Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases
Abstract
:1. Introduction
2. Effect of Phytochemicals and Medicinal Plants on Paraoxonase-1 in Health and Disease
2.1. Moringa oleifera (Munga) and Its Bioactive Compounds (Beta-Sitosterol)
2.2. Euterpe oleracea Mart. (Açai)
2.3. Securigera securidaca (Adasolmolk or Gandeh Talkheh)
2.4. Graptopetalum paraguayense
2.5. Allium cepa L. (Onion) and Its Bioactive Compounds (Quercetin and Catechin)
2.6. Ilex paraguariensis (Yerba Mate)
2.7. Punica granatum (Pomegranate) and Its Bioactive Compounds (Quercetin and Punicalagin)
2.8. Red Wine and Grape Seed Extract (GSE)
2.9. Berberis vulgaris (Barberry)
2.10. Flos Carthamus tinctorius L. (Honghua) and Radix Salvia miltiorrhiza (Danshen)
2.11. Fragaria ananassa (Strawberries)
2.12. Rhus coriaria (Sumac)
2.13. Vaccinium macrocarpon (Red Cranberry)
2.14. Phoenix dactylifera (Date Palm)
2.15. Canola Oil
2.16. Resveratrol (3,5,4’-trihydroxystilbene)
2.17. Quercetin (3,3’,4’,5,7-pentahydroxyflavone)
2.18. Curcumin
3. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
References
- Hadaegh, F.; Fahimfar, N.; Khalili, D.; Sheikholeslami, F.; Azizi, F. New and known type 2 diabetes as coronary heart disease equivalent: Results from 7.6 year follow up in a Middle East population. Cardiovasc. Diabetol. 2010, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Draganov, D.; La Du, B.N. Pharmacogenetics of paraoxonases: A brief review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Wadleigh, D.J.; Grijalva, V.; Ng, C.; Hama, S.; Gangopadhyay, A.; Shih, D.M.; Lusis, A.J.; Navab, M.; Fogelman, A.M. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.J.; Wadleigh, D.J.; Gangopadhyay, A.; Hama, S.; Grijalva, V.R.; Navab, M.; Fogelman, A.M.; Reddy, S.T. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 2001, 276, 44444–44449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahrooz, A.; Mackness, M.; Bagheri, A.; Ghaffari-Cherati, M.; Masoumi, P. The epigenetic regulation of paraoxonase 1 (PON1) as an important enzyme in HDL function: The missing link between environmental and genetic regulation. Clin. Biochem. 2019, 73, 1–10. [Google Scholar] [CrossRef]
- Costa, L.G.; Furlong, C.E. Paraoxonase (PON1) in Health and Disease: Basic and Clinical Aspects; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar]
- Navab, M.; Hama, S.Y.; Wagner, A.C.; Hough, G.; Watson, A.D.; Reddy, S.T.; Van Lenten, B.J.; Laks, H.; Fogelman, A.M. Protective action of HDL-associated PON1 against LDL oxidation. In Paraoxonase (PON1) in Health and Disease; Springer: New York, NY, USA, 2002; pp. 125–136. [Google Scholar]
- Alidadi, M.; Jamialahmadi, T.; Cicero, A.F.G.; Bianconi, V.; Pirro, M.; Banach, M.; Sahebkar, A. The potential role of plant-derived natural products in improving arterial stiffness: A review of dietary intervention studies. Trends Food Sci. Technol. 2020, 99, 426–440. [Google Scholar] [CrossRef]
- Bagherniya, M.; Nobili, V.; Blesso, C.N.; Sahebkar, A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacol. Res. 2018, 130, 213–240. [Google Scholar] [CrossRef]
- Enayati, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Protective role of nutraceuticals against myocarditis. Biomed. Pharmacother. 2022, 146, 112242. [Google Scholar] [CrossRef]
