Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants
Abstract
:1. Introduction
2. Clinical Risk Factors and Treatment of CAVS
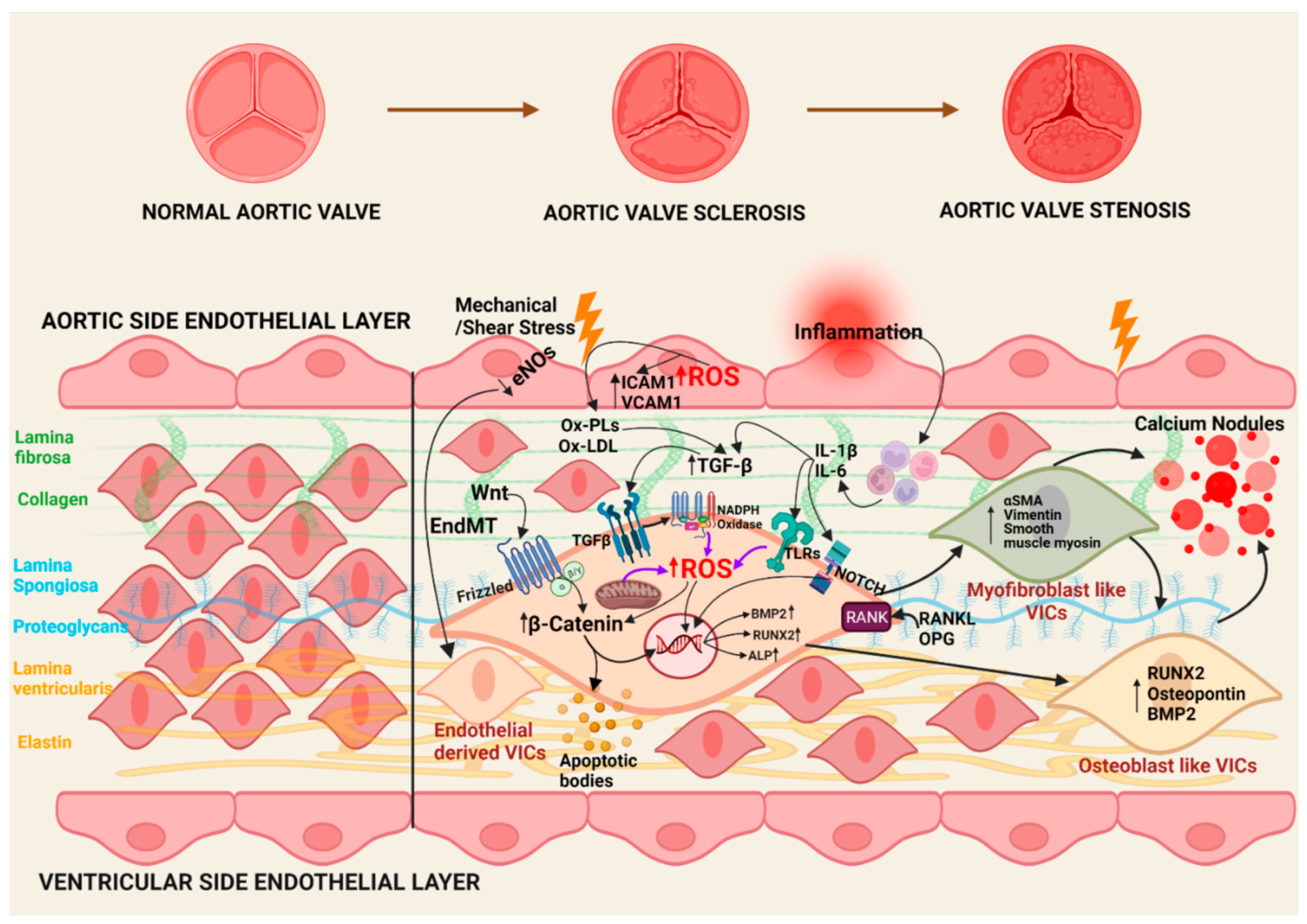
3. Oxidative Stress in the Pathophysiology of CAVS
3.1. Initiation Phase
3.2. Propagation Phase
3.3. Oxidative Stress in CAVS
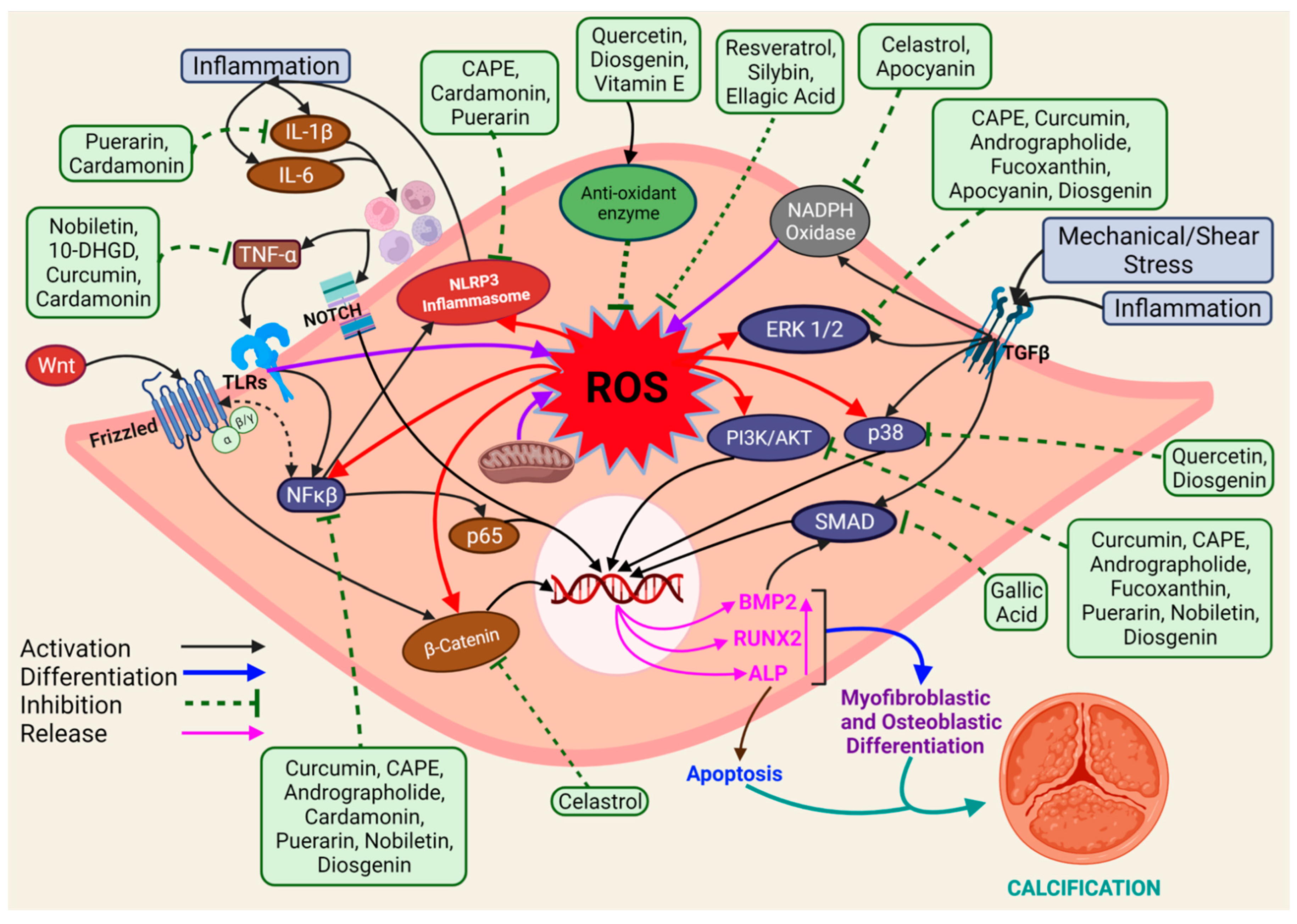
4. Protective Effect of Natural Antioxidants on CAVS Development
4.1. Curcumin
4.2. Nobiletin
4.3. Caffeic Acid Phenethyl Ester
4.4. Celastrol
4.5. Andrographolide
4.6. Fucoxanthin
4.7. Cardamonin
4.8. Others
5. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.-A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Prim. 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerman, D.A.; Prasad, S.; Alotti, N. Calcific Aortic Valve Disease: Molecular Mechanisms and Therapeutic Approaches. Eur. Cardiol. 2015, 10, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific Aortic Stenosis: A Disease of the Valve and the Myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef] [Green Version]
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M.; et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef] [Green Version]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific Aortic Valve Disease: Not Simply a Degenerative Process. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Bogdanova, M.; Kostina, A.; Zihlavnikova Enayati, K.; Zabirnyk, A.; Malashicheva, A.; Stensløkken, K.-O.; Sullivan, G.J.; Kaljusto, M.-L.; Kvitting, J.-P.E.; Kostareva, A.; et al. Inflammation and Mechanical Stress Stimulate Osteogenic Differentiation of Human Aortic Valve Interstitial Cells. Front. Physiol. 2018, 9, 1635. [Google Scholar] [CrossRef]
- Miller, J.D.; Weiss, R.M.; Heistad, D.D. Calcific aortic valve stenosis: Methods, models, and mechanisms. Circ. Res. 2011, 108, 1392–1412. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, H.Z.E.; Zhao, G.; Shah, A.M.; Zhang, M. Role of oxidative stress in calcific aortic valve disease and its therapeutic implications. Cardiovasc. Res. 2022, 118, 1433–1451. [Google Scholar] [CrossRef]
- Faggiano, P.; Antonini-Canterin, F.; Baldessin, F.; Lorusso, R.; D’Aloia, A.; Cas, L.D. Epidemiology and cardiovascular risk factors of aortic stenosis. Cardiovasc. Ultrasound 2006, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Kamceva, G.; Arsova-Sarafinovska, Z.; Ruskovska, T.; Zdravkovska, M.; Kamceva-Panova, L.; Stikova, E. Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced. J. Med. Sci. 2016, 4, 636–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid Oxidation Products Have Opposite Effects on Calcifying Vascular Cell and Bone Cell Differentiation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Grimard, B.H.; Safford, R.E.; Burns, E.L. Aortic Stenosis: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 371–378. [Google Scholar]
- Kimura, N.; Nomura, Y.; Aomatsu, A.; Matsuda, A.; Imamura, Y.; Taniguchi, Y.; Hori, D.; Morishita, Y.; Fujita, H.; Yuri, K.; et al. Effect of Transcatheter Aortic Valve Implantation on the Immune Response Associated With Surgical Aortic Valve Replacement. Am. J. Cardiol. 2020, 128, 35–44. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Elbaz-Greener, G.; Rozen, G.; Kusniec, F.; Marai, I.; Carasso, S.; Ko, D.T.; Wijeysundera, H.C.; Alcalai, R.; Planer, D.; Amir, O. Comparing Trajectory of Surgical Aortic Valve Replacement in the Early vs. Late Transcatheter Aortic Valve Replacement Era. Front. Cardiovasc. Med. 2021, 8, 680123. [Google Scholar] [CrossRef]
