Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study
Abstract
:1. Introduction
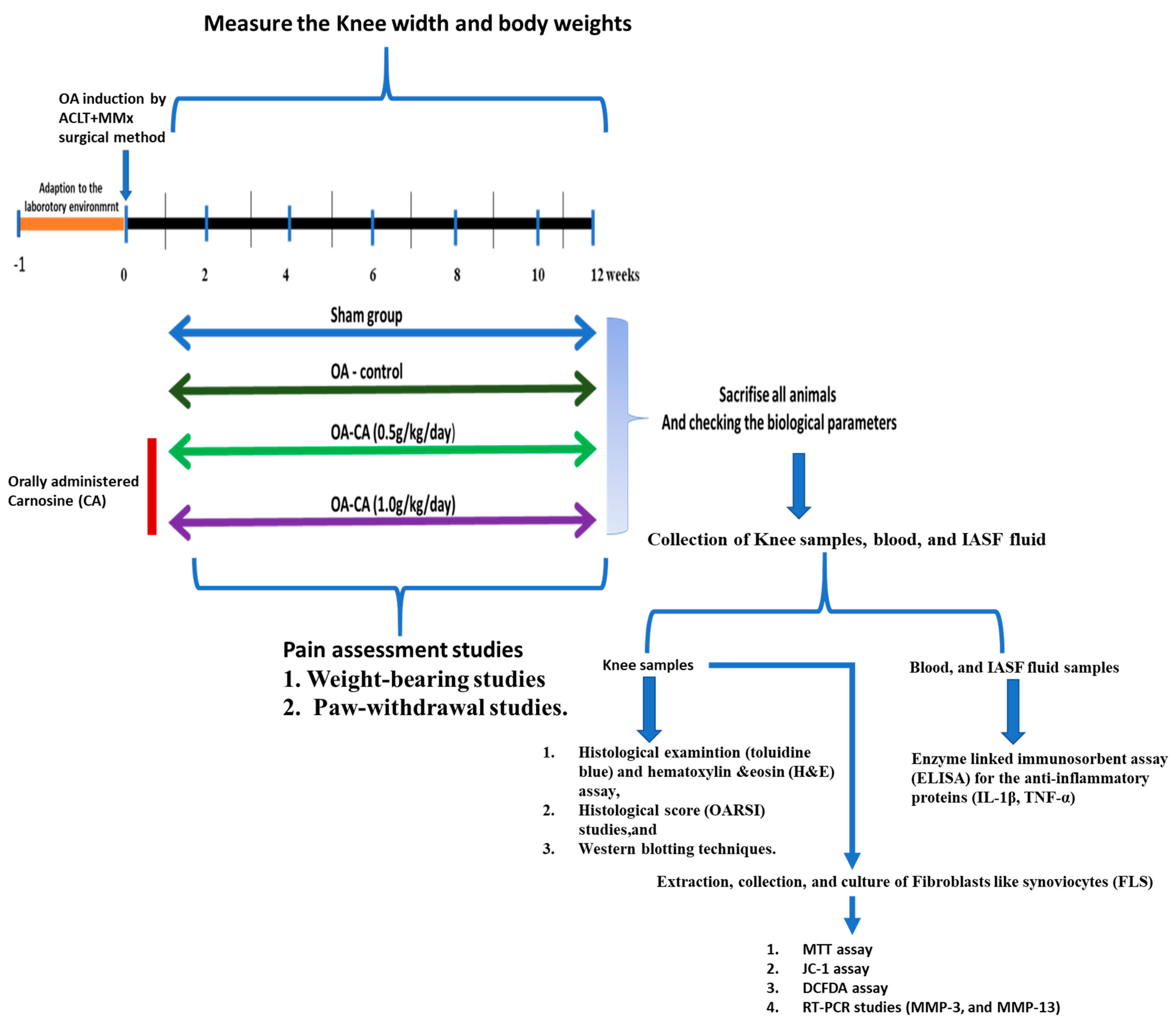
2. Materials and Methods
2.1. Experimental Animals
2.2. ACLT + MMx Method Was Used to Treat Rat-Induced Knee OA
2.3. CA Administration
2.4. Measurements of Knee Width and Weight-Bearing Tests
2.5. Paw-Withdrawal Examination
2.6. Histology Studies
2.7. Measurements of Cytokines
2.8. FLS Cells Culture
2.9. Measurement of Lipid Peroxidation (LPO), Nitrate (NO), GSH, GPx, SOD, and CAT Levels
2.10. Western Blotting Studies
2.11. RT-PCR Studies
2.12. Mitochondrial Membrane Permeability (ΔΨm) Assay
2.13. DCFDA Assay
2.14. Statistical Analysis
3. Results
3.1. CA’s Impact on Weight Bearing and Knee Width in ACLT + MMx-Induced OA
3.2. CA’s Effect on Body Weight and Anti-Nociception in ACLT + MMx-Induced OA Rats
3.3. The Effect of CA on Knee Histopathology Changes in ACLT + MMx-Induced OA in Rats
3.4. The Effect of CA on Antioxidant Parameters on ACLT + MMx-Induced OA Knee Rats
3.5. The Effect of CA on Serum and IASF Fluid and IL-1β and TNF-α Levels in ACLT + MMx-Induced OA Knee Rats
3.6. The Effect of CA on the Nrf2/HO-1 Signaling Pathway in ACLT + MMx-Induced OA Knee Rats, as Well as MMP-3 and MMP-13 mRNA Expression in FLS Cells Stimulated by IL-1β
3.7. The Effect of CA on Cell Viability and IL-1β Stimulated ROS Production in FLS Cells
3.8. CA’s Effect on the Mitochondrial Membrane Permeability (ΔΨm) in FLS Cells Stimulated ROS with IL-1β
3.9. The Effect of CA on ROS Generation Was Inhibition of IL-1β Stimulated ROS Production in FLS Cells
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.M.C.; Pitsillides, A.A.; Meeson, R.L. Moving Beyond the Limits of Detection: The Past, the Present, and the Future of Diagnostic Imaging in Canine Osteoarthritis. Front. Vet. Sci. 2022, 9, 789898. [Google Scholar] [CrossRef] [PubMed]
- Krasnokutsky, S.; Attur, M.; Palmer, G.; Samuels, J.; Abramson, S.B. Current concepts in the pathogenesis of osteoarthritis. Osteoarthr. Cartil. 2008, 16, S1–S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, S.R. The role of bone in osteoarthritis pathogenesis. Rheum. Dis. Clin. N. Am. 2008, 34, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Berenbaum, F. Pathogenesis of osteoarthritis. Ann. Rheum. Dis. 2007, 66, 43. [Google Scholar]
- Towle, C.A.; Hung, H.H.; Bonassar, L.J.; Treadwell, B.V.; Mangham, D.C. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: A possible autocrine/paracrine role in pathogenesis. Osteoarthr. Cartil. 1997, 5, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Segami, N.; Kaneyama, K.; Suzuki, T.; Miyamaru, M. Proinflammatory cytokines and arthroscopic findings of patients with internal derangement and osteoarthritis of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2002, 40, 68–71. [Google Scholar] [CrossRef]
- Ku, J.H.; Lee, C.K.; Joo, B.S.; An, B.M.; Choi, S.H.; Wang, T.H.; Cho, H.L. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin. Rheumatol. 2009, 28, 1431–1435. [Google Scholar] [CrossRef]
- Hui, W.; Bell, M.; Carroll, G. Detection of oncostatin M in synovial fluid from patients with rheumatoid arthritis. Ann. Rheum. Dis. 1997, 56, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.H.; Lin, L.P.; Guo, Y.X.; Zou, R.; Wang, Z.; Shi, Z.P.; Lin, F.Q. Matrix metalloproteinase-13, NF-kappa B p65 and interleukin-1 beta are associated with the severity of knee osteoarthritis. Exp. Ther. Med. 2020, 19, 3620–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondeson, J. Activated Synovial Macrophages as Targets for Osteoarthritis Drug Therapy. Curr. Drug Targets 2010, 11, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, R.L.; Huang, Z.Q.; Ding, L.; Zhang, L.; Li, M.C.; Li, X.C.; Wang, P.M.; Mao, J. Synovial Fibrosis Involvement in Osteoarthritis. Front. Med. 2021, 8, 684389. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, S.; Kersse, K.; Festjens, N.; Lamkanfi, M.; Vandenabeelel, P. Inflammatory caspases: Targets for novel therapies. Curr. Pharm. Des. 2007, 13, 367–385. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappa B as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Weisbrode, S.E. Degenerative Joint Disease: In the Beginning. Vet. Pathol. 2014, 51, 893–894. [Google Scholar] [CrossRef] [Green Version]
