Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity
Abstract
:1. Introduction
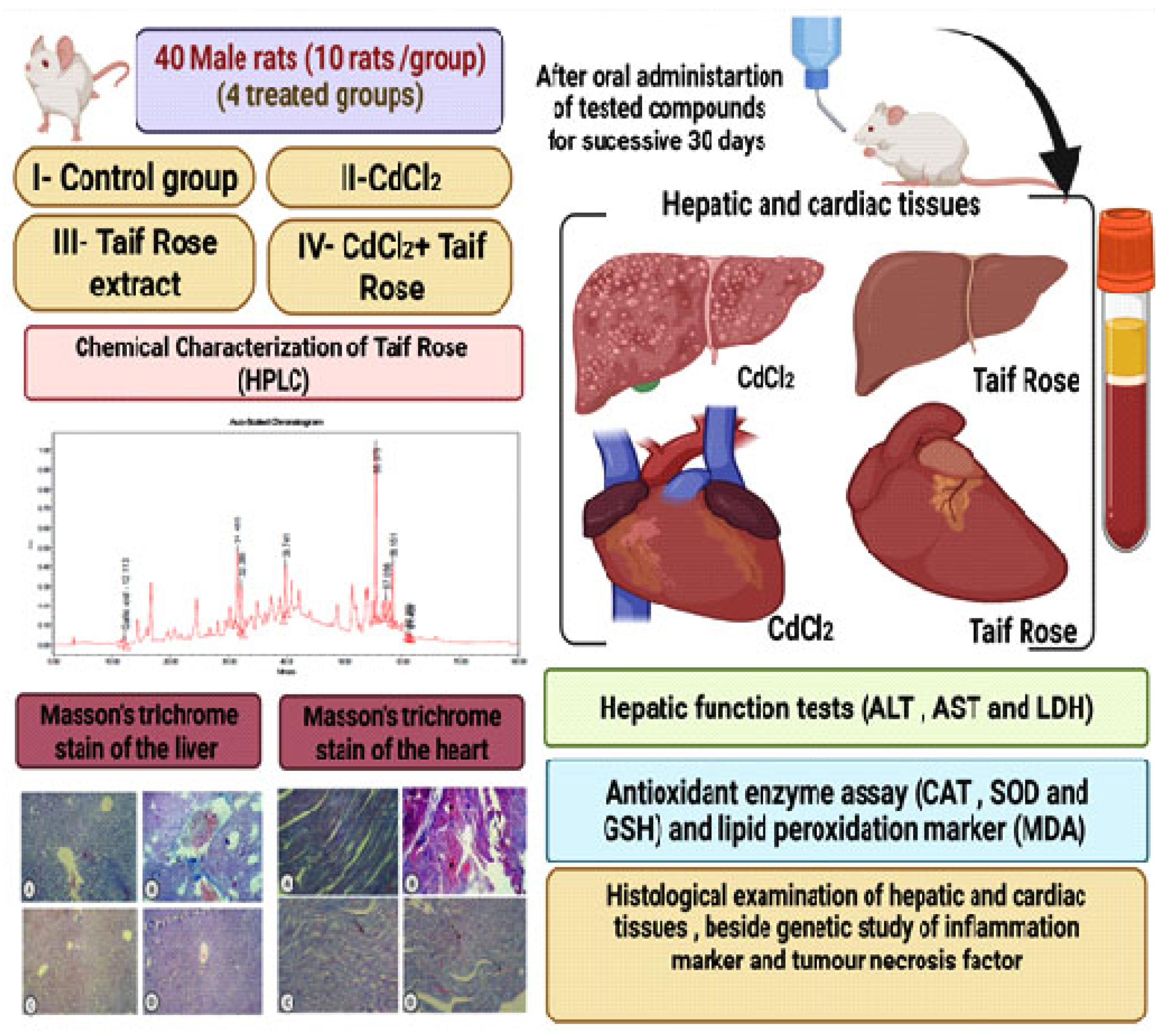
2. Materials and Methods
2.1. Plant Sampling
2.2. Preparation of R. damascena Water Extract and Determination of Flavonoids, Total Phenolics, and ROS Free Radical-Scavenging Activity
2.3. Extraction of R. damascena and Determination of Its Active Components
2.4. HPLC Analysis
2.5. Experimental Animals
2.6. Biochemical Assays
2.7. Determination of Inflammation Markers
2.8. Histopathological Study
2.9. Masson’s Trichrome Stain Study
2.10. Gene Expression
2.11. Statistical Analysis
3. Results
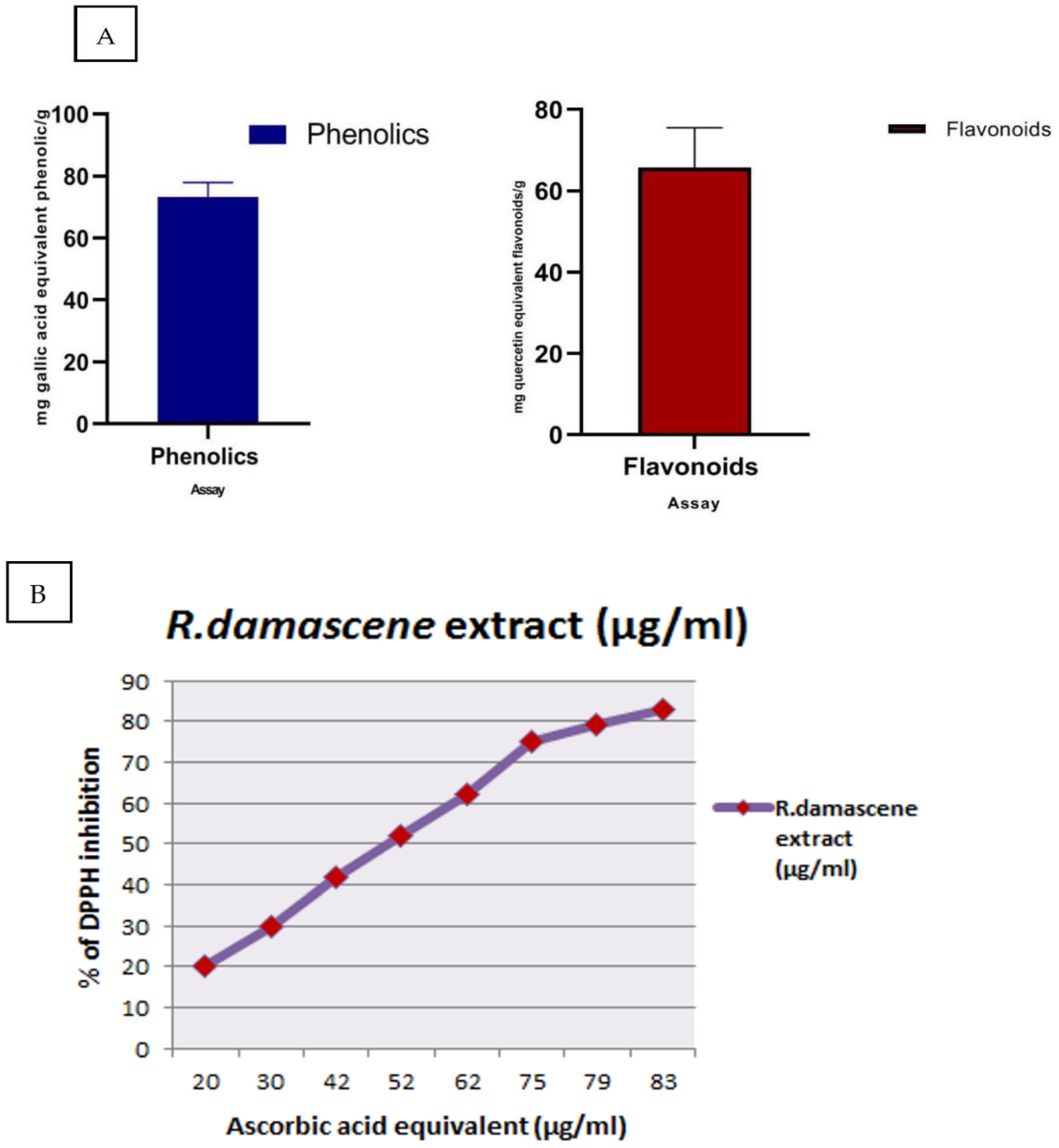
3.1. Total Phenolics, Free Radical Scavenging, and Flavonoids of R. damascena
3.2. HPLC of R. damascena (MeOH)
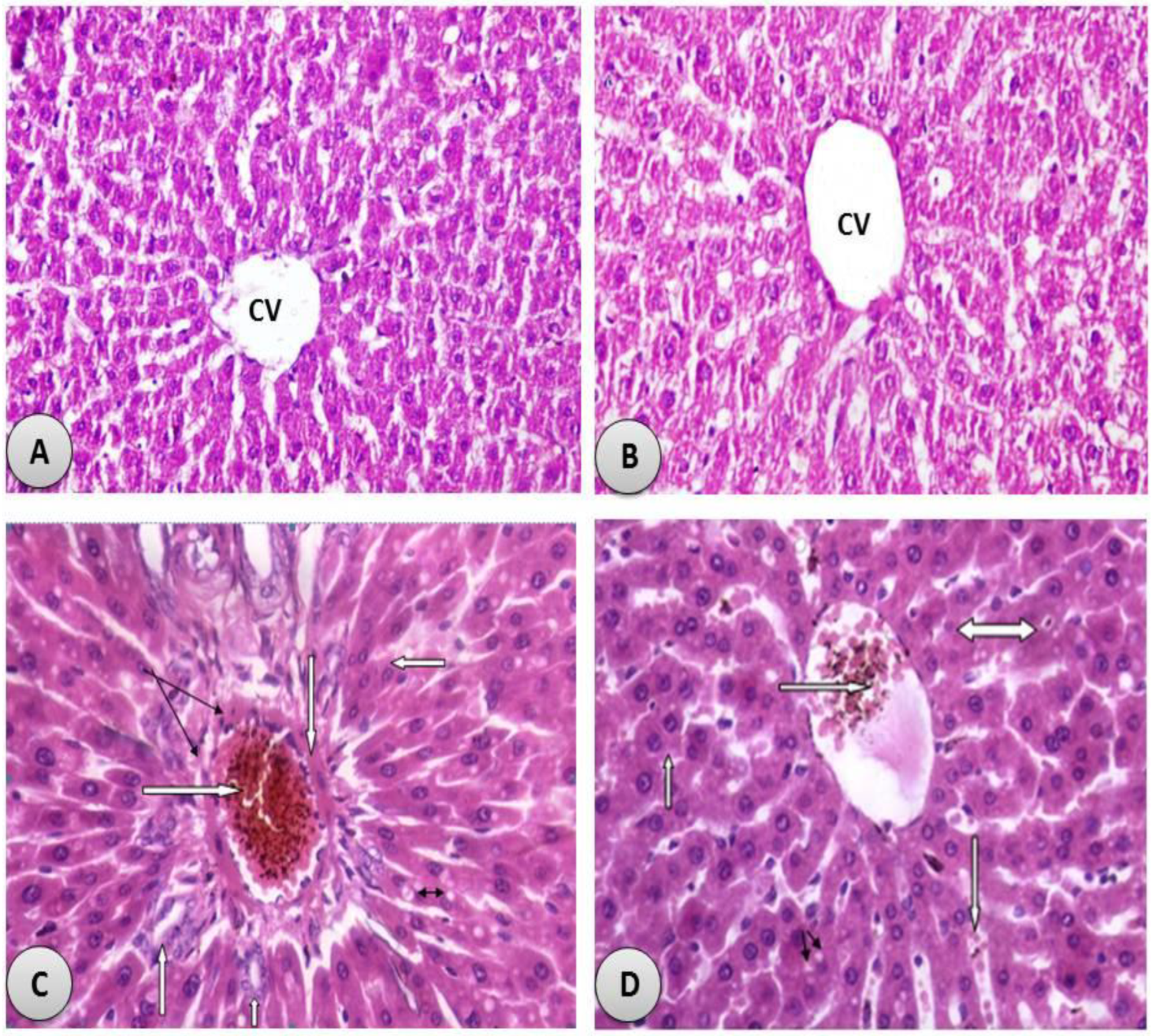
3.3. R. damascena Alleviated Liver Injury in Rats Exposed to CdCl2
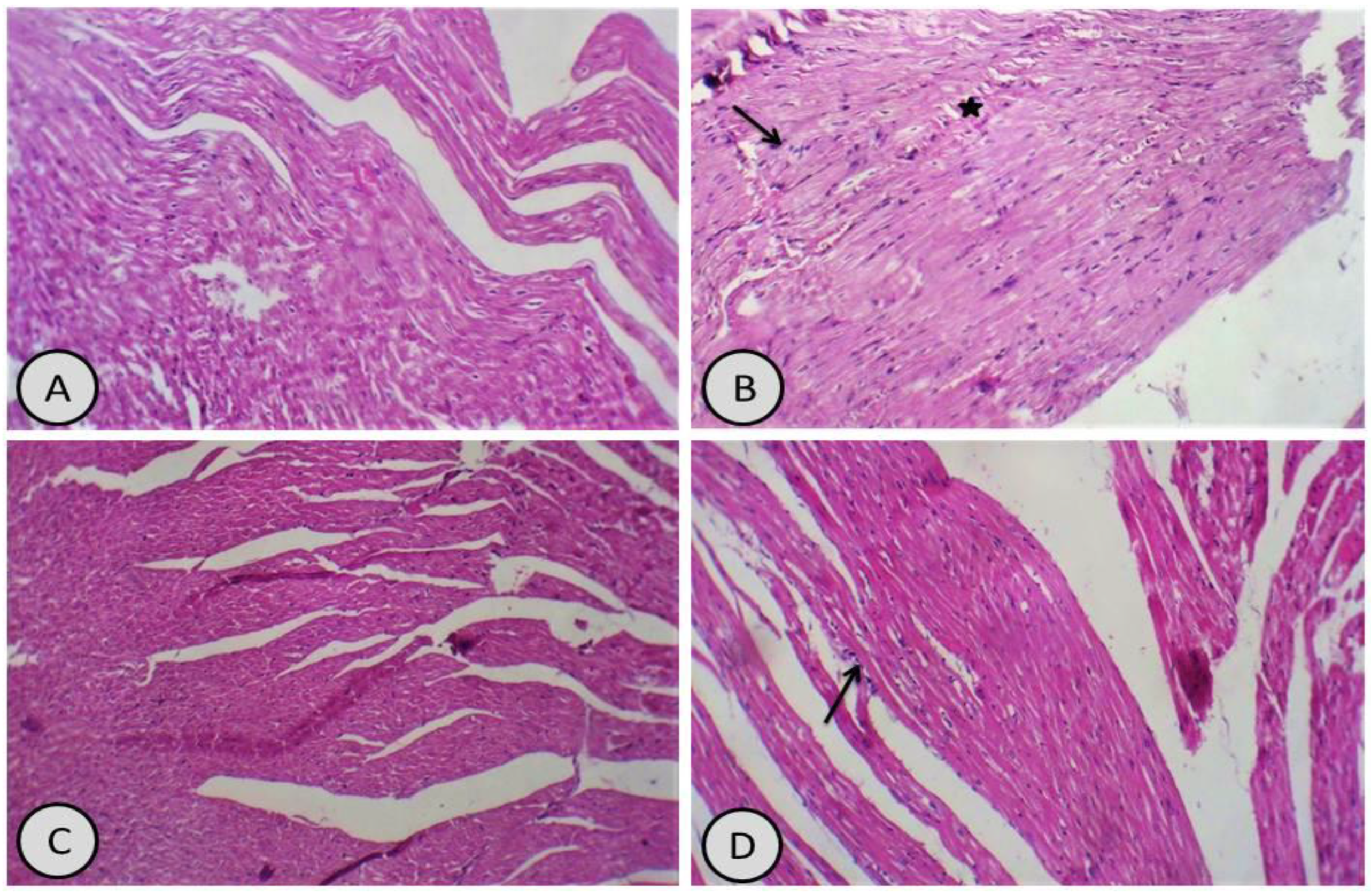
3.4. R. damascena Alleviated Oxidative Injury in the Liver and Heart of Male Rats Exposed to CdCl2
3.5. R. damascene (100 mg Kg−1) Alleviated IL-6 and TNF-α in Groups Exposed to CdCl2
3.6. R. damascena Mitigated Inflammatory Marker Expressions
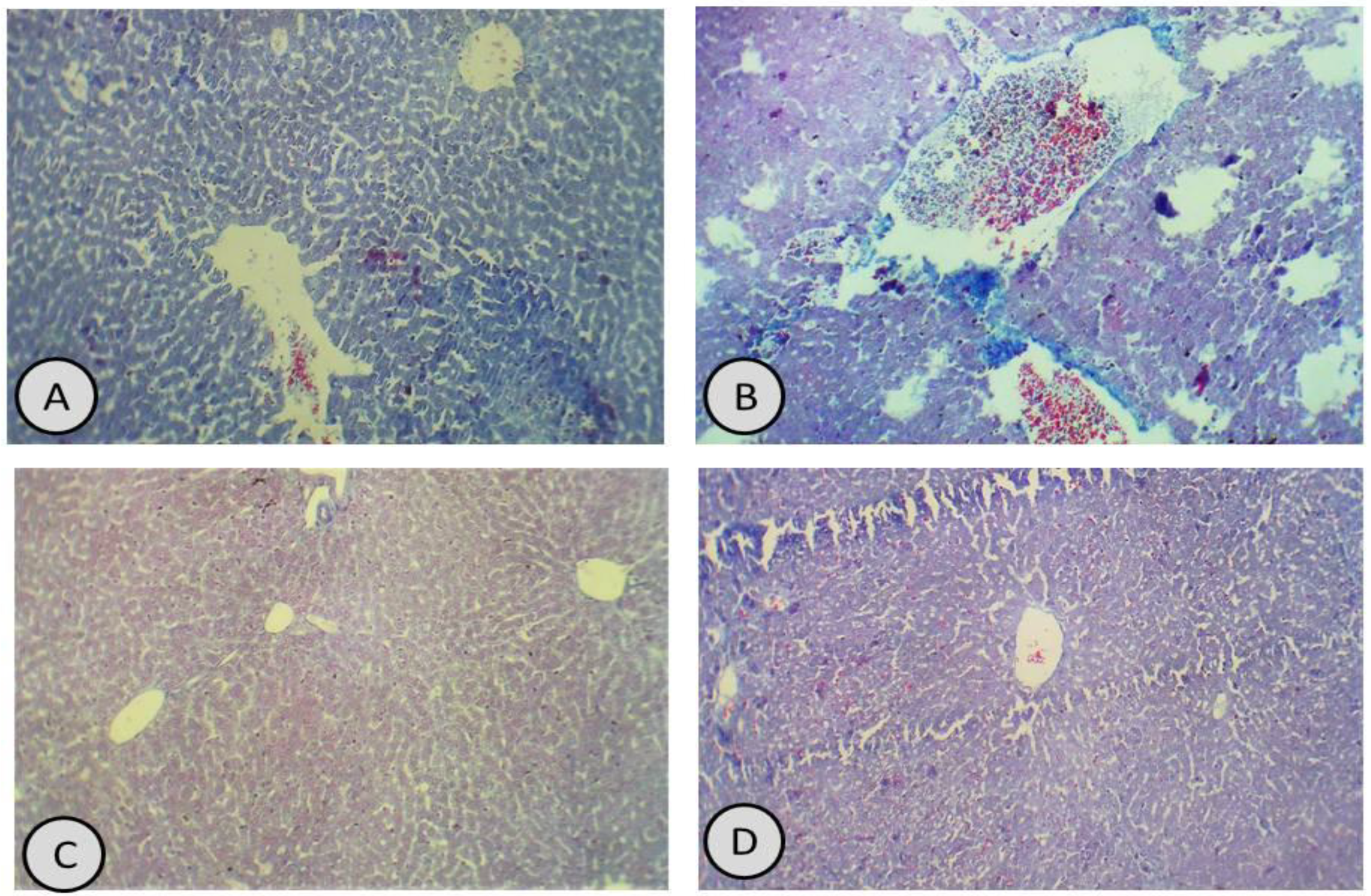
