Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.1.1. Bunch
2.1.2. Fermented Pomace
2.2. Sample Preparation
2.3. Chemical Characterization of the Extract
2.3.1. Total Monomeric Anthocyanin Content
2.3.2. Anthocyanin Analytical Methodology
2.3.3. Oxygen Radical Absorbance Capacity (ORAC)
2.4. Molecular Docking
2.5. Cell Culture
2.5.1. Culture Medium
2.5.2. Reverse Transcription Followed by Polymerase Chain Reaction (RT-qPCR)
Total RNA Extraction
Synthesis of Complementary DNA
Relative Quantification by Real-Time PCR
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Monomeric Anthocyanins and Antioxidant Activity of Pinot Noir Pomace
3.2. In Silico Modelling and Molecular Couplings
3.3. Positive Regulation of HO-1 and NQO1 by HUVEC Pinot Noir Pomace Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Botelho, R.V.; Bennemann, G.D.; Torres, Y.R.; Sato, A.J. Potential for Use of the Residues of the Wine Industry in Human Nutrition and as Agricultural Input. In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; Intech Open: London, UK, 2018. [Google Scholar]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Garrido, R.A.; Lagos, C.; Luna, C.; Sánchez, J.; Díaz, G. Study of the Potential Uses of Hydrochar from Grape Pomace and Walnut Shells Generated from Hydrothermal Carbonization as an Alternative for the Revalorization of Agri-Waste in Chile. Sustainability 2021, 13, 12600. [Google Scholar] [CrossRef]
- Garzón, G.A. Anthocyanins as Natural Colorants and Bioactive Compounds. A Review. Acta Biológ. Colomb. 2010, 13, 27–36. [Google Scholar]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Lee, D.W.; Collins, T.M. Phylogenetic and Ontogenetic Influences on the Distribution of Anthocyanins and Betacyanins in Leaves of Tropical Plants. Int. J. Plant Sci. 2001, 162, 1141–1153. [Google Scholar] [CrossRef]
- Lee, D.W. Anthocyanins in Leaves: Distribution, Phylogeny and Development. Adv. Bot. Res. 2002, 37, 37–53. [Google Scholar]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Lawrence, W.J.C.; Price, J.R.; Robinson, G.M.; Robinson, R. The Distribution of Anthocyanins in Flowers, Fruits and Leaves. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1939, 230, 149–178. [Google Scholar] [CrossRef] [Green Version]
- Timberlake, C.F.; Bridle, P. Distribution of Anthocyanins in Food Plants. In Anthocyanins as Food Colors; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From Plant to Health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin Stability and Recovery: Implications for the Analysis of Clinical and Experimental Samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E.; Skrede, G.; Lea, P.; Enersen, G. Influence of Sugar on Anthocyanin Pigment Stability in Frozen Strawberries. J. Food Sci. 1990, 55, 1064–1065. [Google Scholar] [CrossRef]
