Effects of Barranca yajiagengensis Powder in the Diet of Trachinotus ovatus on the Growth Performance, Antioxidant Capacity, Immunity and Morphology of the Liver and Intestine
Abstract
:1. Introduction
2. Materials and Methods
2.1. B. yajiagengensis Cultivation and Diet Preparation
2.2. Fish and Feeding Trial
2.3. Sampling Collection
2.4. Biochemical Analysis of B. yajiagengensis and Experimental Diets
2.5. Enzyme Activity Assays
2.6. Immune-Related Parameters Assays
2.7. Histological Observation
2.8. Serum Parameters Assays
2.9. RNA Extraction and Gene Expression Analysis
2.10. Statistical Analysis
3. Results
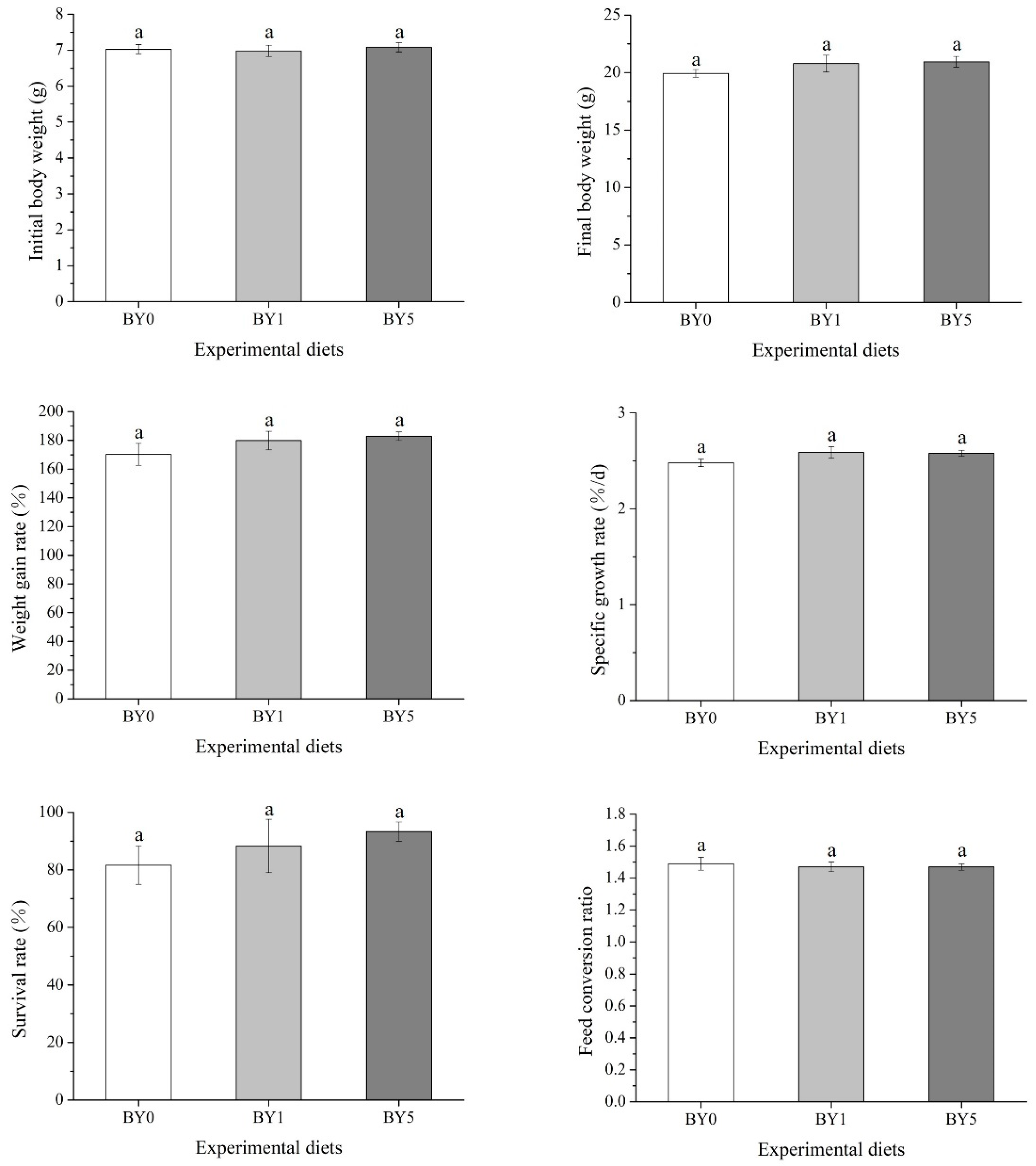
3.1. Biological Performance
3.2. Serum Biochemical Parameters
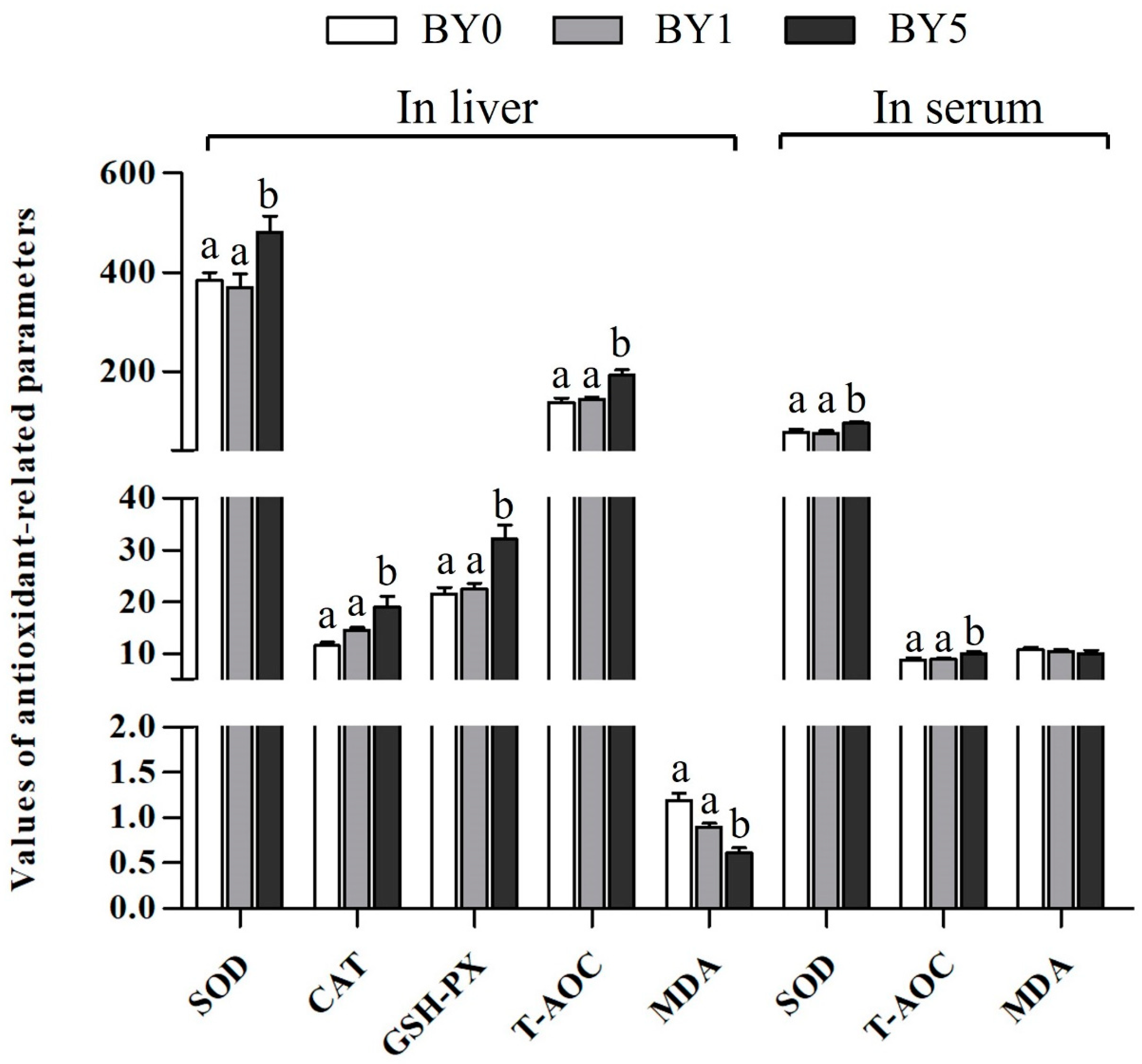
3.3. Antioxidant Parameters
3.4. Immune Biochemical Parameters
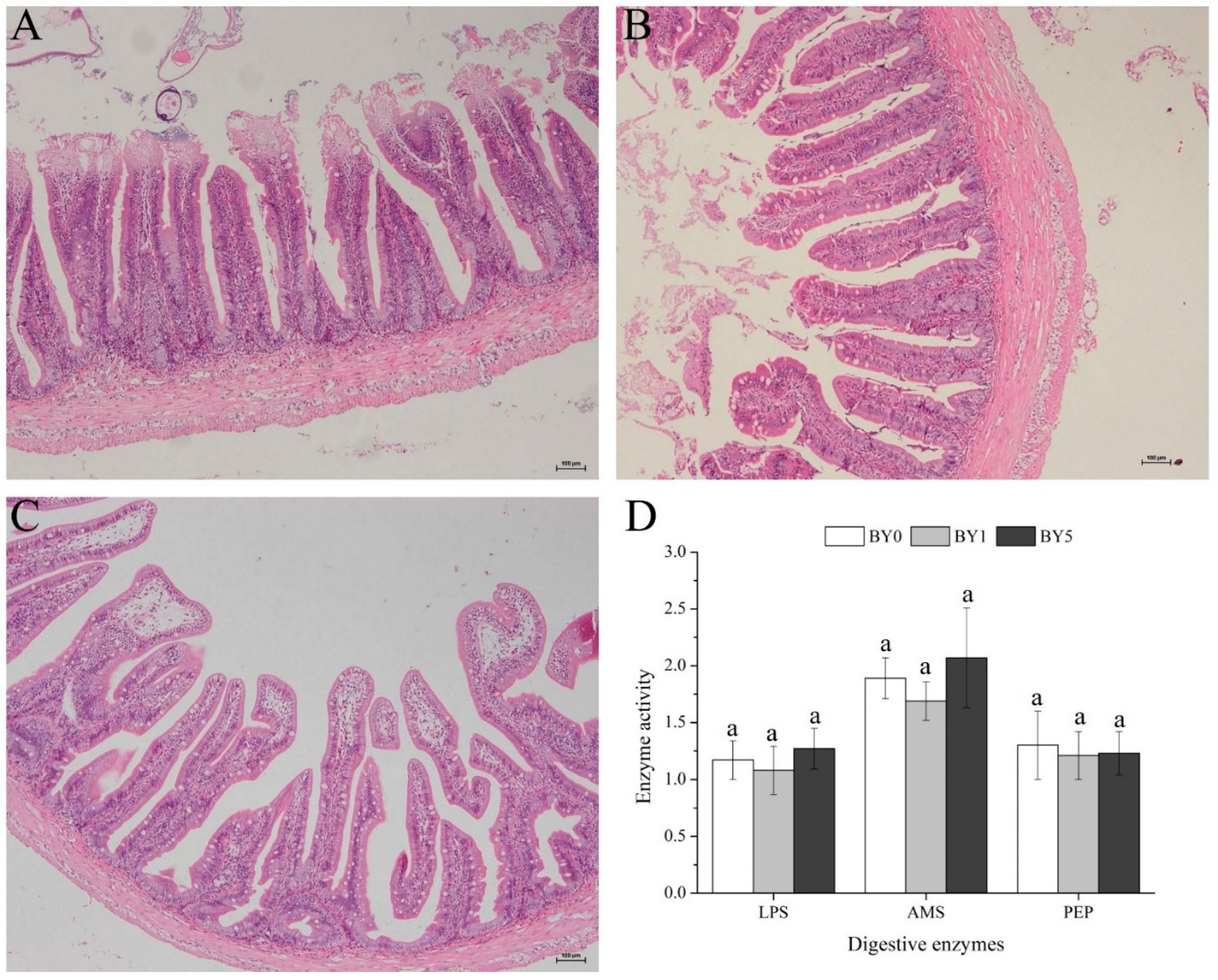
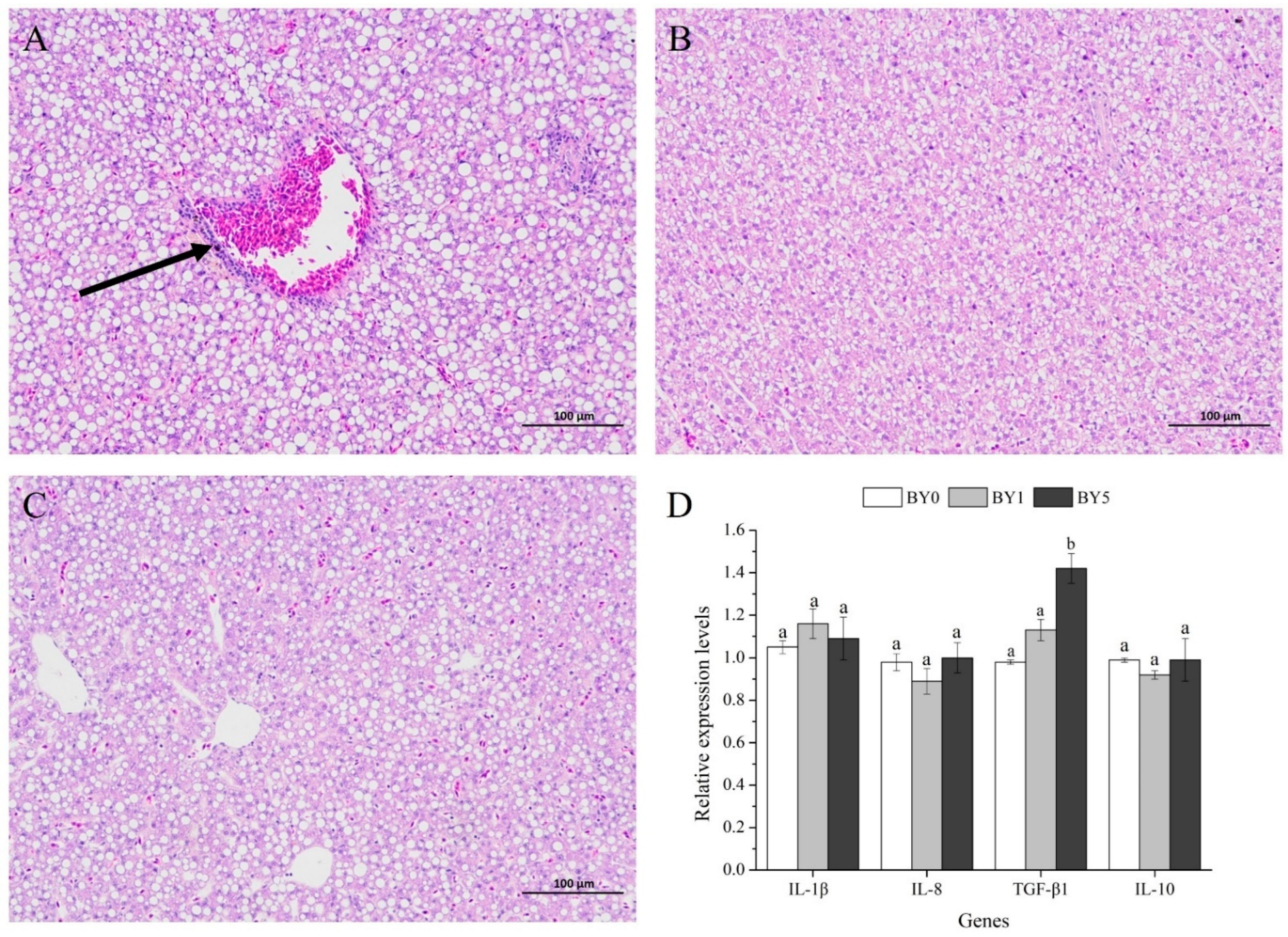
3.5. Morphology of Liver and Midgut and Activities of Digestive Enzymes in Midgut
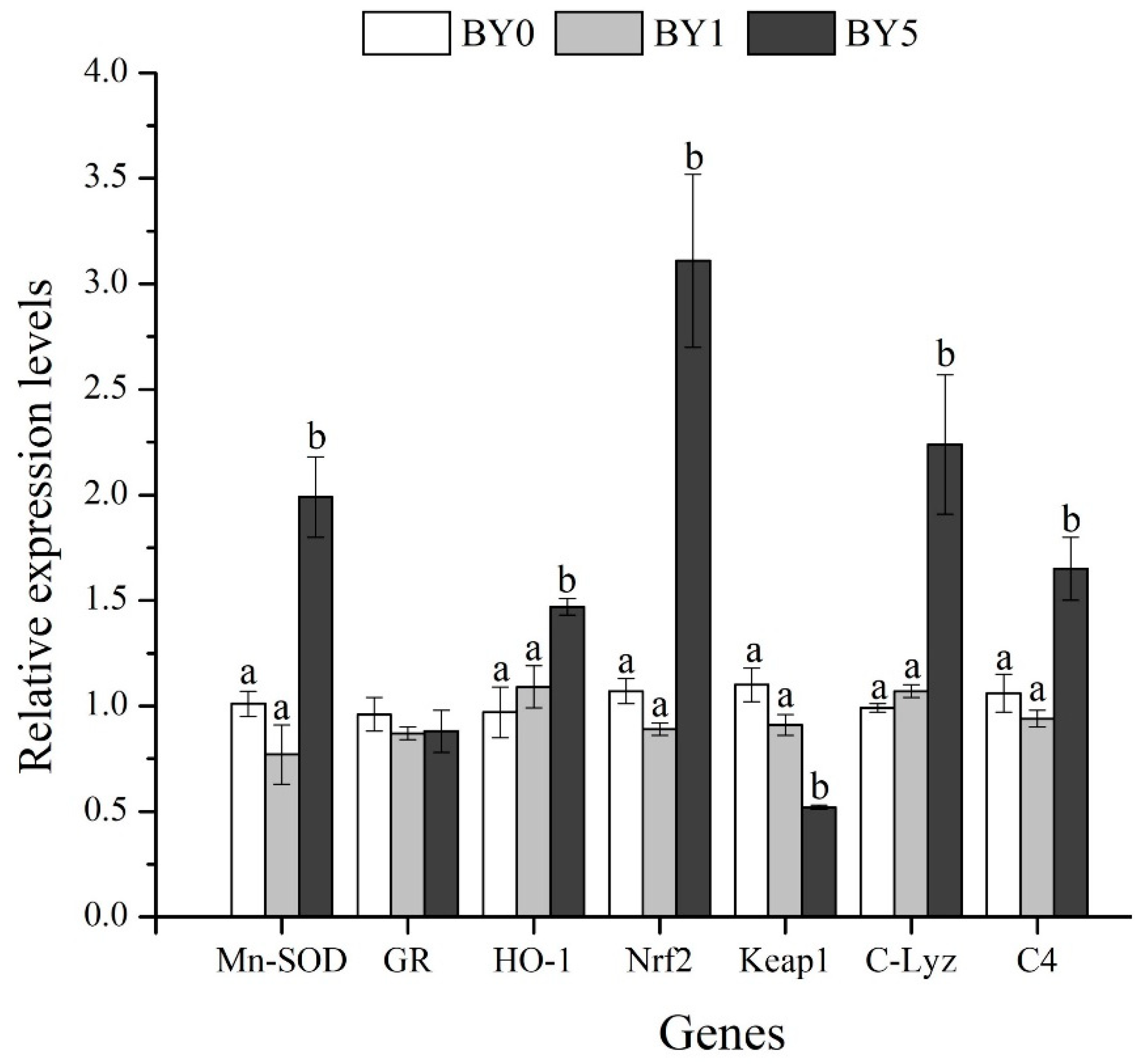
3.6. Expression Analysis of Antioxidant-Related and Immune-Related Genes in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries Administration Department of the Ministry of Agriculture of the People’s Republic of China. Fisheries Statistical Yearbook; China Agriculture Press: Beijing, China, 2021; p. 22. (In Chinese)
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yao, R.; He, X.; Liao, Z.; Liu, Y.; Gao, B.; Zhang, C.; Niu, J. Beneficial contribution of the microalga Odontella aurita to the growth, immune response, antioxidant capacity, and hepatic health of juvenile golden pompano (Trachinotus ovatus). Aquaculture 2022, 555, 738206. [Google Scholar] [CrossRef]