3.7. Histological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Siddiqi, N.J.; Alrashood, S.T.; Khan, H.A.; Dubey, A.; Sharma, B. Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomed. Pharmacother. 2021, 139, 111588. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.M.; Hina, A.; Saeed, A.; Sabahat, S.; Jannat, F.T.; Aslam, M. Impact of heavy metals on plants and animals in relation to sewage water. Sci. Technol. Dev. 2017, 36, 215–226. [Google Scholar]
- Kumar, A.; Pandey, R.; Siddiqi, N.J.; Sharma, B. Oxidative stress biomarkers of cadmium toxicity in mammalian systems and their distinct ameliorative strategy. J. Appl. Biotechnol. Bioeng. 2019, 6, 126–135. [Google Scholar]
- Gupta, V.K.; Kumar, A.; Siddiqi, N.J.; Sharma, B. Rat brain acetyl cholinesterase as a biomarker of cadmium induced neurotoxicity. Open Access J. Toxicol. 2016, 1, 1–7. [Google Scholar]
- Kumar, A.; Sharma, B. Consequences of heavy metals pollution in environment and bioremediation practices. In Recent Advances in Environmental Management; Bharagava, R.N., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 247–273. [Google Scholar]
- Abu-El-Zahab, H.S.H.; Hamza, R.Z.; Montaserd, M.M.; El-Mahdia, M.M.; Al-Harthi, W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef]