- Baquedano, J.; Lainez, C.; Pedro, A.; Carabante, D.; Mira, M.; Vila, A.; Fernandez, J.; Sanches-Reyes, A.; Sanchiz, F. Anti-inflamatory radiotherapy for refractory degenerative joints (osteoarthritis). Radiother. Oncol. 2006, 81, S242. [Google Scholar]
- Ozeki, N.; Koga, H.; Sekiya, I. Degenerative Meniscus in Knee Osteoarthritis: From Pathology to Treatment. Life 2022, 12, 603. [Google Scholar] [CrossRef]
- Chen, C.; Bao, G.F.; Xu, G.H.; Sun, Y.Y.; Cui, Z.M. Altered Wnt and NF-kappa B Signaling in Facet Joint Osteoarthritis: Insights from RNA Deep Sequencing. Tohoku J. Exp. Med. 2018, 245, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.S.; Meng, Y.F.; Hu, S.F.; Botchway, B.O.A.; Zhang, Y.; Liu, X.H. Saikosaponin D: A potential therapeutic drug for osteoarthritis. J. Tissue Eng. Regen. Med. 2020, 14, 1175–1184. [Google Scholar] [CrossRef]
- Qu, Y.L.; Wang, C.L.; Liu, N.; Gao, C.Z.; Liu, F. Morin Exhibits Anti-Inflammatory Effects on IL-1 beta-Stimulated Human Osteoarthritis Chondrocytes by Activating the Nrf2 Signaling Pathway. Cell. Physiol. Biochem. 2018, 51, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.D.; Yan, Z.J.; Kong, X.J.; Liu, J.L.; Lin, Z.; Qi, W.H.; Wu, Y.F.; Lin, J.; Pan, X.Y.; Xue, X.H. Lycopene inhibits IL-1 beta-induced inflammation in mouse chondrocytes and mediates murine osteoarthritis. J. Cell. Mol. Med. 2021, 25, 3573–3584. [Google Scholar] [CrossRef]
- Pan, X.X.; Chen, T.T.; Zhang, Z.J.; Chen, X.W.; Chen, C.S.; Chen, L.; Wang, X.Y.; Ying, X.Z. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.Y.; Hu, S.Q.; Pan, S.H.; Wang, C.L. Polygonatum sibiricum polysaccharide inhibits IL-1 beta-induced inflammation in human chondrocytes. Food Sci. Technol. 2022, 42, e40421. [Google Scholar] [CrossRef]
- Chen, P.; Ruan, A.M.; Zhou, J.; Huang, L.W.; Zhang, X.Z.; Ma, Y.F.; Wang, Q.F. Cinnamic Aldehyde Inhibits Lipopolysaccharide-Induced Chondrocyte Inflammation and Reduces Cartilage Degeneration by Blocking the Nuclear Factor-Kappa B Signaling Pathway. Front. Pharmacol. 2020, 11, 949. [Google Scholar] [CrossRef]
- Xu, X.L.; Liu, X.D.; Yang, Y.C.; He, J.Y.; Gu, H.L.; Jiang, M.Q.; Huang, Y.; Liu, X.T.; Liu, L. Resveratrol inhibits the development of obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling pathways. Connect. Tissue Res. 2019, 60, 571–582. [Google Scholar] [CrossRef]
- D’Arcy, Y.; Mantyh, P.; Yaksh, T.; Donevan, S.; Hall, J.; Sadrarhami, M.; Viktrup, L. Treating osteoarthritis pain: Mechanisms of action of acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, and nerve growth factor antibodies. Postgrad. Med. 2021, 133, 879–894. [Google Scholar] [CrossRef]
- Gregori, D.; Giacovelli, G.; Minto, C.; Barbetta, B.; Gualtieri, F.; Azzolina, D.; Vaghi, P.; Rovati, L.C. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis A Systematic Review and Meta-analysis. JAMA-J. Am. Med. Assoc. 2018, 320, 2564–2579. [Google Scholar] [CrossRef] [Green Version]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2016, 15, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, M.C.; Dougados, M. Pharmacological therapy of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2001, 15, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Jukic, I.; Kolobaric, N.; Stupin, A.; Matic, A.; Kozina, N.; Mihaljevic, Z.; Mihalj, M.; Susnjara, P.; Stupin, M.; Curic, Z.B.; et al. Carnosine, Small but Mighty-Prospect of Use as Functional Ingredient for Functional Food Formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of Pro-Oxidant and Pro-Inflammatory Activities of M1 Macrophages by the Natural Dipeptide Carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, B.Y.; Lee, H.Y.; Park, C.G.; Kang, J.; Yu, S.L.; Choi, D.R.; Han, S.Y.; Park, M.H.; Cho, S.; Lee, S.Y.; et al. Oxidative stress caused by activation of NADPH oxidase 4 promotes contrast-induced acute kidney injury. PLoS ONE 2018, 13, e0191034. