Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans
Abstract
:1. Introduction
2. Material and Methods
2.1. Honey Samples
2.2. Physicochemical Parameters
2.3. Hydroxymethylfurfural (HMF) Analysis
2.4. Total Phenolic Content
2.5. DPPH Radical Scavenging Activity
2.6. Antibacterial Activity
2.7. Antiproliferative Activity
2.8. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characterisation of Honey
3.2. Antioxidative Potential of Honey
3.3. Antibacterial Activity
3.4. Antiproliferative Activity
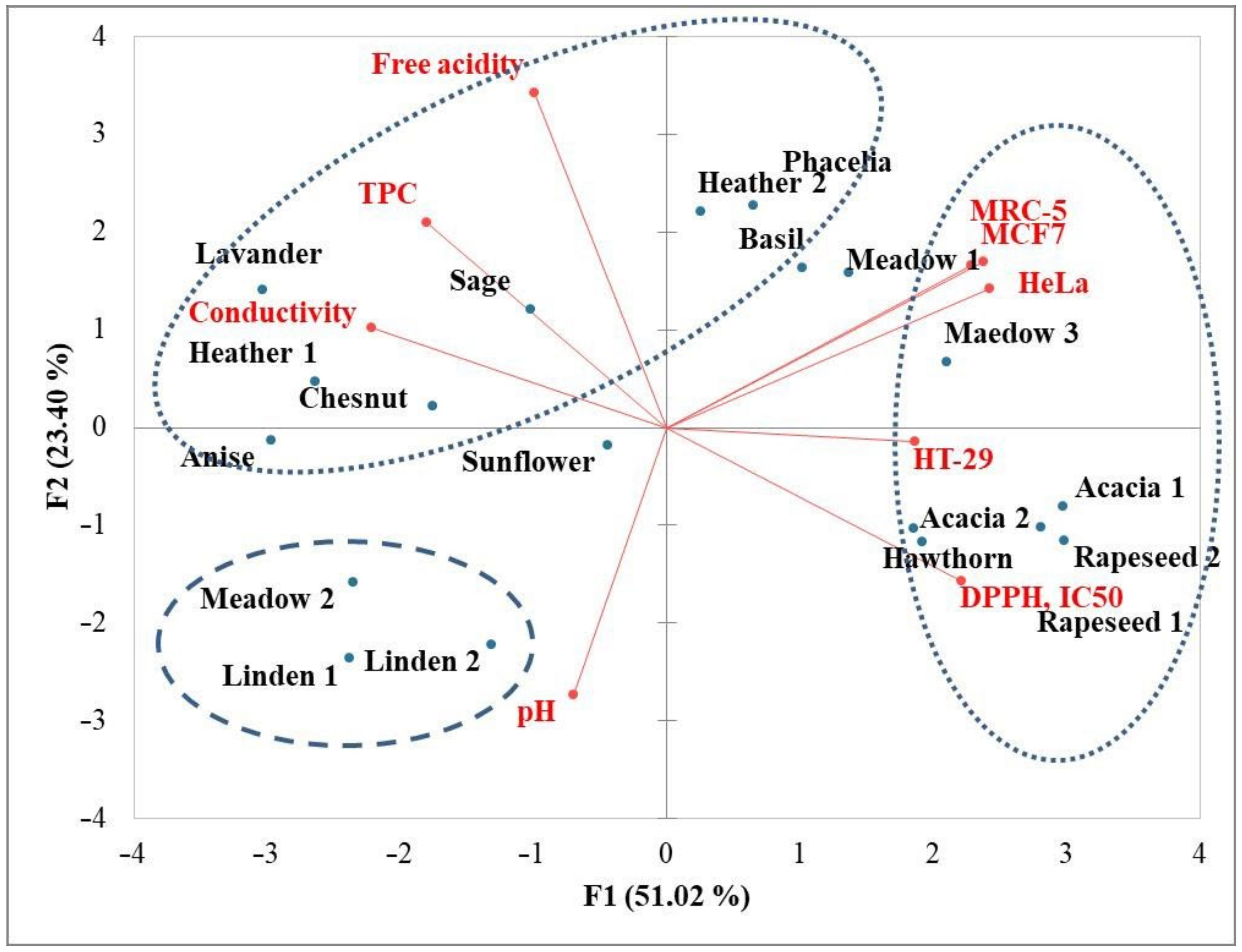
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.B.; Jovanov, P.T.; Marić, A.Z.; Pezo, L.L.; Kevrešan, Ž.S.; Novaković, A.R.; Nedeljković, N.M. Physicochemical properties and mineral content of honey samples from Vojvodina (Republic of Serbia). Food Chem. 2019, 276, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, S.; Koeling-Speer, I.; Speer, K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Hegazi, A.; Al Tahtawi, R.H.M.; Abd-Allah, F.; Abdou, A.M. Antitumor and antioxidant activity of honey in mice bearing Ehrlich Ascite carcinoma. Acad. J. Cancer Res. 2014, 7, 208–214. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Hua Gan, S.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Bose, B. Honey or sugar in treatment of infected wounds? Lancet 1982, 319, 963. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef]
- Russell, K.M.; Molan, P.C.; Wilkins, A.L.; Holland, P.T. Identification of some antibacterial constituents of New Zealand manuka honey. J. Agric. Food Chem. 1990, 38, 10–13. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Eshak, Z.; Ismail, W.I.W. Acacia honey induces apoptosis in human breast adenocarcinoma cell lines (MCF-7). J. Teknol. 2017, 79, 9–16. [Google Scholar]
- Moskwa, J.; Borawska, M.H.; Markiewicz-Zukowska, R.; Puscion-Jakubik, A.; Naliwajko, S.K.; Socha, K.; Soroczynska, J. Polish natural bee honeys are anti-proliferative and anti-metastatic agents in human glioblastoma multiforme U87MG cell line. PLoS ONE 2014, 9, e90533. [Google Scholar] [CrossRef] [PubMed]
- Premratanachai, P.; Chanchao, C. Review of anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarević, K.B.; Andrić, F.; Trifković, J.; Tešić, Ž.; Milojković-Opsenica, D. Characterisation of Serbian unifloral honeys according to their physicochemical parameters. Food Chem. 2012, 132, 2060–2064. [Google Scholar] [CrossRef]
- Đogo Mračević, S.; Krstić, M.; Lolić, A.; Ražić, S. Comparative study of the chemical composition and biological potential of honey from different regions in Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Codex Standard 12-1981; Revised Codex Standards for Honey. Codex Alimentarius Commission: Italy, Rome, 2019.
- Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bogdanov, S. Harmonised Methods of the International Honey Commission, Bern, Switzerland. 2009. Available online: http://www.ihc-platform.net/publications.html (accessed on 26 January 2022).
- Rufián-Henares, J.A.; de la Cueva, S.P. Assessment of hydroxymethylfurfural intake in the Spanish diet. Food Addit. Contam. A 2008, 25, 1306–1312. [Google Scholar] [CrossRef]
- Petisca, C.; Henriques, A.R.; Pérez-Palacios, T.; Pinho, O.; Ferreira, I.M.P.L.V.O. Assessment of hydroxymethylfurfural and furfural in commercial bakery products. J. Food Compos. Anal. 2014, 33, 20–25. [Google Scholar] [CrossRef]
- Ariffin, A.A.; Ghazali, H.M.; Kavousi, P. Validation of a HPLC method for determination of hydroxymethylfurfural in crude palm oil. Food Chem. 2014, 154, 102–107. [Google Scholar] [CrossRef]
- Tomasini, D.; Sampaio, M.R.F.; Caldas, S.S.; Buffon, J.G.; Duarte, F.A.; Primel, E.G. Simultaneous determination of pesticides and 5-hydroxymethylfurfural in honey by the modified QuEChERS method and liquid chromatography coupled to tandem mass spectrometry. Talanta 2012, 99, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szweda, P. Antimicrobial activity of honey. Honey Anal. 2017, 1, 215–232. [Google Scholar] [CrossRef] [Green Version]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Cetojevic-Simin, D.D.; Bogdanovic, G.M.; Cvetkovic, D.D.; Velicanski, A.S. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha. J. BUON 2008, 13, 395–401. [Google Scholar]
- Karabagias, I.K.; Vavoura, M.V.; Nikolaou, C.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 62, 753–760. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant activity, quality parameters and mineral content of Portuguese monofloral honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Terrab, A.; Diez, M.J.; Heredia, F.J. Characterization of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physicochemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A. Physicochemical characteristics, total phenols and pigments of national and international honeys in Saudi Arabia. Arab. J. Chem. 2016, 9, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Ghidotti, M.; Fiamegos, Y.; Dumitrascu, C.; de la Calle, M.B. Use of elemental profiles to verify geographical origin and botanical variety of Spanish honeys with a protected denomination of origin. Food Chem. 2021, 324, 128350. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Ye, J.-L.; Lin, T.-Y.; Wang, Y.-W.; Peng, C.