- Reis, G.M.; Faccin, H.; Viana, C.; da Rosa, M.B.; de Carvalho, L.M. Vitis vinifera L. Cv Pinot Noir Pomace and Lees as Potential Sources of Bioactive Compounds. Int. J. Food Sci. Nutr. 2016, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of Blueberry Anthocyanins Malvidin and Glycosides on the Antioxidant Properties in Endothelial Cells. Oxid. Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE Signaling Pathway: An Update on Its Regulation and Possible Role in Cancer Prevention and Treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Panda, H.; Wen, H.; Suzuki, M.; Yamamoto, M. Multifaceted Roles of the KEAP1–NRF2 System in Cancer and Inflammatory Disease Milieu. Antioxidants 2022, 11, 538. [Google Scholar] [CrossRef]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxid. Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 Mutations: Permanent Activation of an Adaptive Response in Cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef]
- Alonso-Piñeiro, J.A.; Gonzalez-Rovira, A.; Sánchez-Gomar, I.; Moreno, J.A.; Durán-Ruiz, M.C. Nrf2 and Heme Oxygenase-1 Involvement in Atherosclerosis Related Oxidative Stress. Antioxidants 2021, 10, 1463. [Google Scholar] [CrossRef]
- Ooi, B.K.; Chan, K.G.; Goh, B.H.; Yap, W.H. The Role of Natural Products in Targeting Cardiovascular Diseases via Nrf2 Pathway: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.J.; Yang, H.J.; Li, W.; Oh, Y.C.; Go, Y. Menthae Herba Attenuates Neuroinflammation by Regulating CREB/Nrf2/HO-1 Pathway in BV2 Microglial Cells. Antioxidants 2022, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ha, L.-K.; Oh, S.; Fang, M.; Zheng, S.; Bellere, A.D.; Jeong, J.; Yi, T.-H. Antiphotoaging Effects of Damiana (Turnera diffusa) Leaves Extract via Regulation AP-1 and Nrf2/ARE Signaling Pathways. Plants 2022, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Sandanuwan Kirindage, K.G.I.; Fernando, I.P.S.; Jayasinghe, A.M.K.; Han, E.J.; Madhawa Dias, M.K.H.; Kang, K.P.; Moon, S.I.; Shin, T.S.; Ma, A.; Ahn, G. Moringa Oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling. Foods 2022, 11, 420. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, J.-H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Vo, T.T.T.; Lin, W.N.; Huang, H.W.; Chuang, C.C.; Chu, P.M.; Lee, I.T. Nrf2/HO-1 Partially Regulates Cytoprotective Effects of Carbon Monoxide against Urban Particulate Matter-Induced Inflammatory Responses in Oral Keratinocytes. Cytokine 2020, 133, 155185. [Google Scholar] [CrossRef]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.-Y.; Bach, F.H. Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef] [Green Version]