- Khalil, C.A.; Ignatiuk, B.; Erdem, G.; Chemaitelly, H.; Barilli, F.; El-Shazly, M.; Al Suwaidi, J.; Aboulsoud, S.; Kofler, M.; Stastny, L.; et al. Aortic valve function post-replacement of severe aortic stenosis by transcatheter procedure versus surgery: A systematic review and metanalysis. Sci. Rep. 2021, 11, 11975. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yearwood, T.L.; Misbach, G.A.; Chandran, K.B. Experimental fluid dynamics of aortic stenosis in a model of the human aorta. Clin. Phys. Physiol. Meas. 1989, 10, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, L.A.; Grego-Bessa, J.; Raya, A.; Bertrán, E.; Pérez-Pomares, J.M.; Díez, J.; Aranda, S.; Palomo, S.; McCormick, F.; Izpisúa-Belmonte, J.C.; et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004, 18, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Wirrig, E.E.; Yutzey, K.E. Conserved Transcriptional Regulatory Mechanisms in Aortic Valve Development and Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, L.M.; Markwald, R.R. Molecular Regulation of Atrioventricular Valvuloseptal Morphogenesis. Circ. Res. 1995, 77, 1–6. [Google Scholar] [CrossRef]
- Aikawa, E.; Whittaker, P.; Farber, M.; Mendelson, K.; Padera, R.F.; Aikawa, M.; Schoen, F.J. Human Semilunar Cardiac Valve Remodeling by Activated Cells From Fetus to Adult. Circulation 2006, 113, 1344–1352. [Google Scholar] [CrossRef]
- Liberman, M.; Bassi, E.; Martinatti, M.K.; Lario, F.C.; Wosniak, J.; Pomerantzeff, P.M.A.; Laurindo, F.R.M. Oxidant Generation Predominates Around Calcifying Foci and Enhances Progression of Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.D.; Chu, Y.; Brooks, R.M.; Richenbacher, W.E.; Peña-Silva, R.; Heistad, D.D. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008, 52, 843–850. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.D.; Reichenbach, D.D.; Marcovina, S.M.; Kuusisto, J.; Alpers, C.E.; Otto, C.M. Apolipoproteins B, (a), and E Accumulate in the Morphologically Early Lesion of ‘Degenerative’ Valvular Aortic Stenosis. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 523–532. [Google Scholar] [CrossRef]
- Mohty, D.; Pibarot, P.; Després, J.-P.; Côté, C.; Arsenault, B.; Cartier, A.; Cosnay, P.; Couture, C.; Mathieu, P. Association Between Plasma LDL Particle Size, Valvular Accumulation of Oxidized LDL, and Inflammation in Patients With Aortic Stenosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of Oxidized Low Density Lipoprotein in Nonrheumatic Stenotic Aortic Valves. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucosky, P.; Balachandran, K.; Elhammali, A.; Jo, H.; Yoganathan, A.P. Altered Shear Stress Stimulates Upregulation of Endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-Dependent Pathway. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelbaky, A.; Corsini, E.; Figueroa, A.L.; Subramanian, S.; Fontanez, S.; Emami, H.; Hoffmann, U.; Narula, J.; Tawakol, A. Early aortic valve inflammation precedes calcification: A longitudinal FDG-PET/CT study. Atherosclerosis 2015, 238, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Bouchareb, R.; Boulanger, M.-C. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J. Immunol. Res. 2015, 2015, 851945. [Google Scholar] [CrossRef]