- Al-Baqami, N.M.; Hamza, R.Z. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.A.; Saad, H.A.; Gobouri, A.A.; Azab, E.; Al-Salmi, F.A.; Altalhi, T.A.; Khojah, E.; Gaber, A.; et al. Antioxidant, Antigenotoxic, and Hepatic Ameliorative Effects of Quercetin/Zinc Complex on Cadmium-Induced Hepatotoxicity and Alterations in Hepatic Tissue Structure. Coatings 2021, 11, 501. [Google Scholar] [CrossRef]
- Alharthi, W.A.; Hamza, R.Z.; Elmahdi, M.M.; Abuelzahab, H.S.H.; Saleh, H. Selenium and L-carnitine ameliorate reproductive toxicity induced by cadmium in male mice. Biol. Trace Elem. Res. 2020, 197, 619–627. [Google Scholar] [CrossRef]
- Almeer, R.S.; Alarifi, S.; Alkahtani, S.; Ibrahim, S.R.; Ali, D.; Moneim, A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef]
- Lin, C.; Yon, J.M.; Hong, J.T.; Lee, J.K.; Jeong, J.; Baek, I.J.; Lee, B.J.; Yun, Y.W.; Nam, S.Y. 4-o-methylhonokiol inhibits serious embryo anomalies caused by nicotine via modulations of oxidative stress, apoptosis, and inflammation. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2014, 101, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Saad, S.; Pollock, C.; Oliver, B.; Al-Odat, I.; Zaky, A.A.; Jones, N.; Chen, H. Impact of maternal cigarette smoke exposure on brain inflammation and oxidative stress in male mice offspring. Sci. Rep. 2016, 6, 