- Otterbein, L.E.; Foresti, R.; Motterlini, R. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act between Danger Signaling and Pro-Survival. Circ. Res. 2016, 118, 1940–1959. [Google Scholar] [CrossRef] [Green Version]
- Ross, D.; Siegel, D. The Diverse Functionality of NQO1 and Its Roles in Redox Control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; Dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.F.D.S.; Rosalen, P.L.; De Alencar, S.M. Winery By-Products: Extraction Optimization, Phenolic Composition and Cytotoxic Evaluation to Act as a New Source of Scavenging of Reactive Oxygen Species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Bravo, J.; Beltrán-Lissabet, J.F.; Saavedra, K.; Saavedra, N.; Hevia, M.; Alvear, M.; Lanas, F.; Salazar, L.A. Protective Effect of Pinot Noir Pomace Extract against the Cytotoxicity Induced by Polycyclic Aromatic Hydrocarbons on Endothelial Cells. Food Chem. Toxicol. 2021, 148, 111947. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Wang, Z.-J.; Bai, F.; Qin, X.-J.; Cao, J.; Lv, J.-Y.; Zhang, M.-S. Epigallocatechin-3-Gallate Protects HUVECs from PM2.5-Induced Oxidative Stress Injury by Activating Critical Antioxidant Pathways. Molecules 2015, 20, 6626–6639. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, R.; Toral, M.; Gómez-Guzmán, M.; Romero, M.; Sanchez, M.; Mahmoud, A.M.; Duarte, J. The Role of Nrf2 Signaling in PPARβ/δ-Mediated Vascular Protection against Hyperglycemia-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 5852706. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, T.; de Bruijn, J.; Loyola, C.; Bustamante, L.; Vergara, C.; von Baer, D.; Mardones, C.; Serra, I. Characterization of an Antioxidant-Enriched Beverage from Grape Musts and Extracts of Winery and Grapevine by-Products. Beverages 2018, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Lillo, A.; Carvajal, F.; Nuñez, D.; Balboa, N.; Alvear, M. Cuantificación Espectrofotométrica de Compuestos Fenólicos y Actividad Antioxidante En Distintos Berries Nativos Del Cono Sur de América. Rev. Investig. Agropecu. 2016, 42, 168–174. [Google Scholar]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, K.; Charles, A.L. In Vitro Antioxidant Activity of Kyoho Grape Extracts in DPPH∙ and ABTS∙ Assays: Estimation Methods for EC 50 Using Advanced Statistical Programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (Cv Negramaro) and White (Cv Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH Assay Comparison to Assess Antioxidant Capacity of Tea Infusions: Relationship between Total Polyphenol and Individual Catechin Content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M.E. Polymeric Nanoparticles as Oral Delivery Systems for a Grape Pomace Extract towards the Improvement of Biological Activities. Mater. Sci. Eng. C 2021, 119, 111551. [Google Scholar] [CrossRef]
