The Impact of Controlled Ovarian Stimulation on Serum Oxidative Stress Markers in Infertile Women with Endometriosis Undergoing ICSI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Duration
2.3. Participants—Eligibility Criteria
2.4. Assisted Reproduction Methodology
2.4.1. Stimulation Protocol
2.4.2. Oocyte Retrieval and Denudation
2.4.3. ICSI and Fertilization, Implantation, Clinical Pregnancy, and Live Birth Rates
2.4.4. Sample Collection and Processing
2.4.5. Methodology
- Total hydroperoxides (FOX1)As previously described by our group and other researchers [18,21,46], the FOX1 method was applied to determine the total peroxide concentration based on the conversion of Fe+2 (ferrous ions) into Fe+3 (ferric ions) by peroxides in the analyzed samples. The results were represented as µmol/g of protein.
- SOD
- VitE
- GSH—total concentration of thiols and sulfhydryl groupsSerum GSH was determined as described previously [18,21,48], in which thiol groups reacted with dithionitrobenzoic acid (DTNB) to form a deeply colored anion with a maximum peak at 412 nm (e412 = 13,600 M−1cm−1). The concentration of sulfhydryl groups was calculated using GSH, and the results were reported as nmol/g of protein.
- TAC
- AOPP
- MDA
- 8OHdG
- Determination of total proteins (FOX, MDA, and GSH, reported as g of protein)
2.5. Study Size
2.6. Statistical Analysis
3. Results
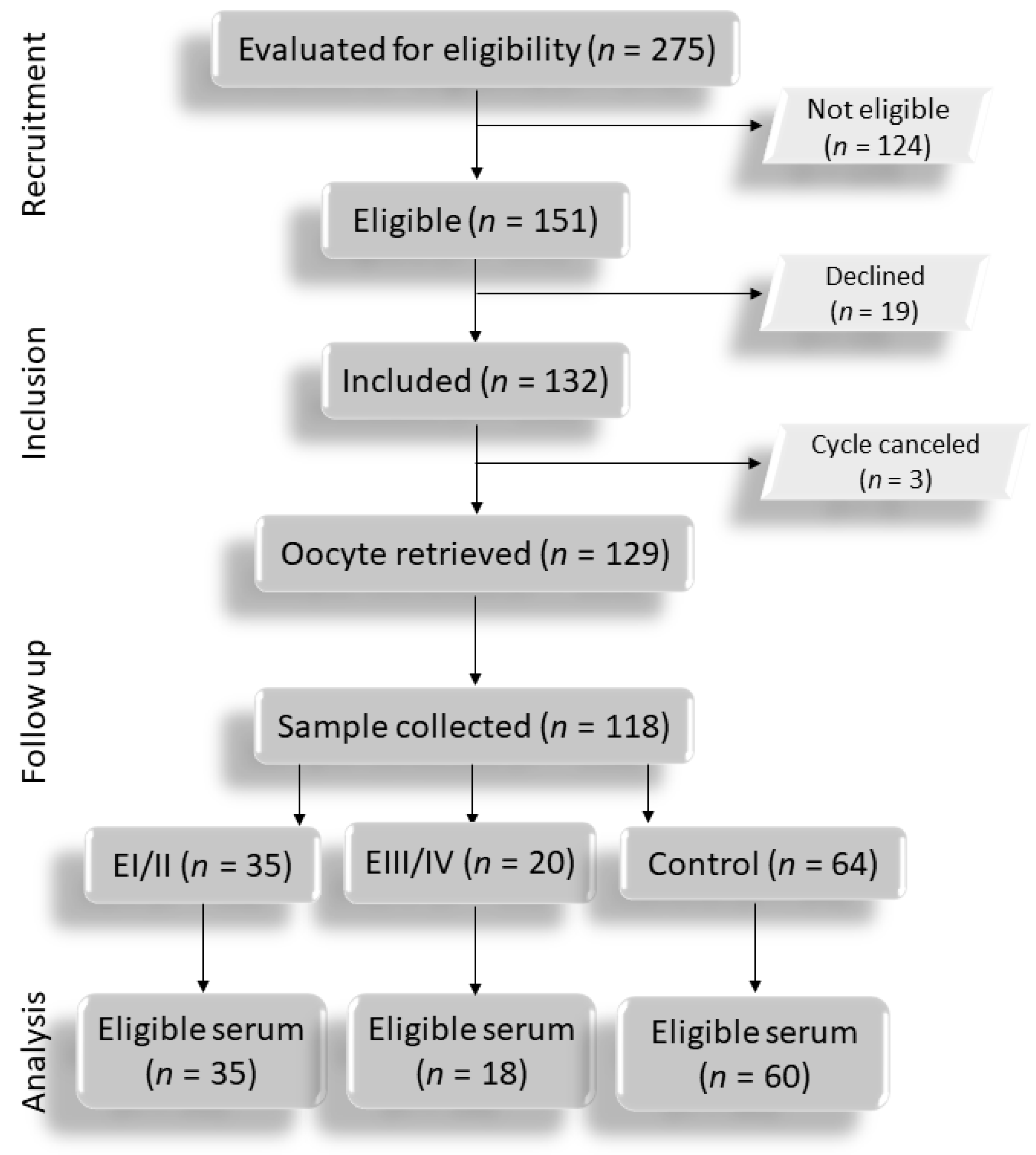
3.1. Flowchart
3.2. Clinical Variables, Response to Ovarian Stimulation, and ICSI Results in Infertile Patients with Endometriosis (Regardless of Disease Staging; E), with Endometriosis I/II (EI/II), and with Endometriosis III/IV (EIII/IV) and the Controls
3.3. Serum Oxidative Stress Markers among Infertile Patients with E, EI/II, and EIII/IV and the Controls at Different Timepoints of Controlled Ovarian Stimulation for ICSI
3.4. Serum Oxidative Stress Markers among the Different Timepoints of Controlled Ovarian Stimulation for ICSI within the Same Group of Infertile Patients with E, EI/II, and EIII/IV and the Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fassbender, A.; Vodolazkaia, A.; Saunders, P.; Lebovic, D.; Waelkens, E.; De Moor, B.; D’Hooghe, T. Biomarkers of endometriosis. Fertil. Steril. 2013, 99, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Navarro, J.; Remohi, J.; Simon, C.; Pellicer, A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum. Reprod. Update 2000, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Gupta, S.; Goldberg, J.M.; Aziz, N.; Goldberg, E.; Krajcir, N.; Agarwal, A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil. Steril. 2008, 90, 247–257. [Google Scholar] [CrossRef]