- West, X.Z.; Malinin, N.L.; Merkulova, A.A.; Tischenko, M.; Kerr, B.A.; Borden, E.C.; Podrez, E.A.; Salomon, R.G.; Byzova, T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 2010, 467, 972–976. [Google Scholar] [CrossRef]
- Hsu, H.; Shu, H.-B.; Pan, M.-G.; Goeddel, D.V. TRADD–TRAF2 and TRADD–FADD Interactions Define Two Distinct TNF Receptor 1 Signal Transduction Pathways. Cell 1996, 84, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-L.; Woo, K.M.; Ryoo, H.-M.; Baek, J.-H. Tumor necrosis factor-α increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem. Biophys. Res. Commun. 2010, 391, 1087–1092. [Google Scholar] [CrossRef]
- Isoda, K.; Matsuki, T.; Kondo, H.; Iwakura, Y.; Ohsuzu, F. Deficiency of Interleukin-1 Receptor Antagonist Induces Aortic Valve Disease in BALB/c Mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Galeone, A.; Brunetti, G.; Oranger, A.; Greco, G.; Di Benedetto, A.; Mori, G.; Colucci, S.; Zallone, A.; Paparella, D.; Grano, M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int. J. Cardiol. 2013, 169, 296–304. [Google Scholar] [CrossRef]
- New, S.E.P.; Aikawa, E. Cardiovascular Calcification—An Inflammatory Disease. Circ. J. 2011, 75, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.C.; Joag, V.R.; Gotlieb, A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 2007, 171, 1407–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, L.; Yacoub, M.H.; Latif, N.; Amrani, M.; Chester, A.H. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation 2006, 114, I547–I552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahair, M.M.; Howe, C.J.; Rodriguez-Mora, O.; McCubrey, J.A.; Franklin, R.A. Molecular pathways leading to oxidative stress-induced phosphorylation of Akt. Antioxid. Redox Signal. 2006, 8, 1749–1756. [Google Scholar] [CrossRef]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef]
- Kaden, J.J.; Bickelhaupt, S.; Grobholz, R.; Haase, K.K.; Sarιkoç, A.; Brueckmann, M.; Lang, S.; Zahn, I.; Vahl, C.; Hagl, S. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulate aortic valve calcification. J. Mol. Cell. Cardiol. 2004, 36, 57–66. [Google Scholar] [CrossRef]
- Caira, F.C.; Stock, S.R.; Gleason, T.G.; McGee, E.C.; Huang, J.; Bonow, R.O.; Spelsberg, T.C.; McCarthy, P.M.; Rahimtoola, S.H.; Rajamannan, N.M. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J. Am. Coll. Cardiol. 2006, 47, 1707–1712. [Google Scholar] [CrossRef] [Green Version]
- Côté, N.; El Husseini, D.; Pépin, A.; Guauque-Olarte, S.; Ducharme, V.; Bouchard-Cannon, P.; Audet, A.; Fournier, D.; Gaudreault, N.; Derbali, H. ATP acts as a survival signal and prevents the mineralization of aortic valve. J. Mol. Cell. Cardiol. 2012, 52, 1191–1202. [Google Scholar] [CrossRef]
- El Husseini, D.; Boulanger, M.-C.; Mahmut, A.; Bouchareb, R.; Laflamme, M.-H.; Fournier, D.; Pibarot, P.; Bossé, Y.; Mathieu, P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: Implication for calcific aortic valve disease. J. Mol. Cell. Cardiol. 2014, 72, 146–156. [Google Scholar] [CrossRef]