- Chen, W.; Luo, L.; Han, D.; Long, F.; Chi, Q.; Hu, Q. Effect of dietary supplementation with Chlorella sorokiniana meal on the growth performance, antioxidant status, and immune response of rainbow trout (Oncorhynchus mykiss). J. Appl. Phycol. 2021, 33, 3113–3122. [Google Scholar] [CrossRef]
- Charoonnart, P.; Purton, S.; Saksmerprome, V. Applications of microalgal biotechnology for disease control in aquaculture. Biology 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, P.R.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S.; Zilberg, D. Dietary supplementation with the microalgae Parietochloris incisa increases survival and stress resistance in guppy (Poecilia reticulata) fry. Aquac. Nutr. 2012, 18, 167–180. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, J.; Chi, Q.; Li, Y.; Wang, H.; Gong, Y.; Liu, G.; Hu, Z.; Han, D.; Hu, Q. Critical assessment of the filamentous green microalga Oedocladium carolinianum for astaxanthin and oil production. Algal Res. 2022, 61, 102599. [Google Scholar] [CrossRef]
- Fang, H.; He, X.; Zeng, H.; Liu, Y.; Tian, L.; Niu, J. Replacement of astaxanthin with lutein in diets of juvenile Litopenaeus vannamei: Effects on growth performance, antioxidant capacity, and immune response. Front. Mar. Sci. 2021, 8, 803748. [Google Scholar] [CrossRef]
- Ettefaghdoost, M.; Haghighi, H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2021, 115, 86–94. [Google Scholar] [CrossRef]