- The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Amreen, S.; Kumar, P.; Gupta, P.; Rao, P. Evaluation of Oxidative Stress and Severity of Endometriosis. J. Hum. Reprod. Sci. 2019, 12, 40–46. [Google Scholar] [CrossRef]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. 2017, 18, 582–587. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Polak, G.; Wertel, I.; Barczyński, B.; Kwaśniewski, W.; Bednarek, W.; Kotarski, J. Increased levels of oxidative stress markers in the peritoneal fluid of women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 187–190. [Google Scholar] [CrossRef]
- Polak, G.; Barczyński, B.; Wertel, I.; Kwaśniewski, W.; Bednarek, W.; Derewianka-Polak, M.; Frąszczak, K.; Olajossy, M.; Kotarski, J. Disrupted iron metabolism in peritoneal fluid may induce oxidative stress in the peritoneal cavity of women with endometriosis. Ann. Agric. Environ. Med. 2018, 25, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Chen, H.Y.; Chen, W.; Liu, Y.N.; Fu, Y.; Wang, L.N. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol. Endocrinol. 2018, 34, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Polak, G.; Barczyński, B.; Bednarek, W.; Kwaśniewski, W.; Wertell, I.; Derewianka-Polak, M.; Makara-Studzińska, M.; Kotarski, J. Increased levels of proteins of the acute inflammatory phase in the peritoneal fluid of women with advanced stages of endometriosis. Ginekol. Pol. 2015, 86, 414–418. [Google Scholar] [CrossRef]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, L.; Liu, Y.; Shi, Y.; Du, H. The expression and role of oxidative stress markers in the serum and follicular fluid of patients with endometriosis. Clin. Exp. Obstet. Gynecol. 2013, 40, 372–376. [Google Scholar]
- Choi, Y.S.; Cho, S.; Seo, S.K.; Park, J.H.; Kim, S.H.; Lee, B.S. Alteration in the intrafollicular thiol-redox system in infertile women with endometriosis. Reproduction 2015, 149, 155–162. [Google Scholar] [CrossRef]
- Da Broi, M.G.; de Albuquerque, F.O.; de Andrade, A.Z.; Cardoso, R.L.; Jordao Junior, A.A.; Navarro, P.A. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016, 366, 231–242. [Google Scholar] [CrossRef]
- Andrade, A.Z.; Rodrigues, J.K.; Dib, L.A.; Romão, G.S.; Ferriani, R.A.; Jordão Junior, A.A.; Navarro, P.A. Serum markers of oxidative stress in infertile women with endometriosis. Rev. Bras. Ginecol. Obstet. 2010, 32, 279–285. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Giorgi, V.S.I.; Rodrigues, J.K.; de Andrade, A.Z.; Junior, A.A.J.; Navarro, P.A. Systemic oxidative stress as a possible mechanism underlying the pathogenesis of mild endometriosis-related infertility. Reprod. Biomed. Online 2019, 39, 785–794. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Jordão, A.A.; Ferriani, R.A.; Navarro, P.A. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol. Reprod. Dev. 2018, 85, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Malvezzi, H.; Paz, C.C.; Ferriani, R.A.; Navarro, P.A. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Hum. Reprod. 