- Migdal, C.; Serres, M. Reactive oxygen species and oxidative stress. Med. Sci. 2011, 27, 405–412. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Adhikari, D.; Lee, J.P.; Kang, K.-W.; Lee, I.-S.; Kim, H.J.; Oak, M.-H. Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds. Antioxidants 2020, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-T.; Shao, Y.-D.; Liu, Y.-Z.; Xiao, X.; Cheng, Z.-B.; Qu, S.-L.; Huang, L.; Zhang, C. Oxidative stress in vascular calcification. Clin. Chim. Acta 2021, 519, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Santos Sánchez, N.; Salas-Coronado, R.; Villanueva, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Radovanovic, J.; Banjac, K.; Obradovic, M.; Isenovic, E.R. Antioxidant enzymes and vascular diseases. Explor. Med. 2021, 2, 544–555. [Google Scholar] [CrossRef]
- Tóth, A.; Balogh, E.; Jeney, V. Regulation of Vascular Calcification by Reactive Oxygen Species. Antioxidants 2020, 9, 963. [Google Scholar] [CrossRef]
- Das, D.; Holmes, A.; Murphy, G.A.; Mishra, K.; Rosenkranz, A.C.; Horowitz, J.D.; Kennedy, J.A. TGF-beta1-Induced MAPK activation promotes collagen synthesis, nodule formation, redox stress and cellular senescence in porcine aortic valve interstitial cells. J. Heart Valve Dis. 2013, 22, 621–630. [Google Scholar]
- Liu, H.; Wang, L.; Pan, Y.; Wang, X.; Ding, Y.; Zhou, C.; Shah, A.M.; Zhao, G.; Zhang, M. Celastrol Alleviates Aortic Valve Calcification Via Inhibition of NADPH Oxidase 2 in Valvular Interstitial Cells. JACC Basic Transl. Sci. 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Branchetti, E.; Sainger, R.; Poggio, P.; Grau, J.B.; Patterson-Fortin, J.; Bavaria, J.E.; Chorny, M.; Lai, E.; Gorman, R.C.; Levy, R.J.; et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e66–e74. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; St Hilaire, C.; Hortells, L.; Phillippi, J.A.; Sant, V.; Sant, S. Shape-Specific Nanoceria Mitigate Oxidative Stress-Induced Calcification in Primary Human Valvular Interstitial Cell Culture. Cell. Mol. Bioeng. 2017, 10, 483–500. [Google Scholar] [CrossRef]
- Nawar, W.W. Lipids. In Food Chemistry; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 225–314. [Google Scholar]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Asif, M. Chemistry and antioxidant activity of plants containing some phenolic compounds. Chem. Int. 2015, 1, 35–52. [Google Scholar]
- Zhou, T.; Wang, Y.; Liu, M.; Huang, Y.; Shi, J.; Dong, N.; Xu, K. Curcumin inhibits calcification of human aortic valve interstitial cells by interfering NF-κB, AKT, and ERK pathways. Phytother. Res. 2020, 34, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Huang, Y.; Zhou, T.; Wang, C.; Chi, Q.; Shi, J.; Zhu, P.; Dong, N. Nobiletin exhibits potent inhibition on tumor necrosis factor alpha-induced calcification of human aortic valve interstitial cells via targeting ABCG2 and AKR1B1. Phytother. Res. 2019, 33, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, F.; Huang, Y.; Zhou, T.; Chen, S.; Li, G.; Shi, J.; Dong, N.; Xu, K. Caffeic Acid Phenethyl Ester Ameliorates Calcification by Inhibiting Activation of the AKT/NF-κB/NLRP3 Inflammasome Pathway in Human Aortic Valve Interstitial Cells. Front. Pharmacol. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, X.; Liu, M.; Zhou, T.; Shi, J.; Dong, N.; Xu, K. The natural compound andrographolide inhibits human aortic valve interstitial cell calcification via the NF-kappa B/Akt/ERK pathway. Biomed. Pharmacother. 2020, 125, 109985. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-F.; Tsai, C.-H.; Chen, H.-Y.; Wang, K.-L.; Chang, H.-Y.; Huang, Y.-J.; Hong, Y.-H.; Ali, M.; Shieh, T.-M.; Huang, T.-C.; et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs 2021, 19, 307. [Google Scholar] [CrossRef]
