thamarai, K.; Kandimalla, R.; Manczak, M.; Yin, X.; Kumar, S.; Vijayan, M.; Reddy, P.H. Mitochondria-Targeted Small Peptide, SS31 Ameliorates Diabetes Induced Mitochondrial Dynamics in Male TallyHO/JngJ Mice. Mol. Neurobiol. 2021, 58, 795–808. [Google Scholar] [CrossRef]
- Kim, J.H.; Sen, S.; Avery, C.S.; Simpson, E.; Chandler, P.; Nishina, P.M.; Churchill, G.A.; Naggert, J.K. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics 2001, 74, 273–286. [Google Scholar] [CrossRef]
- Duseja, A.; Thumburu, K.K.; Das, A.; Dhiman, R.K.; Chawla, Y.K.; Bhadada, S.; Bhansali, A. Insulin tolerance test is comparable to homeostasis model assessment for insulin resistance in patients with nonalcoholic fatty liver disease. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2007, 26, 170–173. [Google Scholar]
- Keshk, W.A.; Ibrahim, M.A.; Shalaby, S.M.; Zalat, Z.A.; Elseady, W.S. Redox status, inflammation, necroptosis and inflammasome as indispensable contributors to high fat diet (HFD)-induced neurodegeneration; Effect of N-acetylcysteine (NAC). Arch. Biochem. Biophys. 2020, 680, 108227. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.E.; Zhang, H.; Lassance-Soares, R.M.; Prabhakar, P.; Najafi, A.H.; Burnett, M.S.; Epstein, S.E. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1748–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Hao, H.; Xu, H.; Fichman, Y.; Cui, Y.; Yang, C.; Wang, M.; Mittler, R.; Hill, M.A.; Cowan, P.J.; et al. Combination of Antioxidant Enzyme Overexpression and N-Acetylcysteine Treatment Enhances the Survival of Bone Marrow Mesenchymal Stromal Cells in Ischemic Limb in Mice With Type 2 Diabetes. J. Am. Heart Assoc. 2021, 10, e023491. [Google Scholar] [CrossRef]
- Cui, Y.; Narasimhulu, C.A.; Liu, L.; Zhang, Q.; Liu, P.Z.; Li, X.; Xiao, Y.; Zhang, J.; Hao, H.; Xie, X.; et al. N-acetylcysteine inhibits in vivo oxidation of native low-density lipoprotein. Sci. Rep. 2015, 5, 16339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Xia, Z.; Leo, J.M.; Pang, C.C. The effect of N-acetylcysteine on cardiac contractility to dobutamine in rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 2005, 519, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.Y.; Sharkis, S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007, 110, 3056–3063. [Google Scholar] [CrossRef] [Green Version]
- Luttun, A.; Tjwa, M.; Moons, L.; Wu, Y.; Angelillo-Scherrer, A.; Liao, F.; Nagy, J.A.; Hooper, A.; Priller, J.; De Klerck, B.; et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 2002, 8, 831–840. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Liu, C.; Wang, Y.; Ma, J.; Mao, X.; Li, Q. Netrin-1 promotes mesenchymal stem cell revascularization of limb ischaemia. Diabetes Vasc. Dis. Res. 2016, 13, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.D.; Wang, Y.Y.; Fu, W.L.; Wu, J.; Chen, A.F. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 2004, 110, 2484–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Zhang, L.; Chi, J.; Li, H.; Liu, X.; Hu, T.; Li, R.; Guo, Y.; Zhang, X.; Wang, H.; et al. Helicobacter pylori Infection Impairs Endothelial Function Through an Exosome-Mediated Mechanism. J. Am. Heart Assoc. 2020, 9, e014120. [Google Scholar] [CrossRef] [PubMed]