-C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Marić, A.; Jovanov, P.; Sakač, M.; Novaković, A.; Hadnađev, M.; Pezo, L.; Mandić, A.; Milićević, N.; Đurović, A.; Gadžurić, S. A comprehensive study of parameters correlated with honey health benefits. RSC Adv. 2021, 11, 12434–12441. [Google Scholar] [CrossRef] [PubMed]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Tezcan, F.; Erim, F.B.; Yildiz, O.; Sahin, H.; Can, H.; Kolayli, S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT-Food Sci. Technol. 2016, 68, 273–279. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT-Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Stipčević, T.; Belščak, A. Antioxidant properties and phenolic content of different floral origin honeys. J. ApiProd. ApiMed. Sci. 2009, 1, 43–50. [Google Scholar] [CrossRef]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics in honey. LWT-Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2017, 41, e12345. [Google Scholar] [CrossRef]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; de la Luz Cadiz-Gurrea, M.; Mahmoodi-Khaledi, E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT-Food Sci. Technol. 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The antibacterial effect in vitro of honey derived from various Danish flora. Dermatol. Res. Pract. 2018, 2018, 7021713. [Google Scholar] [CrossRef] [Green Version]
- Junie, L.M.; Vică, M.L.; Glevitzky, M.; Matei, H.V. Physico-chemical characterization and antibacterial activity of different types of honey tested on strains isolated from hospitalized patients. J. Apic. Sci. 2016, 60, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Nemo, R.; Bacha, K. Microbial quality, physicochemical characteristics, proximate analysis, and antimicrobial activities of honey from Anfilo district. Food Biosci. 2021, 42, 101132. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, L.; Kong, L.; Dong, J.; Zhou, Z.; Zhang, H. Characteristic components and authenticity evaluation of rape, acacia, and linden honey. J. Agric. Food Chem. 2020, 68, 9776–9788. [Google Scholar] [CrossRef]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Res. Int. 2009, 2009, 830616. [Google Scholar] [CrossRef] [Green Version]
- Četojević-Simin, D. Tumor cell growth activity of fruit and pomace extracts. In Fruit and Pomace Extracts: Biological Activity, Potential Applications and Beneficial Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 241–253. [Google Scholar]
- Seyhan, M.F.; Yılmaz, E.; Timirci-Kahraman, Ö.; Saygılı, N.; Kısakesen, H.İ.; Eronat, A.P.; Ceviz, A.B.; Bilgiç Gazioğlu, S.; Yılmaz-Aydoğan, H.; Öztürk, O. Anatolian honey is not only sweet but can also protect from breast cancer: Elixir for women from artemis to present. IUBMB Life 2017, 69, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; Boccard, J.; Carrupt, P.A.; Nicolle, E.; Blanc, M.; Geze, A.; Choisnard, L.; Wouessidjewe, D.; Matera, E.L.; Dumontet, C. Antimitotic and antiproliferative activities of chalcones: Forward structure-activity relationship. J. Med. Chem. 2008, 51, 2307–2310. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Park, Y.S.; Heo, B.G.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Ham, K.S.; Cho, J.Y.; Kang, S.G. A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. Eur. Food Res. Technol. 2009, 228, 903–911. [Google Scholar] [CrossRef]
| Honey Type | Location | Region | Dominant/Secondary Pollen |
|---|---|---|---|
| Acacia 1 | Alibunar | Bačka, Vojvodina | Robinia pseudoacacia |
| Acacia 2 | Fruška Gora | Srem, Vojvodina | Robinia pseudoacacia |
| Linden 1 | Fruška Gora | Srem, Vojvodina | Tilia sp. |
| Linden 2 | Đerdap | northeastern Serbia | Tilia sp. |
| Heather 1 | Popovo Polje | eastern Herzegovina | Calluna vulgaris |
| Heather 2 | Ljubuški | Bosnia and Herzegovina | Calluna vulgaris |
| Rapeseed 1 | Sečanj | Banat, Vojvodina | Brassica napus |
| Rapeseed 2 | Tovariševo | Bačka, Vojvodina | Brassica napus |
| Sunflower | Apatin | Bačka, Vojvodina | Helianthus annuus |
| Phacelia | Šajkaš | Bačka, Vojvodina | Phacelia tanacetifolia |
| Basil | Novi Kneževac | Banat, Vojvodina | Stachys annua |
| Anise | Probištip | Northern Macedonia | Pinpinella sp. |
| Sage | Ljubuški | Bosnia and Herzegovina | Salvia officinalis |
| Chestnut | Cazin | Bosnia and Herzegovina | Castanea sp. |
| Hawthorn | Cer | northwestern Serbia | Crataegus monogyna |
| Lavender | Fruška Gora | Srem, Vojvodina | Lavandula stoechas |
| Meadow 1 | Trebinje | Republic of Srpska, BH | |
| Meadow 2 | Leposavić | Kosovo | |
| Meadow 3 | Plandište | Banat, Vojvodina |
| Honey Type | Moisture (%) | pH | Electrical Conductivity (μS/cm) | Free Acidity (meq/kg) | HMF (mg/kg) |
|---|---|---|---|---|---|
| Acacia 1 | 17.3 ± 0.10 c | 3.90 ± 0.01 gh | 136 ± 3.51 no | 13.8 ± 0.26 h | 4.02 ± 0.04 fg |
| Acacia 2 | 16.4 ± 0.35 ef | 4.51 ± 0.02 bc | 114 ± 2.65 o | 16.3 ± 0.30 g | 3.23 ± 0.11 gh |
| Linden 1 | 15.8 ± 0.06 hi | 4.62 ± 0.02 ab | 488 ± 10.5 h | 16.1 ± 0.21 g | 7.04 ± 0.98 c |
| Linden 2 | 17.1 ± 0.23 cd | 4.72 ± 0.02 a | 608 ± 2.08 f | 14.5 ± 0.12 gh | 5.46 ± 0.21 d |
| Heather 1 | 16.7 ± 0.21 de | 4.32 ± 0.11 d | 834 ± 8.39 d | 39.2 ± 2.19 a | 5.41 ± 0.17 de |
| Heather 2 | 15.6 ± 0.21 i | 3.36 ± 0.03 l | 809 ± 7.77 d | 30.7 ± 1.30 bc | 3.27 ± 0.06 gh |
| Rapeseed 1 | 19.4 ± 0.15 a | 4.01 ± 0.04 efg | 224 ± 3.79 l | 21.3 ± 0.68 f | 2.42 ± 0.09 hi |
| Rapeseed 2 | 18.4 ± 0.26 b | 4.10 ± 0.03 e | 191 ± 7.77 m | 16.3 ± 0.46 g | 7.15 ± 1.00 c |
| Sunflower | 17.0 ± 0.53 cd | 3.38 ± 0.22 kl | 366 ± 13.1 j | 28.9 ± 1.78 cde | 9.41 ± 0.70 a |