- Wang, C.; Xia, Y.; Qu, L.; Liu, Y.; Liu, X.; Xu, K. Cardamonin inhibits osteogenic differentiation of human valve interstitial cells and ameliorates aortic valve calcification via interfering in the NF-κB/NLRP3 inflammasome pathway. Food Funct. 2021, 12, 11808–11818. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, K.; Liu, Y.; Chen, J.; Cai, Q.; Zhang, Y.; Wang, M.; Wang, J.; Huang, H. Apocynin attenuates angiotensin II-induced vascular smooth muscle cells osteogenic switching via suppressing extracellular signal-regulated kinase 1/2. Oncotarget 2016, 7, 83588–83600. [Google Scholar] [CrossRef] [Green Version]
- Al Hariri, M.; Zibara, K.; Farhat, W.; Hashem, Y.; Soudani, N.; Al Ibrahim, F.; Hamade, E.; Zeidan, A.; Husari, A.; Kobeissy, F. Cigarette Smoking-Induced Cardiac Hypertrophy, Vascular Inflammation and Injury Are Attenuated by Antioxidant Supplementation in an Animal Model. Front. Pharmacol. 2016, 7, 397. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Fujii, H.; Kono, K.; Watanabe, K.; Goto, S.; Nishi, S. Influence of oxidative stress on vascular calcification in the setting of coexisting chronic kidney disease and diabetes mellitus. Sci. Rep. 2020, 10, 20708. [Google Scholar] [CrossRef]
- Jordão, J.B.R.; Porto, H.K.P.; Lopes, F.M.; Batista, A.C.; Rocha, M.L. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017, 83, 830–836. [Google Scholar] [CrossRef]
- Kee, H.J.; Cho, S.N.; Kim, G.R.; Choi, S.Y.; Ryu, Y.; Kim, I.K.; Hong, Y.J.; Park, H.W.; Ahn, Y.; Cho, J.G.; et al. Gallic acid inhibits vascular calcification through the blockade of BMP2-Smad1/5/8 signaling pathway. Vasc. Pharmacol. 2014, 63, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Zhong, X.; Li, Z.; Cai, S.; Yang, P.; Ou, C.; Chen, M. Puerarin inhibits vascular calcification of uremic rats. Eur. J. Pharmacol. 2019, 855, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xiang, D.X.; Yuan, H.Y.; Xiao, Y.; Yuan, L.Q.; Li, H.B. Puerarin attenuates calcification of vascular smooth muscle cells. Am. J. Chin. Med. 2014, 42, 337–347. [Google Scholar] [CrossRef]
- Roman-Garcia, P.; Barrio-Vazquez, S.; Fernandez-Martin, J.L.; Ruiz-Torres, M.P.; Cannata-Andia, J.B. Natural antioxidants and vascular calcification: A possible benefit. J. Nephrol. 2011, 24, 669–672. [Google Scholar] [CrossRef]
- Chang, X.Y.; Cui, L.; Wang, X.Z.; Zhang, L.; Zhu, D.; Zhou, X.R. Quercetin Attenuates Vascular Calcification through Suppressed Oxidative Stress in Adenine-Induced Chronic Renal Failure Rats. BioMed. Res. Int. 2017, 2017, 5716204. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, J.; Barathkumar, T.R.; Sivasubramanian, J.; Arunagiri, P.; Raja, B.; Balamurugan, E. Diosgenin attenuates vascular calcification in chronic renal failure rats. Mol. Cell. Biochem. 2013, 378, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Ramírez, A.; Montes de Oca, A.; Raya, A.I.; Pineda, C.; López, I.; Guerrero, F.; Diez, E.; Muñoz-Castañeda, J.R.; Martinez, J.; Almaden, Y.; et al. Vitamin E protection of obesity-enhanced vascular calcification in uremic rats. Am. J. Physiol. Ren. Physiol. 2014, 306, F422–F429. [Google Scholar] [CrossRef] [Green Version]