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ye, L.H.; Li, W.J.; Li, D.Z.; Li, F. Hyperoside protects human kidney-2 cells against oxidative damage induced by oxalic acid. Mol. Med. Rep. 2018, 18, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Bortolatto, G.P.; de Medeiros, H.C.D.; Guelfi, M.; Tavares, M.A.; Mazzo, M.; Mingatto, F.E. Carnosine avoids the oxidative damage caused by intense exercise on rat soleus muscle. Rev. Bras. Med. Esporte 2020, 26, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Busa, P.; Kuthati, Y.; Huang, N.; Wong, C.S. New Advances on Pathophysiology of Diabetes Neuropathy and Pain Management: Potential Role of Melatonin and DPP-4 Inhibitors. Front. Pharmacol. 2022, 13, 864088. [Google Scholar] [CrossRef]
- Prokopieva, V.D.; Yarygina, E.G.; Bokhan, N.A.; Ivanova, S.A. Use of Carnosine for Oxidative Stress Reduction in Different Pathologies. Oxidative Med. Cell. Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef] [Green Version]
- Hasanein, P.; Felegari, Z. Chelating effects of carnosine in ameliorating nickel-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2017, 95, 1426–1432. [Google Scholar] [CrossRef]
- Calabrese, V.; Scuto, M.; Salinaro, A.T.; Dionisio, G.; Modafferi, S.; Ontario, M.L.; Greco, V.; Sciuto, S.; Schmitt, C.P.; Calabrese, E.J.; et al. Hydrogen Sulfide and Carnosine: Modulation of Oxidative Stress and Inflammation in Kidney and Brain Axis. Antioxidants 2020, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, H.A.; Ramdan, E.; Elmazar, M.M.; Azzazy, H.M.E.; Abdelnaser, A. Comparing the protective effects of resveratrol, curcumin and sulforaphane against LPS/IFN-gamma-mediated inflammation in doxorubicin-treated macrophages. Sci. Rep. 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Andou, A.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kamada, N.; Kobayashi, T.; Hashimoto, M.; Okutsu, T.; Shimbo, K.; Takeda, T.; et al. Dietary Histidine Ameliorates Murine Colitis by Inhibition of Proinflammatory Cytokine Production From Macrophages. Gastroenterology 2009, 136, 564–574. [Google Scholar] [CrossRef]
- Tallon, M.J.; Harris, R.C.; Maffulli, N.; Tarnopolsky, M.A. Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology 2007, 8, 129–137. [Google Scholar] [CrossRef]
- Kubota, M.; Kobayashi, N.; Sugizaki, T.; Shimoda, M.; Kawahara, M.; Tanaka, K. Carnosine suppresses neuronal cell death and inflammation induced by 6-hydroxydopamine in an in vitro model of Parkinson’s disease. PLoS ONE 2020, 15, e0240448. [Google Scholar] [CrossRef]
- Pickarski, M.; Hayami, T.; Zhuo, Y.; Duong, L.T. Molecular changes in articular cartilage and subchondral bone in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. BMC Musculoskelet. Disord. 2011, 12, 197. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.C.; Yang, T.S.; Busa, P.; Lin, C.L.; Fang, Y.C.; Chen, J.; Wong, C.S. Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model. Int. J. Mol. Sci. 2021, 22, 10179. [Google Scholar] [CrossRef]
- Tsai, W.Y.; Tsai, R.Y.; Liu, C.C.; Wu, J.L.; Wong, C.S. Sulfasalazine attenuates ACL transection and medial menisectomy-induced cartilage destruction by inhibition of cystine/glutamate antiporter. J. Orthop. Res. 2016, 34, 650–657. [Google Scholar] [CrossRef]