2014, 29, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Shridharani, A.; Sandlow, J.I. Vasectomy reversal versus IVF with sperm retrieval: Which is better? Curr. Opin. Urol. 2010, 20, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bouwmans, C.A.; Lintsen, B.M.; Eijkemans, M.J.; Habbema, J.D.; Braat, D.D.; Hakkaart, L. A detailed cost analysis of in vitro fertilization and intracytoplasmic sperm injection treatment. Fertil. Steril. 2008, 89, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, I.; Ruiz-Sanz, J.I.; Del Agua, A.R.; Navarro, R.; Hernández, M.L.; Matorras, R.; Prieto, B.; Ruiz-Larrea, M.B. Serum oxidizability and antioxidant status in patients undergoing in vitro fertilization. Fertil. Steril. 2010, 94, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Kollmann, Z.; Flück, C.E.; Stute, P.; Marti, U.; Weiss, B.; Bersinger, N.A. Gonadotrophin stimulation for in vitro fertilization significantly alters the hormone milieu in follicular fluid: A comparative study between natural cycle IVF and conventional IVF. Hum. Reprod. 2014, 29, 1049–1057. [Google Scholar] [CrossRef]
- Yeste, M.; Jones, C.; Amdani, S.N.; Patel, S.; Coward, K. Oocyte activation deficiency: A role for an oocyte contribution? Hum. Reprod. Update 2016, 22, 23–47. [Google Scholar] [CrossRef]
- Pizarro, B.M.; Cordeiro, A.; Reginatto, M.W.; Campos, S.P.C.; Mancebo, A.C.A.; Areas, P.C.F.; Antunes, R.A.; Souza, M.D.C.B.; Oliveira, K.J.; Bloise, F.F.; et al. Estradiol and Progesterone Levels are Related to Redox Status in the Follicular Fluid During. J. Endocr. Soc. 2020, 4, bvaa064. [Google Scholar] [CrossRef]
- Pérez-Ruiz, I.; Meijide, S.; Ferrando, M.; Larreategui, Z.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Ovarian stimulated cycles reduce protection of follicular fluid against free radicals. Free Radic. Biol. Med. 2019, 145, 330–335. [Google Scholar] [CrossRef]
- Ayres, S.; Tang, M.; Subbiah, M.T. Estradiol-17beta as.s an antioxidant: Some distinct features when compared with common fat-soluble antioxidants. J. Lab. Clin. Med. 1996, 128, 367–375. [Google Scholar] [CrossRef]
- Reyes, M.R.; Sifuentes-Alvarez, A.; Lazalde, B. Estrogens are potentially the only steroids with an antioxidant role in pregnancy: In vitro evidence. Acta Obstet. Gynecol. Scand. 2006, 85, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Spencer, W.A.; Vadhanam, M.V.; Jeyabalan, J.; Gupta, R.C. Oxidative DNA damage following microsome/Cu(II)-mediated activation of the estrogens, 17β-estradiol, equilenin, and equilin: Role of reactive oxygen species. Chem. Res. Toxicol. 2012, 25, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Barash, A.; Weissman, A.; Manor, M.; Milman, D.; Ben-Arie, A.; Shoham, Z. Prospective evaluation of endometrial thickness as a predictor of pituitary down-regulation after gonadotropin-releasing hormone analogue administration in an in vitro fertilization program. Fertil. Steril. 1998, 69, 496–499. [Google Scholar] [CrossRef]