- Besen, K.P.; Melim, E.W.H.; Cunha, L.D.; Favaretto, E.D.; Moreira, M.; Fabregat, T.E.H.P. Lutein as a natural carotenoid source: Effect on growth, survival and skin pigmentation of goldfish juveniles (Carassius auratus). Aquac. Res. 2019, 50, 2200–2206. [Google Scholar] [CrossRef]
- Lin, J.; Lee, D.; Chang, J. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Dai, C.; Zhang, H.; Zhang, C. Evaluation of a novel oleaginous filamentous green alga, Barranca yajiagengensis (Chlorophyta, Chaetophorales) for biomass, lipids and pigments production. Algal Res. 2022, 64, 102681. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, H.H.; Gao, B.Y.; Dai, C.M.; Liu, Z.Z.; Zhang, C.W.; Niu, J. Dietary Tribonema sp. supplementation increased growth performance, antioxidant capacity, immunity and improved hepatic health in golden pompano (Trachinotus ovatus). Aquaculture 2020, 529, 735667. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Gao, B.; Yang, J.; Lei, X.; Xia, S.; Li, A.; Zhang, C. Characterization of cell structural change, growth, lipid accumulation, and pigment profile of a novel oleaginous microalga, Vischeria stellata (Eustigmatophyceae), cultured with different initial nitrate supplies. J. Appl. Phycol. 2016, 28, 821–830. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, F.F.; Gao, B.Y.; Huang, L.D.; Zhang, C.W. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. 2018, 32, 193–200. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Su, M.; Dai, C.; Huang, L.; Zhang, C. Integrated biorefinery strategy for tofu wastewater biotransformation and biomass valorization with the filamentous microalga Tribonema minus. Bioresour. Technol. 