- Kao, J.H.; Lin, S.H.; Lai, C.F.; Lin, Y.C.; Kong, Z.L.; Wong, C.S. Shea Nut Oil Triterpene Concentrate Attenuates Knee Osteoarthritis Development in Rats: Evidence from Knee Joint Histology. PLoS ONE 2016, 11, e0162022. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chen, T.L.; Chen, Y.P.; Chen, R.M. Traumatic osteoarthritis-induced persistent mechanical hyperalgesia in a rat model of anterior cruciate ligament transection plus a medial meniscectomy. J. Pain Res. 2018, 11, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duc, P.A.; Yudoh, K.; Masuko, K.; Kato, T.; Nishioka, K.; Nakamura, H. Development and characteristics of pannus-like soft tissue in osteoarthritic articular surface in rat osteoarthritis model. Clin. Exp. Rheumatol. 2008, 26, 589–595. [Google Scholar] [PubMed]
- Silva, J.M.D.; Alabarse, P.V.G.; Teixeira, V.D.N.; Freitas, E.C.; de Oliveira, F.H.; Chakr, R.M.D.; Xavier, R.M. Muscle wasting in osteoarthritis model induced by anterior cruciate ligament transection. PLoS ONE 2018, 13, e0196682. [Google Scholar] [CrossRef] [Green Version]
- Szychlinska, M.A.; Trovato, F.M.; Di Rosa, M.; Malaguarnera, L.; Puzzo, L.; Leonardi, R.; Castrogiovanni, P.; Musumeci, G. Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles. Int. J. Mol. Sci. 2016, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Han, D.F.; Fang, Y.L.; Tan, X.W.; Jiang, H.F.; Gong, X.; Wang, X.M.; Hong, W.M.; Tu, J.J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell. Mol. Med. 2020, 24, 9518–9532. [Google Scholar] [CrossRef] [PubMed]
- Saito, M. Dietary docosahexaenoic acid does not promote tissue lipid peroxide formation to the extent expected from the peroxidizability index of the lipids. Biofactors 2000, 13, 15–24. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef] [Green Version]
- Agostini, J.F.; Dal Toe, H.C.Z.; Vieira, K.M.; Baldin, S.L.; Costa, N.L.F.; Cruz, C.U.; Longo, L.; Machado, M.M.; da Silveira, T.R.; Schuck, P.F.; et al. Cholinergic System and Oxidative Stress Changes in the Brain of a Zebrafish Model Chronically Exposed to Ethanol. Neurotox. Res. 2018, 33, 749–758. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Arfuso, F.; Millward, M.; Dharmarajan, A.; Warrier, S. Secreted Frizzled-Related Protein 4 Inhibits Glioma Stem-Like Cells by Reversing Epithelial to Mesenchymal Transition, Inducing Apoptosis and Decreasing Cancer Stem Cell Properties. PLoS ONE 2015, 10, e0127517. [Google Scholar] [CrossRef]
- Shen, W.L.; Chen, J.L.; Zhu, T.; Yin, Z.; Chen, X.; Chen, L.K.; Fang, Z.; Heng, B.C.; Ji, J.F.; Chen, W.S.; et al. Osteoarthritis Prevention Through Meniscal Regeneration Induced by Intra-Articular Injection of Meniscus Stem Cells. Stem Cells Dev. 2013, 22, 2071–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, V.; Yard, B.; Schmitt, C.P. Carnosine and Diabetic Nephropathy. Curr. Med. Chem. 2020, 27, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Zanela, P.B.; Alves, F.D.; de Souza, C.G. Effects of beta-alanine supplementation on performance and muscle fatigue in athletes and non-athletes of different sports: A systematic review. J. Sports Med. Phys. Fit. 2017, 57, 1132–1141. [Google Scholar] [CrossRef]
- Kamei, J.; Ohsawa, M.; Miyata, S.; Tanaka, S. Preventive effect of L-carnosine on changes in the thermal nociceptive threshold in streptozotocin-induced diabetic mice. Eur. J. Pharmacol. 2008, 600, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Nativi, C.; Gualdani, R.; Dragoni, E.; Mannelli, L.D.; Sostegni, S.; Norcini, M.; Gabrielli, G.; la Marca, G.; Richichi, B.; Francesconi, O.; et al. A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci. Rep. 2013, 3, srep02005. [Google Scholar] [CrossRef] [Green Version]