- Uppangala, S.; Fernandes, G.; Salian, S.R.; Kumar, P.; Talevi, R.; Kalthur, G.; Adiga, S.K. Reduced ovarian response to controlled ovarian stimulation is associated with increased oxidative stress in the follicular environment. Reprod. Biol. 2020, 20, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Clower, C.; Nelsen, D.; Butler, W.; Carvalho, A.; Hok, E.; Garelnabi, M. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J. Assist. Reprod. Genet. 2012, 29, 1083–1089. [Google Scholar] [CrossRef]
- Hamdan, M.; Omar, S.Z.; Dunselman, G.; Cheong, Y. Influence of endometriosis on assisted reproductive technology outcomes: A systematic review and meta-analysis. Obstet. Gynecol. 2015, 125, 79–88. [Google Scholar] [CrossRef]
- Harb, H.M.; Gallos, I.D.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 2013, 120, 1308–1320. [Google Scholar] [CrossRef]
- Opøien, H.K.; Fedorcsak, P.; Omland, A.K.; Abyholm, T.; Bjercke, S.; Ertzeid, G.; Oldereid, N.; Mellembakken, J.R.; Tanbo, T. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil. Steril. 2012, 97, 912–918. [Google Scholar] [CrossRef]
- Pop-Trajkovic, S.; Popović, J.; Antić, V.; Radović, D.; Stefanović, M.; Stavanovic, M.; Vukomanović, P. Stages of endometriosis: Does it affect in vitro fertilization outcome. Taiwan. J. Obstet. Gynecol. 2014, 53, 224–226. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Teixeira, D.M.; Navarro, P.A.; Ferriani, R.A.; Nastri, C.O.; Martins, W.P. Impact of endometriosis and its staging on assisted reproduction outcome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2014, 44, 261–278. [Google Scholar] [CrossRef]
- Coelho Neto, M.A.; Martins, W.e.P.; Luz, C.M.; Jianini, B.T.; Ferriani, R.A.; Navarro, P.A. Endometriosis, Ovarian Reserve and Live Birth Rate Following In Vitro Fertilization/Intracytoplasmic Sperm Injection. Rev. Bras. Ginecol. Obstet. 2016, 38, 218–224. [Google Scholar] [CrossRef] [PubMed]
- González-Comadran, M.; Schwarze, J.E.; Zegers-Hochschild, F.; Souza, M.D.; Carreras, R.; Checa, M. The impact of endometriosis on the outcome of Assisted Reproductive Technology. Reprod. Biol. Endocrinol. 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [CrossRef]
- Galli, F.; Piroddi, M.; Annetti, C.; Aisa, C.; Floridi, E.; Floridi, A. Oxidative stress and reactive oxygen species. Contrib. Nephrol. 2005, 149, 240–260. [Google Scholar] [CrossRef]
- Arnaud, J.; Fortis, I.; Blachier, S.; Kia, D.; Favier, A. Simultaneous determination of retinol, alpha-tocopherol and beta-carotene in serum by isocratic high-performance liquid chromatography. J. Chromatogr. 1991, 572, 103–116. [Google Scholar] [CrossRef]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar]