25881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajarem, J.S.; Al-Basher, G.; Allam, A.A.; Mahmoud, A.M. Camellia sinensis prevents perinatal nicotine-induced neurobehavioral alterations, tissue injury, and oxidative stress in male and female mice newborns. Oxidative Med. Cell. Longev. 2017, 2017, 5985219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Basher, G.; Ajarem, J.S.; Allam, A.A.; Mahmoud, A.M. Green tea protects against perinatal nicotine-induced histological, biochemical and hematological alterations in mice offspring. Int. J. Pharmacol. 2017, 13, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, A.R.; Chandran, K.; Marimuthu, S.; Menon, V.P. Ferulic acid modulates altered lipid profiles and prooxidant/antioxidant status in circulation during nicotine-induced toxicity: A dose-dependent study. Toxicol. Mech. Methods 2005, 15, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, A.R.; Muthukumaran, S.; Devipriya, N.; Menon, V.P. Ellagic acid, a natural polyphenol protects rat peripheral blood lymphocytes against nicotine-induced cellular and DNA damage in vitro: With the comparison of n-acetylcysteine. Toxicology 2007, 230, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Lu, B.-N.; Xu, L.-N.; Yin, L.-H.; Wang, X.-N.; Peng, J.-Y.; Liu, K.-X. The antioxidant activity and hypolipidemic activity of the total flavonoids from the fruit of Rosa laevigata Michx. Nat. Sci. 2010, 2, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed. Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hameed, E.S.; Bazaid, S.A.; Salman, M.S. Characterization of the Phytochemical Constituents of Taif Rose and Its Antioxidant and Anticancer Activities. BioMed Res. Int. 2013, 2013, 345465. [Google Scholar] [CrossRef] [Green Version]
- El-Sharnouby, M.E.; Montase, M.M.; Abdallah, S.M. Oil and Flower Production in R.damascena trigintipetala Dieck under Salinity Stress in Taif Region, Saudi Arabia. Sustainability 2021, 13, 4547. [Google Scholar] [CrossRef]
- Koksall, N.; Aslancan, H.; Sadighazadi, S.; Kafkas, E. Chemical investigation on Rose damascena Mill. volatiles; Effects of storage and drying conditions. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Ryu, J.; Lyu, J.I.; Kim, D.-G.; Kim, J.-M.; Jo, Y.D.; Kang, S.-Y.; Kim, J.-B.; Ahn, J.-W.; Kim, S.H. Comparative Analysis of Volatile Compounds of Gamma-Irradiated Mutants of Rose (Rosa hybrida). Plants 2020, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; Al-Shammari, E.; Hussain, T.; Tajuddin, S.; Panda, B.P. In-vitro antimicrobial activity and identification of bioactive components using GC–MS of commercially available essential oils in Saudi Arabia. J. Food Sci. Technol. 2017, 54, 3948–3958. [Google Scholar] [CrossRef] [PubMed]
- Donpunha, W.; Kukongviriyapan, U.; Sompamit, K.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effect of ascorbic acid on cadmium-induced hypertension and vascular dysfunction in mice. BioMetals 2011, 24, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.S.; Bazaid, S.A.; Shohayeb, M.M.; El-Sayed, M.M.; El-Wakil, E.A. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. Eur. J. Med. Plants 2012, 2, 93–112. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Yasi, H.M.; Ali, E.F.; Fawzy, M.A.; Abdelkader, T.G.; Galal, T.M. Chemical Characterization of Taif Rose (Rosa damascene Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2). Crystals 2022, 12, 460. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Elsevier: Beijing, China, 2008. [Google Scholar]
- Foot, N.C. The Masson Trichrome Staining Methods in Routine Laboratory Use. Stain. Technol. 1933, 8, 101–110. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2− δδct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- IBM; IBM SPSS. Statistics for Windows; Version 27; IBM Corp: Armonk, NY, USA, 2020; Available online: http://www-01.ibm.com/support/docview.wss?uid=swg27049428 (accessed on 13 August 2021).