- Kuthati, Y.; Rao, V.N.; Busa, P.; Wong, C.S. Teneligliptin Exerts Antinociceptive Effects in Rat Model of Partial Sciatic Nerve Transection Induced Neuropathic Pain. Antioxidants 2021, 10, 1438. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.E.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine Prevents A beta-Induced Oxidative Stress and Inflammation in Microglial Cells: A Key Role of TGF-beta 1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Ponist, S.; Drafi, F.; Kuncirova, V.; Mihalova, D.; Rackova, L.; Danisovic, L.; Ondrejickova, O.; Tumova, I.; Trunova, O.; Fedorova, T.; et al. Effect of Carnosine in Experimental Arthritis and on Primary Culture Chondrocytes. Oxidative Med. Cell. Longev. 2016, 2016, 8470589. [Google Scholar] [CrossRef] [Green Version]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.B.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y.Z. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2021, 8, 607764. [Google Scholar] [CrossRef]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [Green Version]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Fusco, R.; Crupi, R.; Rizzarelli, E.; Cuzzocrea, S.; et al. Protective effect of a new hyaluronic acid -carnosine conjugate on the modulation of the inflammatory response in mice subjected to collagen-induced arthritis. Biomed. Pharmacother. 2020, 125, 110023. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Fusco, R.; Crupi, R.; Rizzarelli, E.; Cuzzocrea, S.; et al. The Protective Effect of New Carnosine-Hyaluronic Acid Conjugate on the Inflammation and Cartilage Degradation in the Experimental Model of Osteoarthritis. Appl. Sci. 2020, 10, 1324. [Google Scholar] [CrossRef] [Green Version]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlesso, L.C.; Hawker, G.A.; Waugh, E.J.; Davis, A.M. Disease-specific pain and function predict future pain impact in hip and knee osteoarthritis. Clin. Rheumatol. 2016, 35, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, J.C.; Laffon, A.; Blanco, F.J.; Carmona, L.; Episer Study, G. Prevalence, risk factors, and impact of knee pain suggesting osteoarthritis in Spain. Clin. Exp. Rheumatol. 2008, 26, 324–332. [Google Scholar] [PubMed]
- El-Bikai, R.; Welman, M.; Margaron, Y.; Cote, J.F.; Macqueen, L.; Buschmann, M.D.; Fahmi, H.; Shi, Q.; Maghni, K.; Fernandes, J.C.; et al. Perturbation of adhesion molecule-mediated chondrocyte-matrix interactions by 4-hydroxynonenal binding: Implication in osteoarthritis pathogenesis. Arthritis Res. Ther. 2010, 12, R201. [Google Scholar] [CrossRef] [Green Version]
- Maerz, T.; Sherman, E.; Newton, M.; Yilmaz, A.; Kumar, P.; Graham, S.F.; Baker, K.C. Metabolomic serum profiling after ACL injury in rats: A pilot study implicating inflammation and immune dysregulation in post-traumatic osteoarthritis. J. Orthop. Res. 2018, 36, 1969–1979. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.T.; Zhang, X.L.; Zuo, H.N.; Lu, X.; Li, L. Articular cartilage degradation is prevented by tanshinone IIA through inhibiting apoptosis and the expression of inflammatory cytokines. Mol. Med. Rep. 2017, 16, 6285–6289. [Google Scholar] [CrossRef] [Green Version]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Pritzker, K.P.H. Joint instability and OA: Do animal models provide insights? Nat. Rev. Rheumatol. 2011, 7, 444–445. [Google Scholar] [CrossRef]
- Custers, R.J.H.; Creemers, L.B.; Verbout, A.J.; van Rijen, M.H.P.; Dhert, W.J.A.; Saris, D.B.F. Reliability, reproducibility and variability of the traditional Histologic/Histochemical Grading System vs the new OARSI Osteoarthritis Cartilage Histopathology Assessment System. Osteoarthr. Cartil. 2007, 15, 1241–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, Y.; Kong, Y.W.; Zhang, X.N.; Zhang, H.; Gang, Y.; Bai, L.H. Carnosine Prevents Type 2 Diabetes-Induced Osteoarthritis Through the ROS/NF-kappa B Pathway. Front. Pharmacol. 2018, 9, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Shi, L.; Zhang, L.R. Neuroprotective effect of carnosine against salsolinol-induced Parkinson’s disease. Exp. Ther. Med. 2017, 14, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogardus, S.L.; Boissonneault, G.A. Carnosine inhibits in vitro low-density lipoprotein oxidation. Nutr. Res. 2000, 20, 967–976. [Google Scholar] [CrossRef]