| Phacelia | 15.0 ± 0.21 j | 3.66 ± 0.06 i | 295 ± 4.73 k | 37.2 ± 2.00 a | 1.89 ± 0.30 i |
| Basil | 16.0 ± 0.20 gh | 3.84 ± 0.03 h | 413 ± 7.09 i | 27.9 ±0.35 de | 3.14 ± 0.13 gh |
| Anise | 16.4 ± 0.30 efg | 4.34 ± 0.04 d | 879 ± 8.74 c | 31.2 ± 0.89 b | 5.58 ± 0.86 d |
| Sage | 15.1 ± 0.21 j | 4.07 ± 0.06 ef | 557 ± 8.89 g | 37.7 ± 1.94 a | 4.94 ± 0.84 def |
| Chestnut | 16.5 ± 0.25 ef | 4.54 ± 0.11 b | 1251 ± 41.7 a | 27.1 ± 0.26 e | 4.43 ± 0.42 ef |
| Hawthorn | 19.1 ± 0.31 a | 4.41 ± 0.04 cd | 163 ± 12.5 mn | 20.3 ± 0.36 f | 8.25 ± 0.42 b |
| Lavender | 15.8 ± 0.26 hi | 3.65 ± 0.04 i | 1040 ± 54.1 b | 38.9 ± 1.63 a | 7.73 ± 1.01 bc |
| Meadow 1 | 14.9 ± 0.15 j | 3.59 ± 0.09 ij | 353 ± 12.1 j | 37.8 ± 2.76 a | 1.85 ± 0.12 i |
| Meadow 2 | 17.4 ± 0.40 c | 3.95 ± 0.04 fgh | 715 ± 11.9 e | 20.1 ± 0.70 f | 3.41 ± 0.34 gh |
| Meadow 3 | 16.3 ± 0.12 fg | 3.50 ± 0.15 jk | 470 ± 23.7 h | 29.5 ± 0.75 bcd | 1.81 ± 0.58 i |
| Honey Type | Polyphenols (mg GAE/100 g) | DPPH, IC50 (mg/mL) |
|---|---|---|
| Acacia 1 | 14.4 ± 0.49 kl | 442 ± 19.3 b |
| Acacia 2 | 13.5 ± 0.35 lm | 388 ± 10.1 d |
| Linden 1 | 67.3 ± 2.57 f | 115 ± 9.00 l |
| Linden 2 | 53.7 ± 3.37 g | 223 ± 12.3 g |
| Heather 1 | 79.3 ± 1.01 e | 137 ± 7.55 k |
| Heather 2 | 84.0 ± 3.83 g | 156 ± 10.3 j |
| Rapeseed 1 | 11.5 ± 0.70 m | 646 ± 8.72 a |
| Rapeseed 2 | 11.9 ±0.25 lm | 640 ± 22.5 a |
| Sunflower | 27.5 ± 0.50 i | 324 ± 5.51 e |
| Phacelia | 89.7 ±0.99 c | 175 ± 4.36 hi |
| Basil | 101 ± 2.72 a | 162 ± 5.29 ij |
| Anise | 98.7 ± 0.90 a | 186 ± 4.04 h |
| Sage | 90.1 ± 1.76 c | 184 ± 9.29 h |
| Chestnut | 88.8 ± 1.55 c | 193 ± 11.0 h |
| Hawthorn | 36.7 ± 1.34 h | 415 ± 14.0 c |
| Lavender | 95.6 ± 1.06 b | 88.2 ± 2.11 m |
| Meadow 1 | 24.5 ± 1.01 j | 266 ± 6.03 f |
| Meadow 2 | 26.5 ±0.76 ij | 224 ± 6.11 g |
| Meadow 3 | 16.8 ±0.50 k | 428 ± 14.2 bc |
| Honey Type | MIC % against Different Strains of Bacteria | |||||
|---|---|---|---|---|---|---|
| Escherichia Coli (ATCC 8739) | Escherichia coli | Proteus Mirabilis | Staphylococcus Aureus (ATCC 25923) | Staphylococcus Epidermidis | Enterococcus Faecalis (ATCC 29212) | |
| Acacia 1 | 25 | 25 | 25 | 25 | 12.5 | >25 |
| Acacia 2 | 25 | 25 | 25 | 12.5 | 25 | >25 |
| Linden 1 | 25 | 25 | 12.5 | 3.12 | 6.25 | 25 |
| Linden 2 | 25 | 25 | 12.5 | 12.5 | 12.5 | 25 |
| Heather 1 | 25 | 25 | 12.5 | 6.25 | 12.5 | 25 |
| Heather 2 | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| Rapeseed 1 | >25 | >25 | >25 | >25 | >25 | >25 |
| Rapeseed 2 | >25 | >25 | 25 | 25 | 25 | >25 |
| Sunflower | 25 | 25 | 12.5 | 12.5 | 12.5 | 25 |
| Phacelia | 12.5 | 25 | 12.5 | 6.25 | 3.12 | 25 |
| Basil | 25 | 25 | 12.5 | 12.5 | 12.5 | 25 |
| Anise | 25 | 25 | 12.5 | 6.25 | 12.5 | 25 |