- Wang, S.; Amigo-Benavent, M.; Mateos, R.; Bravo, L.; Sarriá, B. Effects of in Vitro Digestion and Storage on the Phenolic Content and Antioxidant Capacity of a Red Grape Pomace. Int. J. Food Sci. Nutr. 2017, 68, 188–200. [Google Scholar] [CrossRef]
- Veličkovska, S.K.; Mirhosseini, H. Isolation of Anthocyanins by High-Speed Countercurrent Chromatography and Application of the Color Activity Concept to Different Varieties of Red Grape Pomace from Macedonia. J. Nutr. Food Sci. 2013, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Blesso, C.N. Antioxidant Properties of Anthocyanins and Their Mechanism of Action in Atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the Biological Activity of Anthocyanins and Polyphenols in Blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Lizio, R.; Izzo, C.; Visco, V.; Damato, A.; Venturini, E.; De Lucia, M.; Galasso, G.; Migliarino, S.; Rasile, B.; et al. A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm®) and Anthocyanins Exerts Beneficial Cardiovascular Effects. Antioxidants 2022, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Cimino, F.; Saija, A.; Canali, R.; Virgili, F. Bioavailability and Molecular Activities of Anthocyanins as Modulators of Endothelial Function. Genes Nutr. 2014, 9, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Chun, K.S.; Kim, D.H.; Kim, S.J.; Kim, S.H.; Cho, N.C.; Na, H.K.; Surh, Y.J. Curcumin Induces Stabilization of Nrf2 Protein through Keap1 Cysteine Modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef]
- Da Silva, D.V.T.; Dos Santos Baião, D.; Ferreira, V.F.; Paschoalin, V.M.F. Betanin as a Multipath Oxidative Stress and Inflammation Modulator: A Beetroot Pigment with Protective Effects on Cardiovascular Disease Pathogenesis. Crit. Rev. Food Sci. Nutr. 2021, 62, 539–554. [Google Scholar] [CrossRef]
- Sthijns, M.M.J.P.E.; Schiffers, P.M.; Janssen, G.M.; Lemmens, K.J.A.; Ides, B.; Vangrieken, P.; Bouwman, F.G.; Mariman, E.C.; Pader, I.; Arnér, E.S.J.; et al. Rutin Protects against H2O2-Triggered Impaired Relaxation of Placental Arterioles and Induces Nrf2-Mediated Adaptation in Human Umbilical Vein Endothelial Cells Exposed to Oxidative Stress. Biochim. Biophys. Acta—Gen. Subj. 2017, 1861, 1177–1189. [Google Scholar] [CrossRef]