- Yay, A.; Akkus, D.; Yapislar, H.; Balcioglu, E.; Sonmez, M.F.; Ozdamar, S. Antioxidant effect of carnosine treatment on renal oxidative stress in streptozotocin-induced diabetic rats. Biotech. Histochem. 2014, 89, 552–557. [Google Scholar] [CrossRef]
- Sirse, M. Effect of Dietary Polyphenols on Osteoarthritis-Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef]
- Kuthati, Y.; Busa, P.; Tummala, S.; Rao, V.N.; Davuluri, V.N.G.; Ho, Y.P.; Wong, C.S. Mesoporous Polydopamine Nanoparticles Attenuate Morphine Tolerance in Neuropathic Pain Rats by Inhibition of Oxidative Stress and Restoration of the Endogenous Antioxidant System. Antioxidants 2021, 10, 195. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Tudorachi, N.B.; Totu, E.E.; Fifere, A.; Ardeleanu, V.; Mocanu, V.; Mircea, C.; Isildak, I.; Smilkov, K.; Carausu, E.M. The Implication of Reactive Oxygen Species and Antioxidants in Knee Osteoarthritis. Antioxidants 2021, 10, 985. [Google Scholar] [CrossRef]
- Busa, P.; Koutavarapu, R.; Kuthati, Y. Polydopamine-Coated Copper-Substituted Mesoporous Silica Nanoparticles for Dual Cancer Therapy. Coatings 2022, 12, 60. [Google Scholar] [CrossRef]
- Poulet, B.; Beier, F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res. Ther. 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busa, P.; Koutavarapu, R.; Lee, D.Y.; Shim, J.; Kuthati, Y. Hierarchical Two-Dimensional Layered Double Hydroxide Coated Polydopamine Nanocarriers for Combined Chemodynamic and Photothermal Tumor Therapy. Coatings 2021, 11, 1008. [Google Scholar] [CrossRef]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Ansari, M.Y.; Bano, S.; Haqqi, T.M. Imperatorin suppresses IL-1 beta-induced iNOS expression via inhibiting ERK-MAPK/AP1 signaling in primary human OA chondrocytes. Int. Immunopharmacol. 2020, 85, 106612. [Google Scholar] [CrossRef]
- Ohsawa, M.; Mutoh, J.; Asato, M.; Yamamoto, S.; Ono, H.; Hisa, H.; Kamei, J. Carnosine has antinociceptive properties in the inflammation-induced nociceptive response in mice. Eur. J. Pharmacol. 2012, 682, 56–61. [Google Scholar] [CrossRef]
- Fleisher-Berkovich, S.; Abramovitch-Dahan, C.; Ben-Shabat, S.; Apte, R.; Beit-Yannai, E. Inhibitory effect of carnosine and N-acetyl carnosine on LPS-induced microglial oxidative stress and inflammation. Peptides 2009, 30, 1306–1312. [Google Scholar] [CrossRef]
- Hashimoto, K.; Akagi, M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: A narrative review. J. Int. Med. Res. 2020, 48, 0300060520931609. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.M.; Cicuttini, F.M.; Alyousef, B.; Wang, Y. Female hormonal factors and osteoarthritis of the knee, hip and hand: A narrative review. Climacteric 2018, 21, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Jiang, W.; Yong, H.; He, M.; Yang, Y.T.; Deng, Z.H.; Li, Y.S. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar] [PubMed]
- Thomson, A.; Hilkens, C.M.U. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front. Immunol. 2021, 12, 1831. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.X.; Li, Y.; Wang, Z.Q.; Han, N.; Wang, Y. Carnosine Protects Mouse Podocytes from High Glucose Induced Apoptosis through PI3K/AKT and Nrf2 Pathways. Biomed Res. Int. 2019, 2019, 4348973. [Google Scholar] [CrossRef]
- Ooi, T.C.; Chan, K.M.; Sharif, R. Zinc L-carnosine suppresses inflammatory responses in lipopolysaccharide-induced RAW 264.7 murine macrophages cell line via activation of Nrf2/HO-1 signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 259–267. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Lin, H.L.; Qin, Y.C.; Li, X.G.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. l-Carnosine Protects Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Stem Cells by Regulating the Keap1/Nrf2 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, 2100406. [Google Scholar] [CrossRef]