- Costa, C.M.; Santos, R.C.C.; Lima, E.S. A simple automated procedure for thiol measurement in human serum samples. J. Bras. Patol. Med. Lab. 2006, 42, 5. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- El-Shahat, K.H.; Kandil, M. Antioxidant capacity of follicular fluid in relation to follicular size and stage of estrous cycle in buffaloes. Theriogenology 2012, 77, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Nishigori, C.; Takeuchi, T.; Oka, S.; Morimoto, K.; Utani, A.; Miyachi, Y. 4-Nitroquinoline 1-oxide forms 8-hydroxydeoxyguanosine in human fibroblasts through reactive oxygen species. Toxicol. Sci. 2006, 91, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shi, Y.F.; Zhou, C.Y. Expressions of aromatase P450 and estrogen receptor in eutopic and ectopic endometrium in endometriosis and their correlation with endometriosis. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 171–174. [Google Scholar]
- Kim, Y.A.; Kim, M.R.; Lee, J.H.; Kim, J.Y.; Hwang, K.J.; Kim, H.S.; Lee, E.S. Gonadotropin-releasing hormone agonist reduces aromatase cytochrome P450 and cyclooxygenase-2 in ovarian endometrioma and eutopic endometrium of patients with endometriosis. Gynecol. Obstet. Investig. 2009, 68, 73–81. [Google Scholar] [CrossRef]
- Kaya, H.; Sezik, M.; Ozkaya, O.; Dittrich, R.; Siebzehnrubl, E.; Wildt, L. Lipid peroxidation at various estradiol concentrations in human circulation during ovarian stimulation with exogenous gonadotropins. Horm. Metab. Res. 2004, 36, 693–695. [Google Scholar] [CrossRef]
- Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Burghardt, R.C.; Banu, S.K. Edaravone mitigates hexavalent chromium-induced oxidative stress and depletion of antioxidant enzymes while estrogen restores antioxidant enzymes in the rat ovary in F1 offspring. Biol. Reprod. 2014, 91, 12. [Google Scholar] [CrossRef]
- Andrade, S.S.; Azevedo, A.E.C.; Monasterio, I.C.; Paredes-Gamero, E.J.; Gonçalves, G.A.; Bonetti, T.C.; Albertoni, G.; Schor, E.; Barreto, J.A.; Luiza Oliva, M.; et al. 17β-Estradiol and steady-state concentrations of H2O2: Antiapoptotic effect in endometrial cells from patients with endometriosis. Free Radic. Biol. Med. 2013, 60, 63–72. [Google Scholar] [CrossRef]
- Vuong, L.N.; Ho, T.M.; Pham, T.D.; Ho, V.N.A.; Andersen, C.Y.; Humaidan, P. The early luteal hormonal profile in IVF patients triggered with hCG. Hum. Reprod. 2020, 35, 157–166. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Joshi, N.; Puscheck, E.; Diamond, M.P.; Abu-Soud, H.M. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil. Steril. 2014, 102, 151–159.e155. [Google Scholar] [CrossRef]
- Donabela, F.C.; Meola, J.; Padovan, C.C.; de Paz, C.C.; Navarro, P.A. Higher SOD1 Gene Expression in Cumulus Cells From Infertile Women With Moderate and Severe Endometriosis. Reprod. Sci. 2015, 22, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Lutosławska, G.; Tkaczyk, J.; Panczenko-Kresowska, B.; Hübner-Woźniak, E.; Skierska, E.; Gajewski, A.K. Plasma TBARS, blood GSH concentrations, and erythrocyte antioxidant enzyme activities in regularly menstruating women with ovulatory and anovulatory menstrual cycles. Clin. Chim. Acta 2003, 331, 159–163. [Google Scholar] [CrossRef]