- Hosseini, A.; Penson, P.E.; Cicero, A.F.G.; Golledge, J.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. Potential benefits of phytochemicals for abdominal aortic aneurysm. Curr. Med. Chem. 2021, 28, 8595–8607. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zahedipour, F.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pulmonary fibrosis: Therapeutic and mechanistic insights into the role of phytochemicals. BioFactors 2021, 47, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Boozari, M.; Cicero, A.F.G.; Jamialahmadi, T.; Sahebkar, A. Effects of phytochemicals on macrophage cholesterol efflux capacity: Impact on atherosclerosis. Phytother. Res. 2021, 35, 2854–2878. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Kaya, E.D.; Erğun, B.; Demir, Y.; Zuhal, A.; Beydemir, Ş. The in vitro impacts of some plant extracts on carbonic anhydrase i, ii and paraoxonase-1. Hacet. J. Biol. Chem. 2019, 47, 51–59. [Google Scholar]
- Gouédard, C.; Barouki, R.; Morel, Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2378–2383. [Google Scholar] [CrossRef] [Green Version]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-γ pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Wu, L.-C.; Lai, J.-M.; Chen, C.-T.; Hsueh, C.-M.; Hsu, S.-L. Ethanol extract of Graptopetalum paraguayense upregulates paraoxonase 1 gene expression via an AKT/NF-κB-dependent pathway. Am. J. Chin. Med. 2012, 40, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Schrader, C.; Schiborr, C.; Frank, J.; Rimbach, G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2011, 105, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Garige, M.; Gong, M.; Varatharajalu, R.; Lakshman, M.R. Quercetin up-regulates paraoxonase 1 gene expression via sterol regulatory element binding protein 2 that translocates from the endoplasmic reticulum to the nucleus where it specifically interacts with sterol responsive element–like sequence in paraoxonase 1 promoter in HuH7 liver cells. Metabolism 2010, 59, 1372–1378. [Google Scholar]
- BinMowyna, M.N.; Binobead, M.A.; Al Badr, N.A.; AlSedairy, S.A.; Elredh, I.A.R.; Alqahtani, W.S. Effect of Saudi and Egyptian pomegranate polyphenols in regulating the activity of Pon1, Pon2 and lipid profile for preventing coronary heart disease. J. Regen. Biol. Med. 2019, 1, 1–12. [Google Scholar]
- Atrahimovich, D.; Samson, A.O.; Khattib, A.; Vaya, J.; Khatib, S. Punicalagin decreases serum glucose levels and increases PON1 activity and HDL anti-inflammatory values in Balb/c mice fed a high-fat diet. Oxidative Med. Cell. Longev. 2018, 2673076. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.R.; Abreu, I.C.M.E.d.; Guerra, J.F.d.C.; Lage, N.N.; Lopes, J.M.M.; Silva, M.; Lima, W.G.d.; Silva, M.E.; Pedrosa, M.L. Açai (Euterpe oleracea Mart.) upregulates paraoxonase 1 gene expression and activity with concomitant reduction of hepatic steatosis in high-fat diet-fed rats. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh-Fanalou, S.; Nazarizadeh, A.; Babaei, M.; Khosravi, M.; Farahmandian, N.; Bahreini, E. Effects of Securigera securidaca (L.) Degen & Dorfl seed extract combined with glibenclamide on paraoxonase1 activity, lipid profile and peroxidation, and cardiovascular risk indices in diabetic rats. BioImpacts BI 2020, 10, 159. [Google Scholar] [PubMed]
- Moustafa, E.M.; Thabet, N.M. Beta-sitosterol upregulated paraoxonase-1 via peroxisome proliferator-activated receptor-γ in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 661–666. [Google Scholar] [CrossRef]
- Moradi, A.; Yousefi, H.; Javidmehr, D.; Karimollah, A. Comparing the Effects of Kaempferol, Galangin and Apigenin Flavanoids on Basis of its Structural Differences in Increasing of Paraoxonase 1 Activity and Attenuating Oxidative Stress Markers in Rats. Int. J. Med. Lab. 2016, 3, 241–248. [Google Scholar]