- Low Wang, C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; Norgren, L.; Jones, W.S.; et al. Cardiovascular and Limb Outcomes in Patients With Diabetes and Peripheral Artery Disease: The EUCLID Trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, W.A.; da Silva, L.A.; Dall’Igna, D.M.; Michels, M.; Manfredini, A.; Dos Santos Cardoso, J.; Constantino, L.; Scaini, G.; Vuolo, F.; Streck, E.L.; et al. N-acetylcysteine effects on a murine model of chronic critical limb ischemia. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Lizarrondo, S.; Gakuba, C.; Herbig, B.A.; Repessé, Y.; Ali, C.; Denis, C.V.; Lenting, P.J.; Touzé, E.; Diamond, S.L.; Vivien, D.; et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation 2017, 136, 646–660. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Jiang, L.; Wei, X.; Niu, C.; Wang, R.; Zhang, J.; Meng, D.; Yao, K. Bach1 Induces Endothelial Cell Apoptosis and Cell-Cycle Arrest through ROS Generation. Oxidative Med. Cell. Longev. 2016, 2016, 6234043. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Pan, W.Y.; Tseng, M.T.; Lin, K.J.; Yang, Y.P.; Tsai, H.W.; Hwang, S.M.; Chang, Y.; Wei, H.J.; Sung, H.W. Enhancement of cell adhesion, retention, and survival of HUVEC/cbMSC aggregates that are transplanted in ischemic tissues by concurrent delivery of an antioxidant for therapeutic angiogenesis. Biomaterials 2016, 74, 53–63. [Google Scholar] [CrossRef]
- Straub, L.G.; Efthymiou, V.; Grandl, G.; Balaz, M.; Challa, T.D.; Truscello, L.; Horvath, C.; Moser, C.; Rachamin, Y.; Arnold, M.; et al. Antioxidants protect against diabetes by improving glucose homeostasis in mouse models of inducible insulin resistance and obesity. Diabetologia 2019, 62, 2094–2105. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Aw, T.Y.; Stokes, K.Y. The protection conferred against ischemia-reperfusion injury in the diabetic brain by N-acetylcysteine is associated with decreased dicarbonyl stress. Free Radic. Biol. Med. 2016, 96, 89–98. [Google Scholar] [CrossRef] [Green Version]
- van de Vyver, M. Intrinsic Mesenchymal Stem Cell Dysfunction in Diabetes Mellitus: Implications for Autologous Cell Therapy. Stem Cells Dev. 2017, 26, 1042–1053. [Google Scholar] [CrossRef]
- Yang, C.T.; Meng, F.H.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.H.; Li, C.C.; Zhang, H. Inhibition of Methylglyoxal-Induced AGEs/RAGE Expression Contributes to Dermal Protection by N-Acetyl-L-Cysteine. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, H.; Chai, Y. Advanced Glycation End Products (AGEs) Induce Apoptosis of Fibroblasts by Activation of NLRP3 Inflammasome via Reactive Oxygen Species (ROS) Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7499–7508. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Lu, A.L.; Yao, X.M.; Hua, Q.; Li, X.Y.; Qin, L.; Zhang, H.M.; Meng, G.X.; Su, Q. Activation of NLRP3 Inflammasome by Advanced Glycation End Products Promotes Pancreatic Islet Damage. Oxidative Med. Cell. Longev. 2017, 2017, 9692546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunkemoeller, B.; Kyriakides, T.R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Richter, K.; Konzack, A.; Pihlajaniemi, T.; Heljasvaara, R.; Kietzmann, T. Redox-fibrosis: Impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol. 2015, 6, 344–352. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiao, Y.; Xing, Y.; Gao, P. Diabetes Mellitus and Risk of Hepatic Fibrosis/Cirrhosis. BioMed Res. Int. 2019, 2019, 5308308. [Google Scholar] [CrossRef] [Green Version]
- Granados, A.; Chan, C.L.; Ode, K.L.; Moheet, A.; Moran, A.; Holl, R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2019, 18 (Suppl. 2), S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Jha, J.C.; Banal, C.; Chow, B.S.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef] [Green Version]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Goldberg, J.L.; Qiao, L.Y.; Mitchell, J.J. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes 1999, 48, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.Y.; Goldberg, J.L.; Russell, J.C.; Sun, X.J. Identification of enhanced serine kinase activity in insulin resistance. J. Biol. Chem. 1999, 274, 10625–10632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; DeFea, K.; Roth, R.A. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 1999, 274, 9351–9356. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hu, Y.; Huang, T.; Zhang, Y.; Li, Z.; Luo, C.; Luo, Y.; Yuan, H.; Hisatome, I.; Yamamoto, T.; et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 2014, 447, 707–714. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Papavassiliou, A.G. Serine phosphorylation of insulin receptor substrate-1: A novel target for the reversal of insulin resistance. Mol. Endocrinol. 2001, 15, 1864–1869. [Google Scholar] [CrossRef]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Taniyama, Y.; Hitomi, H.; Shah, A.; Alexander, R.W.; Griendling, K.K. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recchioni, R.; Marcheselli, F.; Moroni, F.; Pieri, C. Apoptosis in human aortic endothelial cells induced by hyperglycemic condition involves mitochondrial depolarization and is prevented by N-acetyl-L-cysteine. Metab. Clin. Exp. 2002, 51, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Maeda, S.; Takeuchi, M.; Yamagishi, S. Glucose-dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm. Metab. Res. 2012, 44, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Guigas, B.; Chauvin, C.; Batandier, C.; Fontaine, E.; Wiernsperger, N.; Leverve, X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 2005, 54, 2179–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza, C.A.; La Favor, J.D.; Kim, D.H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martina, V.; Masha, A.; Gigliardi, V.R.; Brocato, L.; Manzato, E.; Berchio, A.; Massarenti, P.; Settanni, F.; Della Casa, L.; Bergamini, S.; et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care 2008, 31, 940–944. [Google Scholar] [CrossRef] [Green Version]
- Masha, A.; Brocato, L.; Dinatale, S.; Mascia, C.; Biasi, F.; Martina, V. N-acetylcysteine is able to reduce the oxidation status and the endothelial activation after a high-glucose content meal in patients with Type 2 diabetes mellitus. J. Endocrinol. Investig. 2009, 32, 352–356. [Google Scholar] [CrossRef]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef]
- Lejay, A.; Paradis, S.; Lambert, A.; Charles, A.L.; Talha, S.; Enache, I.; Thaveau, F.; Chakfe, N.; Geny, B. N-Acetyl Cysteine Restores Limb Function, Improves Mitochondrial Respiration, and Reduces Oxidative Stress in a Murine Model of Critical Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 56, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2018, 18, 283–298. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Liu, X.; Zhu, Q.; Liu, Z.; Yang, C.; Wu, H.; Zhang, L.; Xia, X.; Wang, M.; Hao, H.; et al. N-Acetylcysteine Enhances the Recovery of Ischemic Limb in Type-2 Diabetic Mice. Antioxidants 2022, 11, 1097. https://doi.org/10.3390/antiox11061097
Zhu Q, Liu X, Zhu Q, Liu Z, Yang C, Wu H, Zhang L, Xia X, Wang M, Hao H, et al. N-Acetylcysteine Enhances the Recovery of Ischemic Limb in Type-2 Diabetic Mice. Antioxidants. 2022; 11(6):1097. https://doi.org/10.3390/antiox11061097
Chicago/Turabian StyleZhu, Qiang, Xuanyou Liu, Qingyi Zhu, Zehao Liu, Chunlin Yang, Hao Wu, Linfang Zhang, Xiujuan Xia, Meifang Wang, Hong Hao, and et al. 2022. "N-Acetylcysteine Enhances the Recovery of Ischemic Limb in Type-2 Diabetic Mice" Antioxidants 11, no. 6: 1097. https://doi.org/10.3390/antiox11061097
APA StyleZhu, Q., Liu, X., Zhu, Q., Liu, Z., Yang, C., Wu, H., Zhang, L., Xia, X., Wang, M., Hao, H., Cui, Y., Zhang, G., Hill, M. A., Flaker, G. C., Zhou, S., & Liu, Z. (2022). N-Acetylcysteine Enhances the Recovery of Ischemic Limb in Type-2 Diabetic Mice. Antioxidants, 11(6), 1097. https://doi.org/10.3390/antiox11061097