- Jana, S.K.; Dutta, M.; Joshi, M.; Srivastava, S.; Chakravarty, B.; Chaudhury, K. 1H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. Biomed Res. Int. 2013, 2013, 329058. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Sites, C.K.; Matthews, D.E.; Casson, P.R. Ovarian suppression with gonadotropin-releasing hormone agonist reduces whole body protein turnover in women. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E483–E490. [Google Scholar] [CrossRef][Green Version]
- Kodaman, P.H.; Behrman, H.R. Endocrine-regulated and protein kinase C-dependent generation of superoxide by rat preovulatory follicles. Endocrinology 2001, 142, 687–693. [Google Scholar] [CrossRef]
| Variable | Control | Endometriosis (E) | Endometriosis I/II (EI/II) | Endometriosis III/IV (EIII/IV) |
|---|---|---|---|---|
| Age (years) | 33.28 (±4.56) | 32.93 (±3.27) | 32.94 (±3.39) | 32.68 (±3.73) |
| BMI (kg/m2) | 24.12 (±3.4) | 24.05 (2.9) | 24.05 (±2.9) | 23.84 (±4.19) |
| Baseline FSH | 5.52 (±1.94) | 5.74 (±2.3) | 5.78 (±2.3) | 5.48 (±2.85) |
| Total dose of FSH | 1998.45 (±830.2) ab | 2044.43 (±657.39) | 1820.83 (±584.21) ac | 2392.5 (±673.73) bc |
| Days of stimulation | 8.42 (±2.01) | 8.78 (±1.74) | 8.47 (±1.81) | 9.39 (±1.33) |
| Endometrial thickness (mm) | 12.63 | 10.73 (±1.71) | 10.63 (±1.64) | 10.48 (±1.94) |
| Number of retrieved oocytes | 5.89 (±3.54) | 7.36 (±5.06) | 8.03 (±5.23) | 7.44 (±4.97) |
| Number of metafase II oocytes | 4.56 (±3.1) | 5.35 (±3.15) | 5.62 (±2.98) | 5.67 (±3.51) |
| Fertilization rate (%) | 81.08% abc | 72.31% a | 73.19% bd | 67.77% cd |
| Number of high-quality embryos | 1.00 (±1.19) | 1.18 (±1.47) | 1.38 (±1.61) | 1.00 (±1.32) |
| Number of formed embryos | 2.72 (±1.35) | 2.75 (±1.44) | 3.00 (±1.44) | 2.53 (±1.55) |
| Number of cycles with fresh embryo transfer | 30 | 31 | 30 | 17 |
| Clinical pregnancy rate (%) | 16/48 (33.33%) | 19/46 (41.3%) | 11/33 (39.29%) | 6/33 (42.84%) |
| Live birth rate (%) | 13/48 (27.08%) | 12/47 (25.53%) | 7/29 (24.14%) | 3/29 (21.43%) |
| Time-Point | OS Marker | C (n = 60) | E (n = 53) | EI/II (n = 35) | EIII/IV (n = 18) |
|---|---|---|---|---|---|
| D1 | FOX1 | 8.38 (7.85–8.92) | 8.81 (8.32–9.30) | 8.77 (8.18–9.36) | 9.23 (8.00–10.46) |
| SOD | 589.87 (516.44–663.30) | 554.82 (511.10–598.54) | 534.63 (488.73–580.53) | 580.07 (479.07–681.07) | |
| VitE | 22.06 (20.50–23.63) | 22.3 (20.41–24.19) | 21.56 (19.28–23.84) | 22.16 (17.96–26.36) | |
| GSH | 181.91 (167.15–196.67) a | 191.38 (175.29–207.46) | 189.48 (170.37–208.60) ab | 179.26 (142.81–215.70) b | |
| TAC | 0.49 (0.45–0.53) abc | 0.40 (0.34–0.45) a | 0.40 (0.33–0.46) b | 0.39 (0.30–0.48) c | |