2019, 292, 121938. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists (AOAC): Arlington, TX, USA, 1995. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Díaz-Jiménez, L.; Hernández-Vergara, M.P.; Pérez-Rostro, C.I.; Olvera-Novoa, M.Á. The effect of two carotenoid sources, background colour and light spectrum on the body pigmentation of the clownfish Amphiprion ocellaris. Aquac. Res. 2021, 52, 3052–3061. [Google Scholar] [CrossRef]
- Yi, X.; Li, J.; Xu, W.; Zhang, W.; Mai, K. Effects of dietary lutein/canthaxanthin ratio on the growth and pigmentation of large yellow croaker Larimichthys croceus. Aquac. Nutr. 2016, 22, 683–690. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Zhou, Y. Dietary administration of Bacillus subtilis hainup40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, L.; Jiang, W.D.; Wu, P.; Kuang, S.Y.; Jiang, J.; Zhou, X. Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-kappa B, TOR and Nrf2 signaling molecules in the gills of fish: Preventive role of arginine. Aquat. Toxicol. 2015, 158, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Depalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Effect of different anthocyanidin glucosides on lutein uptake by caco-2 cells, and their combined activities on anti-oxidation and anti-inflammation in vitro and ex vivo. Molecules 2018, 23, 2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Jiang, P.; Gao, Y. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xie, Q.; Jin, L.; Sun, B.; Ji, J.; Chen, F.; Ma, J.; Bi, Y. Supplementation of xanthophylls decreased proinflammatory and increased anti-inflammatory cytokines in hens and chicks. Brit. J. Nutr. 2015, 114, 1533. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Kobayashi, M.; Kaneko, H.; Nakajima-Takagi, Y.; Nakayama, Y.; Yamamoto, M. Molecular evolution of keap1. two keap1 molecules with distinctive intervening region structures are conserved among fish. J. Biol. Chem. 2007, 283, 3248–3255. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, Y.; Jiang, J.; Wu, P.; Chen, G.; Jiang, W.; Li, S.; Tang, L.; Kuang, S.; Feng, L.; et al. Effects of dietary isoleucine on growth, the digestion and absorption capacity and gene expression in hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2012, 368-369, 117–128. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Preethi, K.C.; Kuttan, R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J. Exp. Biol. 2010, 48, 843–848. [Google Scholar]
- Tan, X.; Sun, Z.; Zhou, C.; Zhong, H.; Tan, L.; Xun, P.; Huang, Q.; Lin, H.; Ye, C.; Wang, A. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2018, 73, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacology 1991, 43, 109–142. [Google Scholar]
- Yu, S.; Li, G.; Rui, L.; Ma, D.; Xue, W. Dendritic Fe3O4@Poly(dopamine)@PAMAM nanocomposite as controllable NO-releasing material: A synergistic photothermal and no antibacterial study. Adv. Funct. Mater. 2018, 28, 1707440. [Google Scholar] [CrossRef]