- Galal, T.M.; Al-Yasi, H.M.; Fawzy, M.A.; Abdelkader, T.G.; Hamza, R.Z.; Eid, E.M.; Ali, E.F. Evaluation of the Phytochemical and Pharmacological Potential of Taif’s Rose (Rosa damascene Mill var. trigintipetala) for Possible Recycling of Pruning Wastes. Life 2022, 12, 273. [Google Scholar]
- Alghashama, A.; Salem, T.A.; Meki, A. -R.M. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Khodadadi, I.; Tavilani, H.; Goodarzi, M.T. Effect of dill tablet (anethum graveolens l) on antioxidant status and biochemical factors on carbon tetrachloride-induced liver damage on rat. Int. J. Appl. Basic Med. Res. 2016, 6, 111–114. [Google Scholar]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef] [Green Version]
- Alkatib, A.A.; Cosma, M.; Elamin, M.B.; Erickson, D.; Swiglo, B.A.; Erwin, P.J.; Montori, V.M. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J. Clin. Endocrinol. Metab. 2009, 94, 3676–3681. [Google Scholar] [CrossRef] [Green Version]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant flavone apigenin: An emerging anticancer agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.A.; Saad, H.A.; Gobouri, A.A.; Al-Harbi, F.S.; Al-Salmi, F.A.; Altalhi, T.; El-Megharbel, S.M. Quercetin/Zinc complex and stem cells: A new drug therapy to ameliorate glycometabolic control and pulmonary dysfunction in diabetes mellitus: Structural characterization and genetic studies. PLoS ONE 2021, 16, e0246265. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Thubaiti, E.H.; Omar, A.S. The antioxidant activity of quercetin and its effect on acrylamide hepatotoxicity in liver of rats. Lat. Am. J. Pharm. 2021, 38, 2057–2062. [Google Scholar]
- Zahedi-Amiri, Z.; Taravati, A.; Hejazian, L.B. Protective Effect of R.damascena Against Aluminum Chloride-Induced Oxidative Stress. Biol. Trace Elem. Res. 2019, 187, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.A.; Saad, H.A.; Gobouri, A.A.; Al-Salmi, F.A.; Altalhi, T.A.; El-Megharbel, S.M. Potential Therapeutic Effects of New Ruthenium (III) Complex with Quercetin: Characterization, Structure, Gene Regulation, and Antitumor and Anti-Inflammatory Studies (RuIII/Q Novel Complex Is a Potent Immunoprotective Agent). Crystals 2021, 11, 367. [Google Scholar] [CrossRef]
- Chawla, R. Practical Clinical Biochemistry: Methods and Interpretations, 4th ed.; JP Medical Ltd.: London, UK, 2014. [Google Scholar]
- Newairya, A.A.; El-Sharakya, A.S.; Badreldeena, M.M.; Ewedaa, S.M.; Sheweitab, S.A. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef]
- Renugadevi, J.; MiltonPrabu, S. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef]
- Lakshmi, G.D.; Kumar, P.R.; Bharavi, K.; Annapurna, P.; Rajendar, B.; Patel, P.T.; Rao, G.S. Protective effect of Tribulus terrestris linn on liver and kidney in cadmium intoxicated rats. Indian J. Exp. Biol. 2012, 50, 141–146. [Google Scholar]
- Nirmala, M.J.; Samundeeswari, A.; Sankar, P.D. Natural plant resources in anti-cancer therapy—A review. Res. Plant Biol. 2011, 1, 1–14. [Google Scholar]
- Gaskill, C.L.; Miller, L.M.; Mattoon, J.S.; Hoffmann, W.E.; Burton, S.A.; Gelens, H.C.J.; Ihle, S.L.; Miller, J.B.; Shaw, D.H.; Cribb, A.E. Liver Histopathology and Liver and Serum Alanine Aminotransferase and Alkaline Phosphatase Activities in Epileptic Dogs Receiving Phenobarbital. Vet. Pathol. 2005, 42, 147–160. [Google Scholar] [CrossRef]