| AOPP | 103.45 (87.59–119.32) | 113.84 (100.12–127.55) | 104.96 (89.29–120.62) | 119.97 (92.81–147.14) | |
| MDA | 18.96 (15.85–22.07) | 19.67 (17.17–22.17) | 19.81 (16.18–23.45) | 18.99 (14.14–23.84) | |
| 8OHdG | 21.27 (19.06–23.49) abc | 16.92 (15.12–18.73) a | 15.81 (14.18–17.44) b | 16.92 (11.67–22.18) c | |
| D2 | FOX1 | 7.76 (7.30–8.22) | 7.82 (7.45–8.19) | 8.09 (7.56–8.63) | 7.45 (6.70–8.19) |
| SOD | 560.0 (499.45–620.55) | 520.69 (485.0–556.39) | 507.43 (454.01–560.85) | 537.15 (467.85–606.45) | |
| VitE | 21.4 (19.62–23.18) | 22.55 (20.96–24.14) | 22.83 (20.47–25.19) | 22.74 (19.19–26.29) | |
| GSH | 181.26 (168.74–193.78) a* | 211.13 (199.29–222.96) a | 201.78 (183.82–219.74) | 216.25 (198.29–234.20) * | |
| TAC | 0.50 (0.46–0.53) ab | 0.39 (0.33–0.46) a | 0.44 (0.35–0.53) c | 0.36 (0.23–0.49) bc | |
| AOPP | 136.05 (115.39–156.70) | 146.98 (126.49–167.46) | 146.19 (117.38–177.01) | 152.00 (110.92–193.08) | |
| MDA | 20.66 (16.95–24.38) | 19.26 (16.01–22.50) | 19.29 (14.77–23.82) | 16.63 (10.27–23.00) | |
| 8OHdG | 20.23 (18.25–22.20) | 19.08 (16.66–21.49) | 18.53 (15.59–21.48) | 20.19 (14.28–26.09) | |
| D3 | FOX1 | 7.71 (7.12–8.29) | 7.69 (7.32–8.06) | 7.72 (7.16–8.27) | 7.89 (7.11–8.67) |
| SOD | 481.48 (431.22–531.75) | 499.93 (433.49–566.37) | 526.34 (453.26–599.42) | 485.83 (305.34–666.32) | |
| VitE | 22.22 (19.93–24.51) | 23.05 (20.92–25.17) | 21.94 (19.23–24.66) | 23.98 (19.0–28.97) | |
| GSH | 188.28 (176.88–199.69) ab* | 222.13 (212.58–231.68) a | 214.18 (200.72–227.64) * | 235.45 (217.51–253.38) b | |
| TAC | 0.43 (0.40–0.47) ab | 0.33 (0.27–0.39) a | 0.35 (0.27–0.42) | 0.31 (0.19–0.43) b | |
| AOPP | 124.68 (103-57–145.79) | 132.84 (112.28–153.40) | 135.23 (104.30–166.15) | 126.76 (89.23–164.30) | |
| MDA | 18.94 (15.37–22.51) | 18.81 (15.73–21.88) | 19.67 (15.68–23.65) | 16.43 (9.69–23.18) | |
| 8OHdG | 21.45 (19.19–23.70) ab | 18.37 (16.21–20.53) a | 17.06 (14.33–19.79) b | 18.80 (14.98–22.62) | |
| D4 | FOX1 | 7.66 (7.15–8.17) a* | 8.22 (7.72–8.72) * | 8.38 (7.61–9.14) | 8.36 (7.61–9.12) a |
| SOD | 564.66 (511.83–617.49) ab | 666.69 (576.13–757.25) a | 619.89 (492.77–747.01) c | 812.78 (651.02–974.55) bc | |
| VitE | 20.80 (18.62–22.97) | 23.72 (21.52–25.92) | 23.78 (21.17–26.39) | 23.77 (18.66–28.89) | |
| GSH | 196.83 (184.12–209.55) * | 220.51 (207.54–233.49) * | 219.52 (200.91–238.12) | 231.82 (210.38–253.27) | |
| TAC | 0.45 (0.41–0.50) abc | 0.34 (0.29–0.39) a | 0.34 (0.27–0.42) b | 0.29 (0.20–0.37) c | |
| AOPP | 115.26 (96.23–134.29) a | 133.83 (112.16–155.51) | 149.88 (117.60–182.17) a | 121.47 (84.67–158.28) | |
| MDA | 18.82 (15.5–22.14) | 19.45 (15.64–23.27) | 18.96 (13.51–24.41) | 18.0 (11.62–24–37) | |
| 8OHdG | 19.38 (17.35–21.40) | 16.57 (15.30–17.85) | 16.05 (14.58–17.52) | 17.36 (14.28–10.44) |
| Group | OS Marker | D1 | D2 | D3 | D4 |
|---|---|---|---|---|---|