- Chen, Z.M.; Zhong, H.; Wei, J.S.; Lin, S.E.; Zong, Z.X.; Gong, F.; Huang, X.Q.; Sun, J.H.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Sun, S.; Wang, J.; Li, W.; Wang, X.Q.; Yuan, L.; Li, S.Y. S-Allylmercaptocysteine Targets Nrf2 in Osteoarthritis Treatment Through NOX4/NF-kappa B Pathway. Drug Des. Dev. Ther. 2020, 14, 4533–4546. [Google Scholar] [CrossRef]
- Xue, X.H.; Xue, J.X.; Hu, W.; Shi, F.L.; Yang, Y. Nomilin targets the Keap1-Nrf2 signalling and ameliorates the development of osteoarthritis. J. Cell. Mol. Med. 2020, 24, 8579–8588. [Google Scholar] [CrossRef]
- Shen, P.C.; Chou, S.H.; Lu, C.C.; Huang, H.T.; Chien, S.H.; Huang, P.J.; Liu, Z.M.; Shih, C.L.; Su, S.J.; Chen, L.M.; et al. Shockwave Treatment Enhanced Extracellular Matrix Production in Articular Chondrocytes Through Activation of the ROS/MAPK/Nrf2 Signaling Pathway. Cartilage 2021, 13, 238S–253S. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hao, D.K.; Jiang, H.; Huang, M.G.; Du, Q.J.; Lin, Y.; Liu, F.; Chen, B. Nrf2 Regulates CHI3L1 to Suppress Inflammation and Improve Post-Traumatic Osteoarthritis. J. Inflamm. Res. 2021, 14, 4079–4088. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.W.; Yan, H.Y.; Liu, J.; Chen, S.C.; Jiang, L.H.; Wang, X.X.; Qin, J. Ergosterol limits osteoarthritis development and progression through activation of Nrf2 signaling. Exp. Ther. Med. 2021, 21, 194. [Google Scholar] [CrossRef]
- Peng, Y.J.; Lu, J.W.; Lee, C.H.; Lee, H.S.; Chu, Y.H.; Ho, Y.J.; Liu, F.C.; Huang, C.J.; Wu, C.C.; Wang, C.C. Cardamonin Attenuates Inflammation and Oxidative Stress in Interleukin-1 beta-Stimulated Osteoarthritis Chondrocyte through the Nrf2 Pathway. Antioxidants 2021, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, E.S.; Shin, D.; Kim, D.; Choi, S.; Shin, Y.J.; Kim, K.A.; Noh, D.; Caglayan, A.B.; Rajanikant, G.K.; et al. Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases. Int. J. Mol. Sci. 2021, 22, 7495. [Google Scholar] [CrossRef]
- Kim, S.R.; Eom, T.K.; Byun, H.G. Inhibitory effect of the carnosine-gallic acid synthetic peptide on MMP-2 and MMP-9 in human fibrosarcoma HT1080 cells. J. Pept. Sci. 2014, 20, 716–724. [Google Scholar] [CrossRef]
- Chuang, C.H.; Hu, M.L. L-carnosine inhibits metastasis of SK-Hep-1 cells by inhibition of matrix metaoproteinase-9 expression and induction of an antimetastatic gene, nm23-H1. Nutr. Cancer-Int. J. 2008, 60, 526–533. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Wei, H.; Wu, Q.; Shi, Y.M.; Luo, A.S.; Lin, S.Y.; Feng, X.K.; Jiang, J.T.; Zhang, M.J.; Wang, F.; Tan, W.F. MicroRNA-15a/16/SOX5 axis promotes migration, invasion and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. Aging 2020, 12, 14376–14390. [Google Scholar] [CrossRef]
- Lee, H.R.; Yoo, S.J.; Kim, J.; Yoo, I.S.; Park, C.K.; Kang, S.W. The effect of nicotinamide adenine dinucleotide phosphate oxidase 4 on migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 116. [Google Scholar] [CrossRef]
- Oppermann, H.; Purcz, K.; Birkemeyer, C.; Baran-Schmidt, R.; Meixensberger, J.; Gaunitz, F. Carnosine’s inhibitory effect on glioblastoma cell growth is independent of its cleavage. Amino Acids 2019, 51, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.M.; Jang, E.H.; Ko, J.H.; Kang, J.H.; Park, C.S.; Han, S.B.; Kim, J.S.; Kim, K.H.; Pie, J.E.; Shin, D.W. Inhibition of 6-hydroxydopamine-induced endoplasmic reticulum stress by L-carnosine in SH-SY5Y cells. Neurosci. Lett. 2009, 459, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.L.; Dai, H.B.; Hu, W.W.; Fan, Y.Y.; Shen, Y.; Zhang, W.P.; Chen, Z. Carnosine protects against A beta 42-induced neurotoxicity in differentiated rat PC12 cells. Cell. Mol. Neurobiol. 