- Heyninck, K.; Sabbe, L.; Chirumamilla, C.S.; Szarc Vel Szic, K.; Vander Veken, P.; Lemmens, K.J.A.; Lahtela-Kakkonen, M.; Naulaerts, S.; Op De Beeck, K.; Laukens, K.; et al. Withaferin A Induces Heme Oxygenase (HO-1) Expression in Endothelial Cells via Activation of the Keap1/Nrf2 Pathway. Biochem. Pharmacol. 2016, 109, 48–61. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a Polyphenol Chalcone Present in Hops, Activating Nrf2 Enzymes to Confer Protection against Oxidative Damage in Pc12 Cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.J.; Walters, M.J.; Mitchell, J.A. Carbon Monoxide Inhibits Endothelin-1 Release by Human Pulmonary Artery Smooth Muscle Cells. Eur. J. Pharmacol. 2004, 486, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a Phytocomplex of Bilberry (Vaccinium Myrtillus) and Spirulina (Spirulina platensis), Exerts Both Direct Antioxidant Activity and Modulation of ARE/Nrf2 Pathway in HepG2 Cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-Mediated NQO1 Expression by Quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
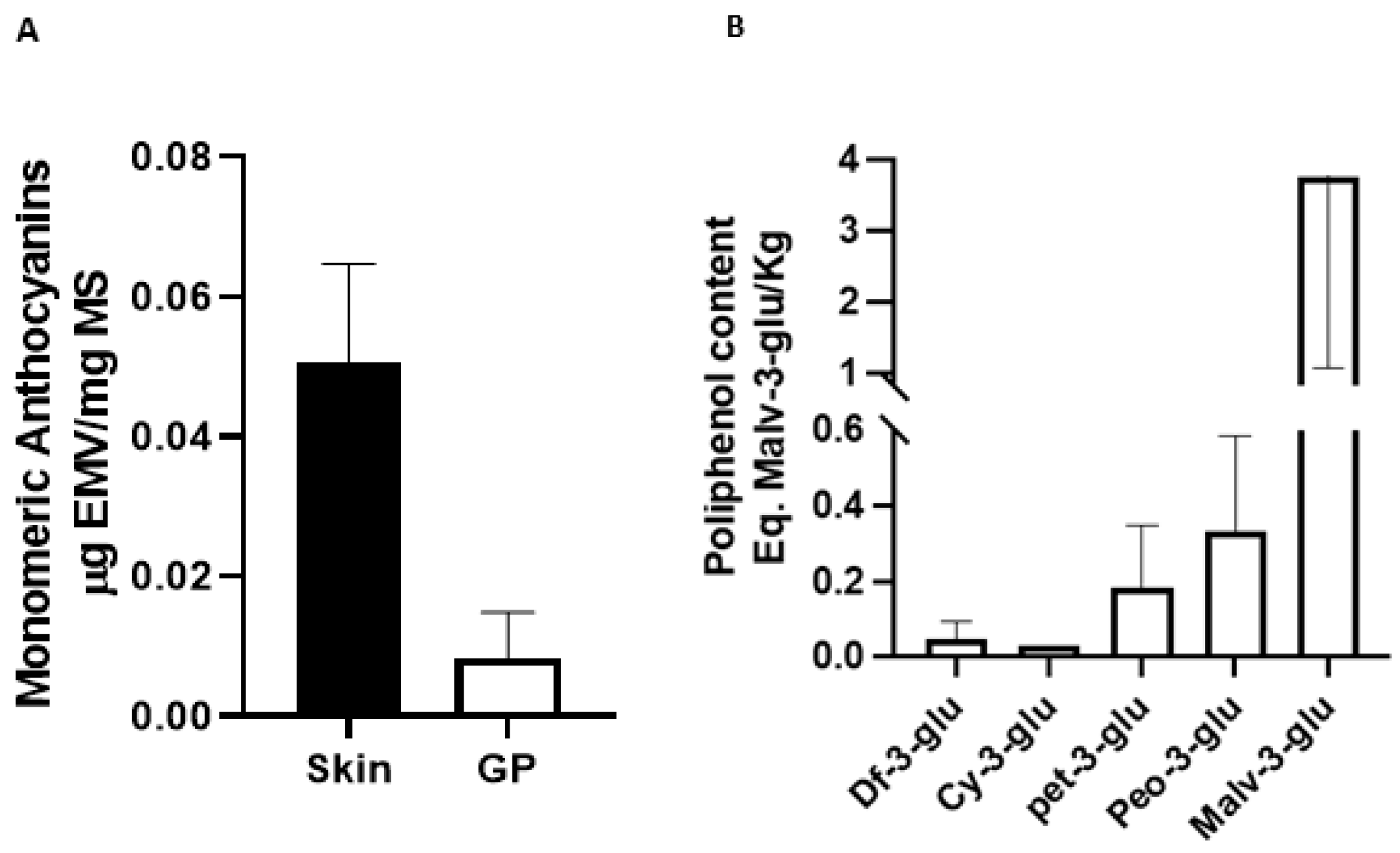
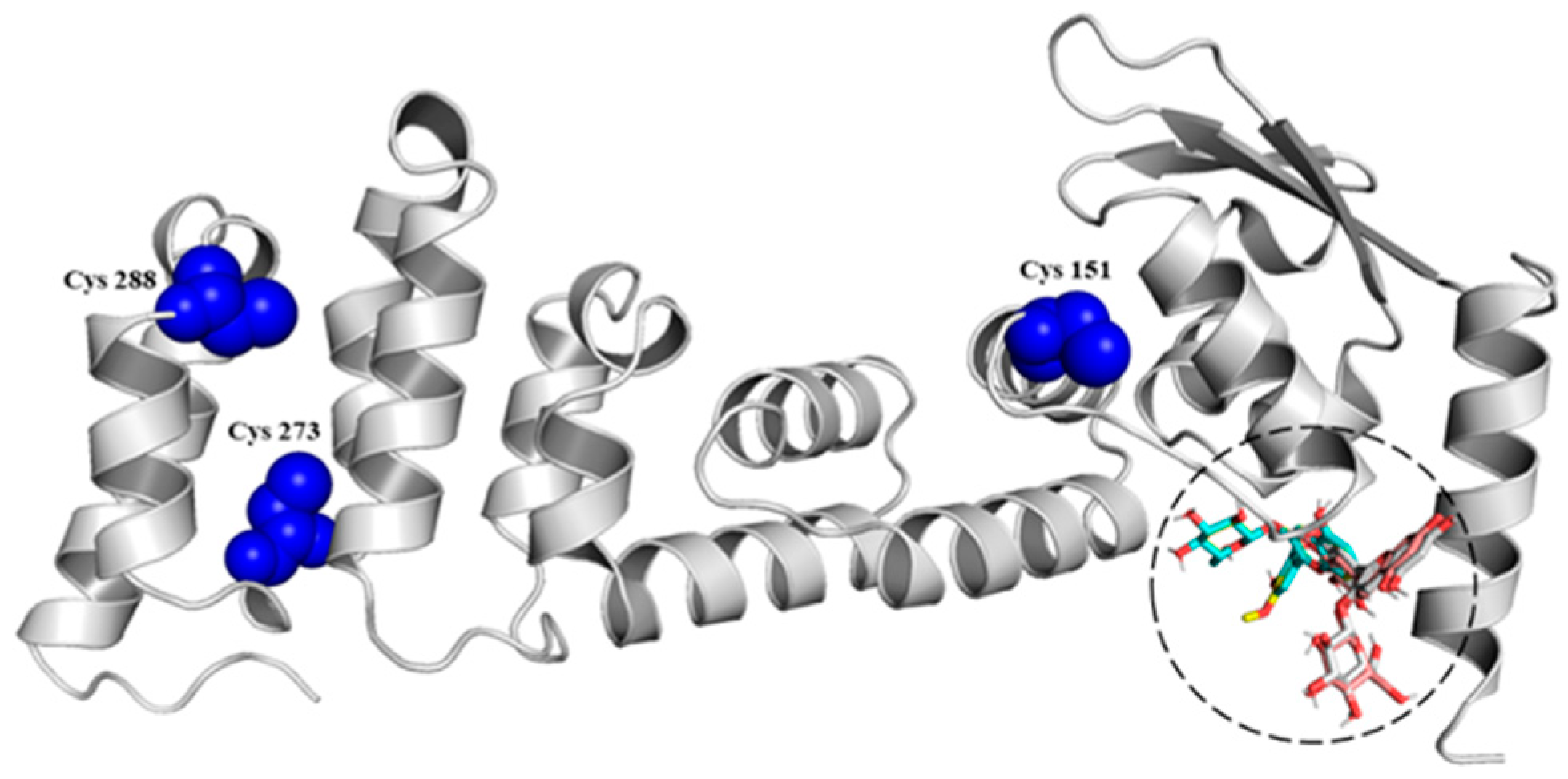

| Gene | Accession Number | Primers | Ref. | |
|---|---|---|---|---|
| Forward | Reverse | |||
| NRF2 | NM_001313903 | GAATTGCCTGTAAGTCCTGGTC | GGTGAAGGCTTTTGTCATTTTC | [40] |
| HO-1 | NM_002133 | CTTCTTCACCTTCCCCAACA | ATTGCCTGGATGTGCTTTTC | [40] |
| NQO1 | NM_001025433 | AGACCTTGTGATATTCCAGTTC | GGCAGCGTAAGTGTAAGC | [41] |
| RPL30 | NM_000989 | ATGGTGGCCGCAAAGAAGA | TCTGCTTGTACCCCAGGACGTACT | -- |
| Ligand | Binding Affinity (kcal/mol) |
|---|---|
| Anthocyanins | |
| Cyanidin-3-glucoside | −6.9 |
| Delphinidin-3-glucoside | −6.5 |
| Malvidin-3-glucoside | −6.6 |
| Peonidin-3-glucoside | −6.6 |
| Petunidin-3-glucoside | −6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Bravo, J.; Beltrán, J.F.; Huard, N.; Saavedra, K.; Saavedra, N.; Alvear, M.; Lanas, F.; Salazar, L.A. Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants 2022, 11, 1239. https://doi.org/10.3390/antiox11071239
Herrera-Bravo J, Beltrán JF, Huard N, Saavedra K, Saavedra N, Alvear M, Lanas F, Salazar LA. Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants. 2022; 11(7):1239. https://doi.org/10.3390/antiox11071239
Chicago/Turabian StyleHerrera-Bravo, Jesús, Jorge F. Beltrán, Nolberto Huard, Kathleen Saavedra, Nicolás Saavedra, Marysol Alvear, Fernando Lanas, and Luis A. Salazar. 2022. "Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells" Antioxidants 11, no. 7: 1239. https://doi.org/10.3390/antiox11071239
APA StyleHerrera-Bravo, J., Beltrán, J. F., Huard, N., Saavedra, K., Saavedra, N., Alvear, M., Lanas, F., & Salazar, L. A. (2022). Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants, 11(7), 1239. https://doi.org/10.3390/antiox11071239