- Privett, B.J.; Broadnax, A.D.; Bauman, S.J.; Riccio, D.A.; Schoenfishch, M.H. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, C.; Kang, H.; Seo, J.S.; Park, H.G.; Rhee, J.; Lee, J. Identification and molecular characterization of nitric oxide synthase (NOS) gene in the intertidal copepod Tigriopus japonicus. Gene 2016, 577, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, Y.; Zhang, j.; Sun, Y.; Wang, J. Dietary effects of succinic acid on the growth, digestive enzymes, immune response and resistance to ammonia stress of Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 78, 10–17. [Google Scholar] [CrossRef]
- Yao, C.L.; Ji, P.F.; Wang, Z.Y.; Li, F.H.; Xiang, J.H. Molecular cloning and expression of NOS in shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2010, 28, 453–460. [Google Scholar] [CrossRef]
- Ghodrati, M.; Islami, H.R.; Shekarabi, S.; Masouleh, A.S.; Mehrgan, M.S. Combined effects of enzymes and probiotics on hemato-biochemical parameters and immunological responses of juvenile Siberian sturgeon (Acipenser baerii). Fish Shellfish Immunol. 2021, 112, 116–124. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.S.; Levine, R.P. Complement mediated opsonisation and phagocytosis of Renibacterium sulmoniwrum. Fish Shellfish Immunol. 1992, 3, 179–190. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Paulsen, S.M.; Engstad, R.E.; Robertsen, B. Enhanced lysozyme production in Atlantic salmon (Salmo salar L.) macrophages treated with yeast beta-glucan and bacterial lipopolysaccharide. Fish Shellfish Immunol. 2001, 11, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Bugla-Poskońska, G.; Kiersnowski, A.; Futoma-Kooch, B.; Doroszkiewicz, W. Killing of Gram-Negative Bacteria with Normal Human Serum and Normal Bovine Serum: Use of Lysozyme and Complement Proteins in the Death of Salmonella Strains O48. Microb. Ecol. 2009, 58, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.M.; Roberts, R.R.; Larsen, H.S.; Tew, J.G. Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect. Immun. 1974, 10, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wu, W.; Zhou, P.; Xie, F.; Zhou, Q.; Mai, K. Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea. Aquaculture 2014, 434, 227–232. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, H.; Liu, Z.; Huang, M.; Su, M.; Zhang, C.; Gao, B.; Niu, J. A newly isolated strain of Haematococcus pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus ovatus). Aquac. Nutr. 2021, 7, 342–354. [Google Scholar] [CrossRef]
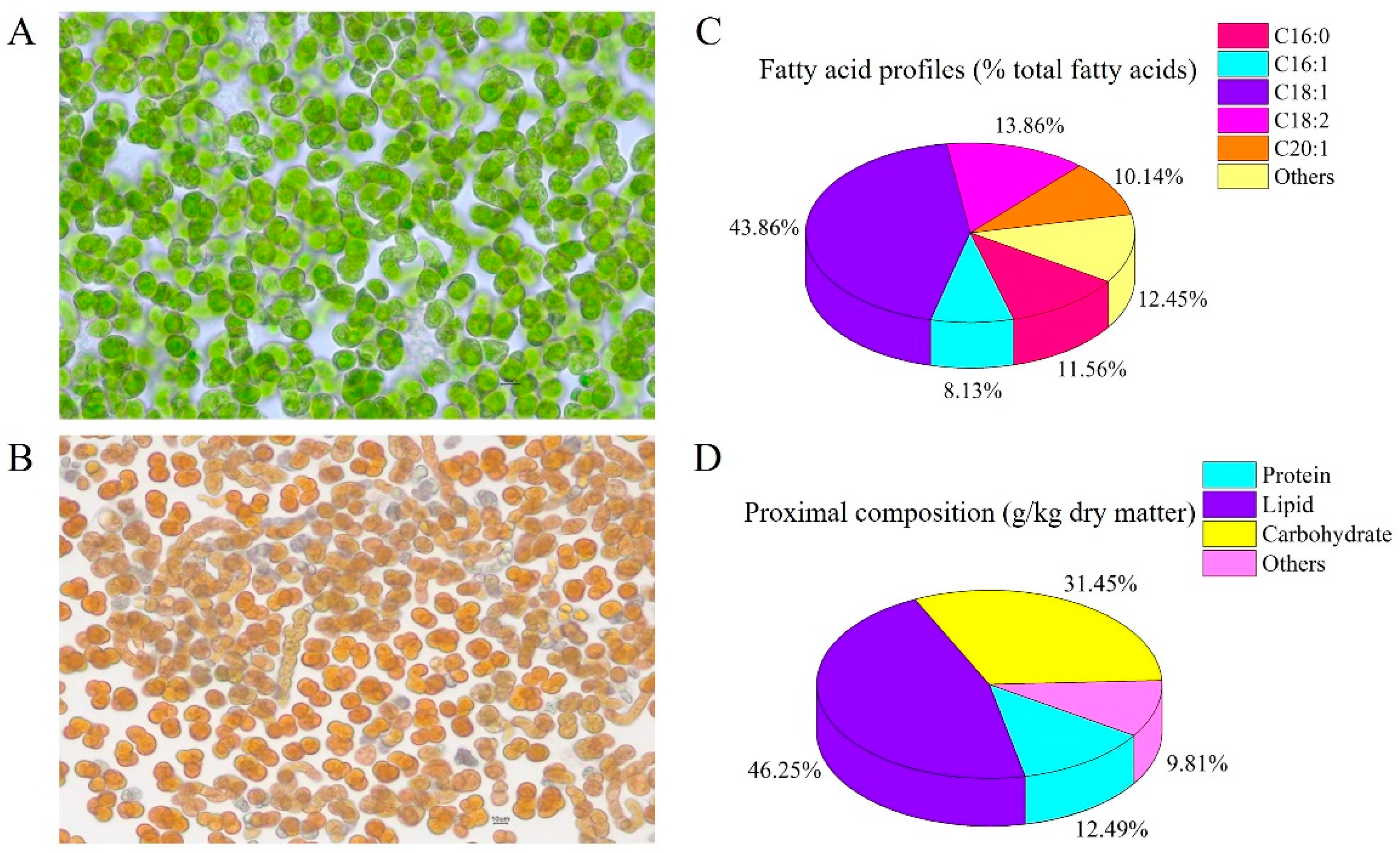

| Ingredients | BY0 | BY1 | BY5 |
|---|---|---|---|
| Fish meal | 45 | 45 | 45 |
| Soybean meal | 16.3 | 16 | 15.4 |
| Wheat flour | 20 | 20 | 20 |
| Beer yeast | 3 | 3 | 3 |
| Microcrystalline Cellulose | 4 | 3.7 | 2.1 |
| Fish oil | 7 | 6.6 | 4.8 |
| Soybean lecithin | 1 | 1 | 1 |
| Ca(H2PO4)2 | 1 | 1 | 1 |
| Vitamin premix a | 1 | 1 | 1 |
| Mineral premix b | 1 | 1 | 1 |
| Choline | 0.5 | 0.5 | 0.5 |
| Vitamin C | 0.2 | 0.2 | 0.2 |
| Barranca yajiagengensis | 0 | 1 | 5 |
| Total | 100 | 100 | 100 |
| Nutrient levels c | |||
| Crude lipid | 12.31 | 12.22 | 12.55 |
| Crude protein | 42.27 | 42.26 | 41.45 |
| Ash | 10.31 | 10.38 | 10.75 |
| Moisture | 8.92 | 9.63 | 9.16 |
| Lutein (mg kg−1 dry matter) | - | 31.20 | 156.18 |
| Items | BY0 | BY1 | BY5 |
|---|---|---|---|
| TG | 2.22 ± 0.15 | 2.44 ± 0.13 | 2.30 ± 0.18 |
| GLU | 3.89 ± 0.50 | 4.11 ± 0.30 | 3.89 ± 0.37 |