- Elseweidy, M.M.; Mohamed, H.E.; Elrashidy, R.A.; Atteia, H.H.; Elnagar, G.M.; Ali, A.E. Potential therapeutic roles of 10-dehydrogingerdione and/or pentoxifylline against calcium deposition in aortic tissues of high dietary cholesterol-fed rabbits. Mol. Cell. Biochem. 2019, 453, 131–142. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, J.; Liu, M.; Yuan, T.; Li, F. Abstract 12300: Sirtuin 1 Prevents Age-associated Aortic Valve Calcification in vivo. Circulation 2019, 140, A12300. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, J.; Li, P.; Zheng, X.; Feng, D. Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. J. Agric. Food Chem. 2018, 66, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.P.; Arcaro, C.A.; Gutierres, V.O.; Oliveira, J.O.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [Green Version]
- Ashrafizadeh, M.; Zarrabi, A.; Saberifar, S.; Hashemi, F.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Mohammadinejad, R.; Najafi, M.; Garg, M. Nobiletin in Cancer Therapy: How This Plant Derived-Natural Compound Targets Various Oncogene and Onco-Suppressor Pathways. Biomedicines 2020, 8, 110. [Google Scholar] [CrossRef]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Tolba, M.F.; Omar, H.A.; Azab, S.S.; Khalifa, A.E.; Abdel-Naim, A.B.; Abdel-Rahman, S.Z. Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-reperfusion Injury and Drug Adverse Reactions. Crit. Rev. Food Sci. Nutr. 2016, 56, 2183–2190. [Google Scholar] [CrossRef]
- Celik, S.; Erdogan, S. Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol. Cell. Biochem. 2008, 312, 39–46. [Google Scholar] [CrossRef]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-κB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Alvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef]
- Choi, B.-S.; Sapkota, K.; Kim, S.; Lee, H.J.; Choi, H.-S.; Kim, S.-J. Antioxidant Activity and Protective Effects of Tripterygium regelii Extract on Hydrogen Peroxide-Induced Injury in Human Dopaminergic Cells, SH-SY5Y. Neurochem. Res. 2010, 35, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Luo, Q.; Alitongbieke, G.; Chong, S.; Xu, C.; Xie, L.; Chen, X.; Zhang, D.; Zhou, Y.; Wang, Z.; et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell 2017, 66, 141–153.e146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Bai, W.; Li, S.; Zhang, Y.; Han, Y.; Gu, Y.; Meng, G.; Xie, L.; Wang, J.; Xiao, Y.; et al. Celastrol Prevents Atherosclerosis via Inhibiting LOX-1 and Oxidative Stress. PLoS ONE 2013, 8, e65477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandanur, S.G.S.; Tamang, N.; Golakoti, N.R.; Nanduri, S. Andrographolide: A natural product template for the generation of structurally and biologically diverse diterpenes. Eur. J. Med. Chem. 2019, 176, 513–533. [Google Scholar] [CrossRef]