2008, 28, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Alhamdani, M.S.S.; Al-Kassir, A.; Abbas, F.K.H.; Jaleel, N.A.; Al-Taee, M.F. Antiglycation and antioxidant effect of carnosine against glucose degradation products in peritoneal mesothelial cells. Nephron Clin. Pract. 2007, 107, C26–C34. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, J.E.; Lee, S.Y.; Lee, E.J.; Kim, D.S.; Moon, S.J.; Lee, J.; Kwok, S.K.; Park, S.H.; Cho, M.L. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017, 8, e2565. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Kim, D.H. Therapeutic efficacy of natural dipeptide carnosine against human cervical carcinoma cells. J. Mol. Recognit. 2016, 29, 426–435. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Ghanbarinejad, V.; Mohammadi, H.R.; Mousavi, K.; Azarpira, N.; Mahboubi, Z.; Ilkhaninasab, F.; Moezi, L.; Heidari, R. The Nephroprotective Role of Carnosine Against Ifosfamide-Induced Renal Injury and Electrolytes Imbalance is Mediated Via the Regulation of Mitochondrial Function and Alleviation of Oxidative Stress. Drug Res. 2020, 70, 49–56. [Google Scholar] [CrossRef]
- Ommati, M.M.; Heidari, R.; Ghanbarinejad, V.; Aminian, A.; Abdoli, N.; Niknahad, H. The neuroprotective properties of carnosine in a mouse model of manganism is mediated via mitochondria regulating and antioxidative mechanisms. Nutr. Neurosci. 2020, 23, 731–743. [Google Scholar] [CrossRef]
- Husain, N.; Mahmood, R. Copper(II)-induced Cytotoxicity and Oxidative Stress in Human Blood Cells and its Attenuation by Carnosine. Free. Radic. Biol. Med. 2017, 108, S21. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yao, K.; Fan, Y.Y.; He, P.; Wang, X.F.; Hu, W.W.; Chen, Z. Carnosine protects brain microvascular endothelial cells against rotenone-induced oxidative stress injury through histamine H-1 and H-2 receptors in vitro. Clin. Exp. Pharmacol. Physiol. 2012, 39, 1019–1025. [Google Scholar] [CrossRef]
- Aydogan, S.; Yapislar, H.; Artis, S.; Aydogan, B. Impaired erythrocytes deformability in H2O2-induced oxidative stress: Protective effect of L-carnosine. Clin. Hemorheol. Microcirc. 2008, 39, 93–98. [Google Scholar] [CrossRef] [PubMed]










| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| MMP-3: | F | 5′-CATAATACACAGCTGACCTGTATAA-3′ |
| R | 5′-ATTTAAGAAATCATAGATAACAGTTACTTA-3′ | |
| MMP-13: | F | 5′-TGATGATGAAACCTGGACAAGCA-3 |
| R | 5′-GAACGTCATCTCTGGGAGCA-3′ | |
| β-actin: | F | 5′-GGAGATTACCTGCCCTGGCTCCTA-3′ |
| R | 5′GACTCATCTACTCCTGCTTGCTG-3′ |
| Histopathological Changes in the Knees | Sham Group (n = 5) | OA-Control (n = 5) | OA-CA (0.5 g/kg/day) (n = 5) | OA-CA (1.0 g/kg/day) (n = 5) |
|---|---|---|---|---|
| 0 ** | 4.0 ± 0.6 | 3.1 ± 0.7 * | 2.4 ± 0.3 * |
| 0 *** | 11.1 ± 1.6 | 5.91 ± 1.5 *** | 3.2 ± 0.2 ** |
| 0 *** | 2.19 ± 0.6 | 1.85 ± 0.2 ** | 1.13 ± 0.1 ** |
| 0 *** | 1.9 ± 0.23 | 0.9 ± 0.6 * | 0.3 ± 0.1 *** |
| 0 ** | 2.1 ± 0.4 | 0.9 ± 0.5 * | 0.5 ± 0.4 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busa, P.; Lee, S.-O.; Huang, N.; Kuthati, Y.; Wong, C.-S. Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study. Antioxidants 2022, 11, 1209. https://doi.org/10.3390/antiox11061209
Busa P, Lee S-O, Huang N, Kuthati Y, Wong C-S. Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study. Antioxidants. 2022; 11(6):1209. https://doi.org/10.3390/antiox11061209
Chicago/Turabian StyleBusa, Prabhakar, Sing-Ong Lee, Niancih Huang, Yaswanth Kuthati, and Chih-Shung Wong. 2022. "Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study" Antioxidants 11, no. 6: 1209. https://doi.org/10.3390/antiox11061209
APA StyleBusa, P., Lee, S.-O., Huang, N., Kuthati, Y., & Wong, C.-S. (2022). Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study. Antioxidants, 11(6), 1209. https://doi.org/10.3390/antiox11061209