| LDL-C | 3.14 ± 0.12 | 3.04 ± 0.10 | 3.26 ± 0.05 |
| HDL-C | 1.05 ± 0.04 | 1.05 ± 0.05 | 1.17 ± 0.04 |
| HDL-C/LDL-C | 0.34 ± 0.02 | 0.34 ± 0.01 | 0.36 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Cui, X.; Wang, Z.-Q.; Yao, R.; Chen, M.-D.; Gao, B.-Y.; Zhang, C.-W.; Niu, J. Effects of Barranca yajiagengensis Powder in the Diet of Trachinotus ovatus on the Growth Performance, Antioxidant Capacity, Immunity and Morphology of the Liver and Intestine. Antioxidants 2022, 11, 1220. https://doi.org/10.3390/antiox11071220
Zhao W, Cui X, Wang Z-Q, Yao R, Chen M-D, Gao B-Y, Zhang C-W, Niu J. Effects of Barranca yajiagengensis Powder in the Diet of Trachinotus ovatus on the Growth Performance, Antioxidant Capacity, Immunity and Morphology of the Liver and Intestine. Antioxidants. 2022; 11(7):1220. https://doi.org/10.3390/antiox11071220
Chicago/Turabian StyleZhao, Wei, Xin Cui, Zi-Qiao Wang, Rong Yao, Meng-Die Chen, Bao-Yan Gao, Cheng-Wu Zhang, and Jin Niu. 2022. "Effects of Barranca yajiagengensis Powder in the Diet of Trachinotus ovatus on the Growth Performance, Antioxidant Capacity, Immunity and Morphology of the Liver and Intestine" Antioxidants 11, no. 7: 1220. https://doi.org/10.3390/antiox11071220
APA StyleZhao, W., Cui, X., Wang, Z.-Q., Yao, R., Chen, M.-D., Gao, B.-Y., Zhang, C.-W., & Niu, J. (2022). Effects of Barranca yajiagengensis Powder in the Diet of Trachinotus ovatus on the Growth Performance, Antioxidant Capacity, Immunity and Morphology of the Liver and Intestine. Antioxidants, 11(7), 1220. https://doi.org/10.3390/antiox11071220