- Jarukamjorn, K.; Nemoto, N. Pharmacological Aspects of Andrographis paniculata on Health and Its Major Diterpenoid Constituent Andrographolide. J. Health Sci. 2008, 54, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Wang, D.-X.; Zhang, W.; Liao, X.-Q.; Guan, X.; Bo, H.; Sun, J.-Y.; Huang, N.-W.; He, J.; Zhang, Y.-K.; et al. Andrographolide Protects against LPS-Induced Acute Lung Injury by Inactivation of NF-κB. PLoS ONE 2013, 8, e56407. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, M.; Zhang, X.; Chen, Q.; Chen, H.; Sun, L.; Liu, G. Protective Effect of Fucoxanthin Isolated from Laminaria japonica against Visible Light-Induced Retinal Damage Both in Vitro and in Vivo. J. Agric. Food Chem. 2016, 64, 416–424. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Gammone, M.A.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; D’Orazio, N. Prevention of cardiovascular diseases with carotenoids. Front. Biosci. 2017, 9, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An overview on cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daimary, U.D.; Parama, D.; Rana, V.; Banik, K.; Kumar, A.; Harsha, C.; Kunnumakkara, A. Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr. Res. Pharmacol. Drug Discov. 2020, 2, 100008. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Israf, D.A.; Lajis, N.H.; Shaari, K.; Mohamed, H.; Wahab, A.A.; Ariffin, K.T.; Hoo, W.Y.; Aziz, N.A.; Kadir, A.A.; et al. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006, 538, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajgai, S.P.; Prachyawarakorn, V.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Hybrid flavan-chalcones, aromatase and lipoxygenase inhibitors, from Desmos cochinchinensis. Phytochemistry 2011, 72, 2062–2067. [Google Scholar] [CrossRef]
- Yang, T.; Zang, D.-W.; Shan, W.; Guo, A.-C.; Wu, J.-P.; Wang, Y.-J.; Wang, Q. Synthesis and Evaluations of Novel Apocynin Derivatives as Anti-Glioma Agents. Front. Pharmacol. 2019, 10, 951. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Apocyanin, a Microglial NADPH Oxidase Inhibitor Prevents Dopaminergic Neuronal Degeneration in Lipopolysaccharide-Induced Parkinson’s Disease Model. Mol. Neurobiol. 2016, 53, 3326–3337. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, Y.; Li, C.; Lu, L. Quercetin attenuates Ox-LDL-induced calcification in vascular smooth muscle cells by regulating ROS-TLR4 signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2018, 38, 980–985. [Google Scholar] [CrossRef]
- Böhm, V. Vitamin E. Antioxidants 2018, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Elseweidy, M.M.; Abdallah, F.R.; Younis, N.N.; Aldohmy, S.; Kassem, H.M. 10-Dehydrogingerdione raises HDL-cholesterol through a CETP inhibition and wards off oxidation and inflammation in dyslipidemic rabbits. Atherosclerosis 2013, 231, 334–340. [Google Scholar] [CrossRef] [PubMed]
- El-Seweidy, M.M.; Asker, M.E.-S.; Eldahmy, S.I.; Atteia, H.H.; Abdallah, M.A. Haemostatic risk factors in dyslipidemic rabbits: Role of 10-dehydrogingerdione as a new hypolipemic agent. J. Thromb. Thrombolysis 2015, 39, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transplant. Proc. 2016, 48, 3378–3386. [Google Scholar] [CrossRef]
- Tomayko, E.J.; Cachia, A.J.; Chung, H.R.; Wilund, K.R. Resveratrol supplementation reduces aortic atherosclerosis and calcification and attenuates loss of aerobic capacity in a mouse model of uremia. J. Med. Food 2014, 17, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Hammad, S.K.; Eissa, R.G.; Shaheen, M.A.; Younis, N.N. Resveratrol Ameliorates Aortic Calcification in Ovariectomized Rats via SIRT1 Signaling. Curr. Issues Mol. Biol. 2021, 43, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