| C (n = 60) | FOX1 | 8.38 abc | 7.76 a | 7.71 b | 7.66 c |
| SOD | 589.87 a | 560.00 b | 481.48 abc | 564.66 c | |
| VITE | 22.06 | 22.30 | 22.22 | 20.80 | |
| GSH | 181.90 | 181.26 | 188.28 | 196.83 | |
| TAC | 0.49 * | 0.50 | 0.43 * | 0.45 | |
| AOPP | 103.45a | 136.05 ab | 124.68 | 115.26 b | |
| MDA | 18.96 * | 20.66 *a | 18.94 | 18.82 a | |
| 8OHdG | 21.27 | 20.23 | 21.45 | 19.38 | |
| E (n = 53) | FOX1 | 8.81 ab | 7.82 a | 7.69 b | 8.22 |
| SOD | 554.82 a | 520.69 b | 499.93 c | 666.69 abc | |
| VITE | 22.30 | 22.55 | 23.05 | 23.72 | |
| GSH | 191.38 *ab | 211.13 * | 222.13 a | 220.51 b | |
| TAC | 0.40 ab | 0.39 * | 0.33 a* | 0.34 b | |
| AOPP | 113.84 a | 146.98 a | 132.84 | 133.83 | |
| MDA | 19.67 | 19.26 | 18.81 | 19.45 | |
| 8OHdG | 16.92 | 19.08 | 18.37 | 16.57 | |
| EI/II (n = 35) | FOX1 | 8.77 *a | 8.09 * | 7.72 a | 8.38 |
| SOD | 534.63 | 507.43 a | 526.34 | 619.89 a | |
| VITE | 21.56 | 22.83 | 21.94 | 23.78 | |
| GSH | 189.48 a | 201.78 | 214.18 | 219.52 a | |
| TAC | 0.40 | 0.44 ab | 0.35 a | 0.34 b | |
| AOPP | 104.96 ab | 147.19 a | 135.23 | 149.88 b | |
| MDA | 19.81 | 19.29 | 19.67 | 18.96 | |
| 8OHdG | 15.81 | 18.53 | 17.06 | 16.05 | |
| EIII/IV (n = 18) | FOX1 | 9.23 a | 7.45 a | 7.89 | 8.36 |
| SOD | 580.07 a | 537.15 b | 485.83 c | 812.78 abc | |
| VITE | 22.16 | 22.74 | 23.98 | 23.77 | |
| GSH | 179.26 ab | 216.25 | 235.45 a | 231.82 b | |
| TAC | 0.39 a | 0.36 | 0.31 | 0.29 a | |
| AOPP | 119.97 | 152.00 | 126.76 | 121.47 | |
| MDA | 18.99 | 16.63 | 16.43 | 18.00 | |
| 8OHdG | 16.92 | 20.19 | 18.80 | 17.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Broi, M.G.; Ferreira, E.M.; Andrade, A.Z.; Jordão, A.A.; Ferriani, R.A.; Navarro, P.A. The Impact of Controlled Ovarian Stimulation on Serum Oxidative Stress Markers in Infertile Women with Endometriosis Undergoing ICSI. Antioxidants 2022, 11, 1161. https://doi.org/10.3390/antiox11061161
Da Broi MG, Ferreira EM, Andrade AZ, Jordão AA, Ferriani RA, Navarro PA. The Impact of Controlled Ovarian Stimulation on Serum Oxidative Stress Markers in Infertile Women with Endometriosis Undergoing ICSI. Antioxidants. 2022; 11(6):1161. https://doi.org/10.3390/antiox11061161
Chicago/Turabian StyleDa Broi, Michele Gomes, Elisa Melo Ferreira, Aline Zyman Andrade, Alceu Afonso Jordão, Rui Alberto Ferriani, and Paula Andrea Navarro. 2022. "The Impact of Controlled Ovarian Stimulation on Serum Oxidative Stress Markers in Infertile Women with Endometriosis Undergoing ICSI" Antioxidants 11, no. 6: 1161. https://doi.org/10.3390/antiox11061161
APA StyleDa Broi, M. G., Ferreira, E. M., Andrade, A. Z., Jordão, A. A., Ferriani, R. A., & Navarro, P. A. (2022). The Impact of Controlled Ovarian Stimulation on Serum Oxidative Stress Markers in Infertile Women with Endometriosis Undergoing ICSI. Antioxidants, 11(6), 1161. https://doi.org/10.3390/antiox11061161