- Takaeidi, M.R.; Jahangiri, A.; Khodayar, M.J.; Siahpoosh, A.; Yaghooti, H.; Rezaei, S.; Salecheh, M.; Mansourzadeh, Z. The effect of date seed (Phoenix dactylifera) extract on paraoxonase and arylesterase activities in hypercholesterolemic rats. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef]
- Ülger, T.G.; Çakıroğlu, F.P. Effect of Allium Cepa on Paraoxonase 1 Activity and Oxidative Stress in Streptozotocin Induced Diabetic Rats. J. Food Nutr. Res. 2018, 6, 689–693. [Google Scholar]
- Sierra-Campos, E.; Valdez-Solana, M.; Avitia-Domínguez, C.; Campos-Almazán, M.; Flores-Molina, I.; García-Arenas, G.; Téllez-Valencia, A. Effects of Moringa oleifera leaf extract on diabetes-induced alterations in paraoxonase 1 and catalase in rats analyzed through progress kinetic and blind docking. Antioxidants 2020, 9, 840. [Google Scholar] [CrossRef]
- Fuhrman, B.; Plat, D.; Herzog, Y.; Aviram, M. Consumption of a novel dietary formula of plant sterol esters of canola oil fatty acids, in a canola oil matrix containing 1, 3-diacylglycerol, reduces oxidative stress in atherosclerotic apolipoprotein E-deficient mice. J. Agric. Food Chem. 2007, 55, 2028–2033. [Google Scholar] [CrossRef]
- Khodarahmi, A.; Javidmehr, D.; Eshaghian, A.; Karimollah, A.; Yousefi, H.; Moradi, A. Curcumin exerts hepatoprotection via overexpression of Paraoxonase-1 and its regulatory genes in rats undergone bile duct ligation. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Altıntoprak, N.; Kar, M.; Acar, M.; Berkoz, M.; Muluk, N.B.; Cingi, C. Antioxidant activities of curcumin in allergic rhinitis. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Protective role of dietary curcumin in the prevention of the oxidative stress induced by chronic alcohol with respect to hepatic injury and antiatherogenic markers. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, H.; Sunay, F.B.; Sinan, S.; Köçkar, F. In vivo effects of curcumin on the paraoxonase, carbonic anhydrase, glucose-6-phosphate dehydrogenase and β-glucosidase enzyme activities in dextran sulphate sodium-induced ulcerative colitis mice. J. Enzym. Inhib. Med. Chem. 2016, 31, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Fatolahi, H.; Azarbayjani, M.A.; Peeri, M.; Matinhomaei, H. The effect of curcumin and exercise rehabilitation on liver paraoxonase-1 and NF-kβ gene expression in the rat induced by forced drinking of ethanol. Clin. Exp. Hepatol. 2020, 6, 49. [Google Scholar] [CrossRef]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; Lima, T.F.O.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndr. 2019, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kıyıcı, A.; Okudan, N.; Gökbel, H.; Belviranlı, M. The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J. Med. Food 2010, 13, 725–728. [Google Scholar] [CrossRef]
- Jaiswal, N.; Rizvi, S.I. Onion extract (Allium cepa L.), quercetin and catechin up-regulate paraoxonase 1 activity with concomitant protection against low-density lipoprotein oxidation in male Wistar rats subjected to oxidative stress. J. Sci. Food Agric. 2014, 94, 2752–2757. [Google Scholar] [CrossRef]
- Noll, C.; Hamelet, J.; Matulewicz, E.; Paul, J.-L.; Delabar, J.-M.; Janel, N. Effects of red wine polyphenolic compounds on paraoxonase-1 and lectin-like oxidized low-density lipoprotein receptor-1 in hyperhomocysteinemic mice. J. Nutr. Biochem. 2009, 20, 586–596. [Google Scholar] [CrossRef]