| Antioxidants | Calcification Inhibition Mechanism | Calcification Model | Reference Number |
|---|---|---|---|
| In vitro experiments conducted in valvular interstitial cells | |||
| Curcumin | Inhibition of NF-κB, AKT, ERK | In vitro; hVIC | [63] |
| Nobiletin | Inhibition of AKT, NF-κB, TNF-α | In vitro; hVIC | [64] |
| Caffeic Acid Phenethyl Ester | Inhibition of ERK/AKT/NF-κB/NLRP3 inflammasome pathway | In vitro; hVIC | [65] |
| Celastrol | Inhibition of NADPH Oxidase 2 and GSK3β/β-catenin pathway | In vitro; porcine AVIC In vivo; rabbit CAVD model | [57] |
| Andrographolide | Inhibition of NF-κB/Akt/ERK pathway | In vitro; hVIC | [66] |
| Fucoxanthin | Inhibition of Akt/ERK-related signaling pathway | In vitro; rat heart VIC In vivo; dog model | [67] |
| Cardamonin | Inhibition of NF-κB/NLRP3 inflammasome pathway | In vitro; hVIC Ex vivo; Human aortic valve leaflet In vivo; ApoE−/− mice model | [68] |
| In vitro experiments conducted in other vascular cells | |||
| Apocynin | Suppressing extracellular signal-regulated kinase 1/2 | In vitro; Vascular smooth muscle cells | [69,70,71] |
| Ellagic acid | Improving nitric oxide bioavailability and reducing the formation of ROS | In vivo; Rat model | [72] |
| Gallic acid | Blocking BMP2-SMAD1/5/8 signaling pathway | In vitro; Vascular smooth muscle cell | [73] |
| Puerarin | NLRP3/CASPASE1/IL-1Β AND NF-ΚB signaling pathways and inhibition of reactive oxygen species ER/PI3K-AKT signal pathway | In vitro; Rat vascular smooth muscle cells, Mice vascular smooth muscle cells In vivo; uremic rats | [74,75] |
| Silybin | Reducing the formation of ROS | In vitro; Vascular smooth muscle cell | [76] |
| Quercetin | Oxidative stress and INOS/P38mapk pathway | In vivo; Adenine-induced chronic renal failure rats | [77] |
| Diosgenin | Reducing the formation of ROS, inhibition of NF-κB/Akt/ERK, p38 pathway | In vivo; Adenine-induced chronic renal failure rats | [78] |
| Vitamin E | Reducing the formation of ROS | In vivo; Uremic obese rats | [79] |
| 10 dehydrogingerdione (10-DHGD) | HDL-raising effect and attenuation of associated inflammation | In vivo; Rabbit model | [80] |
| Resveratrol | Mitochondrial ROS inhibition and SIRT1 activation | In vivo; ApoE−/− mice model | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, R.; Shiwakoti, S.; Ko, J.-Y.; Dhakal, B.; Park, S.-H.; Choi, I.J.; Kim, H.J.; Oak, M.-H. Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants. Antioxidants 2022, 11, 1169. https://doi.org/10.3390/antiox11061169
Adhikari R, Shiwakoti S, Ko J-Y, Dhakal B, Park S-H, Choi IJ, Kim HJ, Oak M-H. Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants. Antioxidants. 2022; 11(6):1169. https://doi.org/10.3390/antiox11061169
Chicago/Turabian StyleAdhikari, Radhika, Saugat Shiwakoti, Ju-Young Ko, Bikalpa Dhakal, Sin-Hee Park, Ik Jun Choi, Hyun Jung Kim, and Min-Ho Oak. 2022. "Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants" Antioxidants 11, no. 6: 1169. https://doi.org/10.3390/antiox11061169
APA StyleAdhikari, R., Shiwakoti, S., Ko, J.-Y., Dhakal, B., Park, S.-H., Choi, I. J., Kim, H. J., & Oak, M.-H. (2022). Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants. Antioxidants, 11(6), 1169. https://doi.org/10.3390/antiox11061169