| Mobile Phase (A) | Concentration % of Eluent | Time (min) | Mobile Phase (B) | Concentration% of Eluent | Time (min) |
|---|---|---|---|---|---|
| Mobile phase (A) 65% water and 35% methanol | 84% | 0–10 | Mobile phase (B) 50% water and 50% methanol | 80% | 0–10 |
| 73% | 10–14 | 70% | 10–13 | ||
| 68% | 14–19 | 61% | 13–18 | ||
| 60% | 19–21 | 57% | 18–20 | ||
| 48% | 21–23 | 40% | 20–22 | ||
| 40% | 23–25 | 35% | 22–24 | ||
| 35% | 1 | 30% | 1 | ||
| 5% | 5 | 4% | 5 | ||
| 85 | 4 | 75 | 4 |
| Gene | GenBank Accession Number | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|---|
| TNF-α | NM_001278601.1 | F: CCCTCACACTCACAAACCAC R: ACAAGGTACAACCCATCGGC | 133 |
| IL-6 | NM_031168.2 | F: ACAAAGCGAGAGTCCTTCAGAG R: GAGCATTGGAAATTGGGGTAGG | 108 |
| β-actin | NM_007393.5 | F: GTGCTATGTTGCTCTAGACTTCG R: ATGCCACAGGATTCCATACC | 174 |
| Peak Name | RT (min) | Area % |
|---|---|---|
| Gallic acid | 12.113 | 20.5 |
| Phthalic acid | 31.450 | 21.84 |
| Querctin | 32.388 | 13.12 |
| Apigenin | 39.741 | 11.35 |
| Nonadecane | 55.375 | 30.48 |
| Β-Sitosterol | 57.056 | 2.36 |
| Prasterone acetate | 60.925 | 0.11 |
| Eugenol | 61.426 | 0.23 |
| Parameters | Control | CdCl2 | R. damascena | CdCl2+ R. damascena |
|---|---|---|---|---|
| ALT (U/L) | 13.24 ± 1.26 | 143.02 ± 5.62 a | 13.02 ± 1.01 b | 18.02 ± 1.15 a,b |
| AST (U/L) | 12.52 ± 1.46 | 110.25 ± 7.45 a | 12.45 ± 0.34 b | 14.17 ± 1.39 a,b |
| Parameters | Control | CdCl2 | R. damascena | CdCl2+ R. damascena |
|---|---|---|---|---|
| CAT (U/g) | 11.02 ± 0.26 | 2.42 ± 0.16 a | 11.05 ± 1.11 b | 9.01 ± 0.35 a, b |
| SOD (U/g) | 16.20 ± 0.26 | 10.07 ± 0.45 a | 16.02 ± 0.34 b | 14.60 ± 0.39 a, b |
| GSH (U/g) | 15.78 ± 2.38 | 8.10 ± 0.88 a | 16.12 ± 0.33 b | 15.64 ± 0.45 a, b |
| MDA(µg/mg) | 3.01 ± 0.15 | 14.01 ± 1.30 a | 2.05 ± 0.68 b | 6.01 ± 0.66 a, b |
| Parameters | Control | CdCl2 | R. damascena | CdCl2+ R. damascena |
|---|---|---|---|---|
| CAT (U/g) | 11.20 ± 0.26 | 3.79 ± 0.16 a | 11.02 ± 1.11 b | 10.05 ± 0.35 a, b |
| SOD (U/g) | 13.19 ± 1.26 | 7.04 ± 1.45 a | 13.45 ± 1.34 b | 12.87 ± 2.39 a,b |
| GSH (U/g) | 13.10 ± 2.38 | 7.10 ± 0.88 a | 13.22 ± 0.33 b | 11.64 ± 0.45 a,b |
| MDA(µg/mg) | 4.87 ± 0.15 | 18.30 ± 1.30 a | 4.25 ± 0.68 b | 6.21 ± 0.68 a, b |
| Parameters | Control | CdCl2 | R. damascena | CdCl2+ R. damascena |
|---|---|---|---|---|
| TNF-α (pg/mL) | 5.69 ± 0.54 | 22.36 ± 0.21 a | 4.36 ± 0.74 b | 7.02 ± 1.20 a,b |
| IL-6 (pg/mL) | 3.65 ± 0.14 | 15.69 ± 1.02 a | 3.02 ± 0.69 b | 5.25 ± 0.36 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, R.Z.; Al-Malki, N.A.; Alharthi, S.; Alharthy, S.A.; Albogami, B.; El-Megharbel, S.M. Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity. Antioxidants 2022, 11, 1229. https://doi.org/10.3390/antiox11071229
Hamza RZ, Al-Malki NA, Alharthi S, Alharthy SA, Albogami B, El-Megharbel SM. Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity. Antioxidants. 2022; 11(7):1229. https://doi.org/10.3390/antiox11071229
Chicago/Turabian StyleHamza, Reham Z., Njood A. Al-Malki, Sarah Alharthi, Saif A. Alharthy, Bander Albogami, and Samy M. El-Megharbel. 2022. "Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity" Antioxidants 11, no. 7: 1229. https://doi.org/10.3390/antiox11071229
APA StyleHamza, R. Z., Al-Malki, N. A., Alharthi, S., Alharthy, S. A., Albogami, B., & El-Megharbel, S. M. (2022). Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity. Antioxidants, 11(7), 1229. https://doi.org/10.3390/antiox11071229