| Sage | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| Chestnut | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| Hawthorn | >25 | >25 | 25 | 25 | 25 | 25 |
| Lavender | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| Meadow 1 | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| Meadow 2 | 25 | 25 | 12.5 | 6.25 | 6.25 | 25 |
| Meadow 3 | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| Honey Type | IC50 (mg/mL) * | |||
|---|---|---|---|---|
| HeLa | MCF7 | HT-29 | MRC-5 | |
| Acacia 1 | >50 g | >50 f | >50 e | >50 f |
| Acacia 2 | >50 g | >50 f | 30.4 ± 5.95 b | >50 f |
| Linden 1 | 12.4 ± 2.00 a | 7.46 ± 1.18 a | 43.3 ± 3.68 d | 9.93 ± 0.68 a |
| Linden 2 | 25.9 ± 1.68 d | 17.2 ± 5.24 c | 41.5 ± 2.36 d | 19.8 ± 1.38 b |
| Heather 1 | 24.0 ± 1.54 d | 18.5 ± 0.52 c | 30.0 ± 2.49 b | 25.2 ± 0.41 c |
| Heather 2 | >50 g | 40.4 ± 8.75 e | 42.9 ± 1.36 d | >50 f |
| Rapeseed 1 | >50 g | 35.8 ± 10.1 e | >50 e | >50 f |
| Rapeseed 2 | >50 g | >50 f | 42.3 ± 3.58 d | >50 f |
| Sunflower | 24.8 ± 0.28 d | 27.7 ± 1.95 d | 35.6 ± 2.68 c | 20.7 ± 2.56 b |
| Phacelia | >50 g | >50 f | 36.7 ± 2.16 c | >50 f |
| Basil | >50 g | >50 f | >50 e | >50 f |
| Anise | 21.0 ± 0.56 c | 14.7 ± 2.42 b | 28.4 ± 6.64 b | 21.9 ± 2.58 b |
| Sage | 35.2 ± 7.50 e | 26.7 ± 0.20 d | 34.3 ± 0.82 c | 45.0 ± 2.31 e |
| Chestnut | 40.3 ± 1.71 f | 25.6 ± 0.27 d | 37.2 ± 0.86 c | 34.8 ± 0.89 d |
| Hawthorn | >50 g | 35.1 ± 11.7 e | 49.0 ± 0.06 e | 44.7 ± 2.28 e |
| Lavender | 20.8 ± 2.66 c | 24.5 ± 2.52 d | 32.0 ± 4.93 b | 17.3 ± 0.53 b |
| Meadow 1 | >50 g | 49.0 ± 0.71 f | 37.4 ± 1.30 c | >50 f |
| Meadow 2 | 16.9 ± 1.54 b | 12.0 ± 0.57 b | 23.7 ± 1.33 a | 12.9 ± 0.34 a |
| Meadow 3 | >50 g | >50 f | >50 e | 40.3 ± 8.93 e |
| Standard | ||||
| Glucose | 40.0 ± 3.02 f | 33.2 ± 5.57 e | 34.5 ± 0.44 c | 39.8 ± 1.07 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants 2022, 11, 1120. https://doi.org/10.3390/antiox11061120
Sakač M, Jovanov P, Marić A, Četojević-Simin D, Novaković A, Plavšić D, Škrobot D, Kovač R. Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants. 2022; 11(6):1120. https://doi.org/10.3390/antiox11061120
Chicago/Turabian StyleSakač, Marijana, Pavle Jovanov, Aleksandar Marić, Dragana Četojević-Simin, Aleksandra Novaković, Dragana Plavšić, Dubravka Škrobot, and Renata Kovač. 2022. "Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans" Antioxidants 11, no. 6: 1120. https://doi.org/10.3390/antiox11061120
APA StyleSakač, M., Jovanov, P., Marić, A., Četojević-Simin, D., Novaković, A., Plavšić, D., Škrobot, D., & Kovač, R. (2022). Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants, 11(6), 1120. https://doi.org/10.3390/antiox11061120