- Amengual-Cladera, E.; Nadal-Casellas, A.; Gómez-Pérez, Y.; Gomila, I.; Prieto, R.M.; Proenza, A.M.; Lladó, I. Phytotherapy in a rat model of hyperoxaluria: The antioxidant effects of quercetin involve serum paraoxonase 1 activation. Exp. Biol. Med. 2011, 236, 1133–1138. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Eleyan, M.; Khwanes, S.A.; Mohamed, R.A.; Abd El-Rahman, H.A. Quercetin ameliorates the hepatic apoptosis of foetal rats induced by in utero exposure to fenitrothion via the transcriptional regulation of paraoxonase-1 and apoptosis-related genes. Biomarkers 2021, 26, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Niering, J.; Minihane, A.M.; Wiswedel, I.; Gardeman, A.; Wolffram, S.; Rimbach, G. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis 2010, 211, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Leckey, L.C.; Garige, M.; Varatharajalu, R.; Gong, M.; Nagata, T.; Spurney, C.F.; Lakshman, R.M. Quercetin and ethanol attenuate the progression of atherosclerotic plaques with concomitant up regulation of paraoxonase1 (PON1) gene expression and PON1 activity in LDLR−/−mice. Alcohol. Clin. Exp. Res. 2010, 34, 1535–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Garige, M.; Varatharajalu, R.; Marmillot, P.; Gottipatti, C.; Leckey, L.C.; Lakshman, R.M. Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem. Biophys. Res. Commun. 2009, 379, 1001–1004. [Google Scholar] [CrossRef]
- Navarro-García, F.; Ponce-Ruíz, N.; Rojas-García, A.E.; Ávila-Villarreal, G.; Herrera-Moreno, J.F.; Barrón-Vivanco, B.S.; Bernal-Hernández, Y.Y.; González-Arias, C.A.; Medina-Díaz, I.M. The Role of Nutritional Habits and Moderate Red Wine Consumption in PON1 Status in Healthy Population. Appl. Sci. 2021, 11, 9503. [Google Scholar] [CrossRef]
- Zasowska-Nowak, A.; Nowak, P.J.; Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Markowski, J.; Rutkowski, K.P.; Nowak, D. Strawberries added to the usual diet suppress fasting plasma paraoxonase activity and have a weak transient decreasing effect on cholesterol levels in healthy nonobese subjects. J. Am. Coll. Nutr. 2016, 35, 422–435. [Google Scholar] [CrossRef]
- Menini, T.; Heck, C.; Schulze, J.; De Mejia, E.; Gugliucci, A. Protective action of Ilex paraguariensis extract against free radical inactivation of paraoxonase-1 in high-density lipoprotein. Planta Med. 2007, 73, 1141–1147. [Google Scholar] [CrossRef]
- Rahideh, S.T.; Shidfar, F.; Khandozi, N.; Rajab, A.; Hosseini, S.P.; Mirtaher, S.M. The effect of sumac (Rhus coriaria L.) powder on insulin resistance, malondialdehyde, high sensitive C-reactive protein and paraoxonase 1 activity in type 2 diabetic patients. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 933. [Google Scholar]
- Lazavi, F.; Mirmiran, P.; Sohrab, G.; Nikpayam, O.; Angoorani, P.; Hedayati, M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 31, 170–174. [Google Scholar] [CrossRef]
- Tabatabaie, M.; Abdollahi, S.; Salehi-Abargouei, A.; Clark, C.C.; Karimi-Nazari, E.; Fallahzadeh, H.; Rahmanian, M.; Mozaffari-Khosravi, H. The effect of resveratrol supplementation on serum levels of asymmetric de-methyl-arginine and paraoxonase 1 activity in patients with type 2 diabetes: A randomized, double-blind controlled trial. Phytother. Res. 2020, 34, 2023–2031. [Google Scholar] [CrossRef]
- Shidfar, F.; Heydari, I.; Hajimiresmaiel, S.J.; Hosseini, S.; Shidfar, S.; Amiri, F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp (a), and Paraoxonase-1 activity in type 2 diabetic male patients. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 355. [Google Scholar]
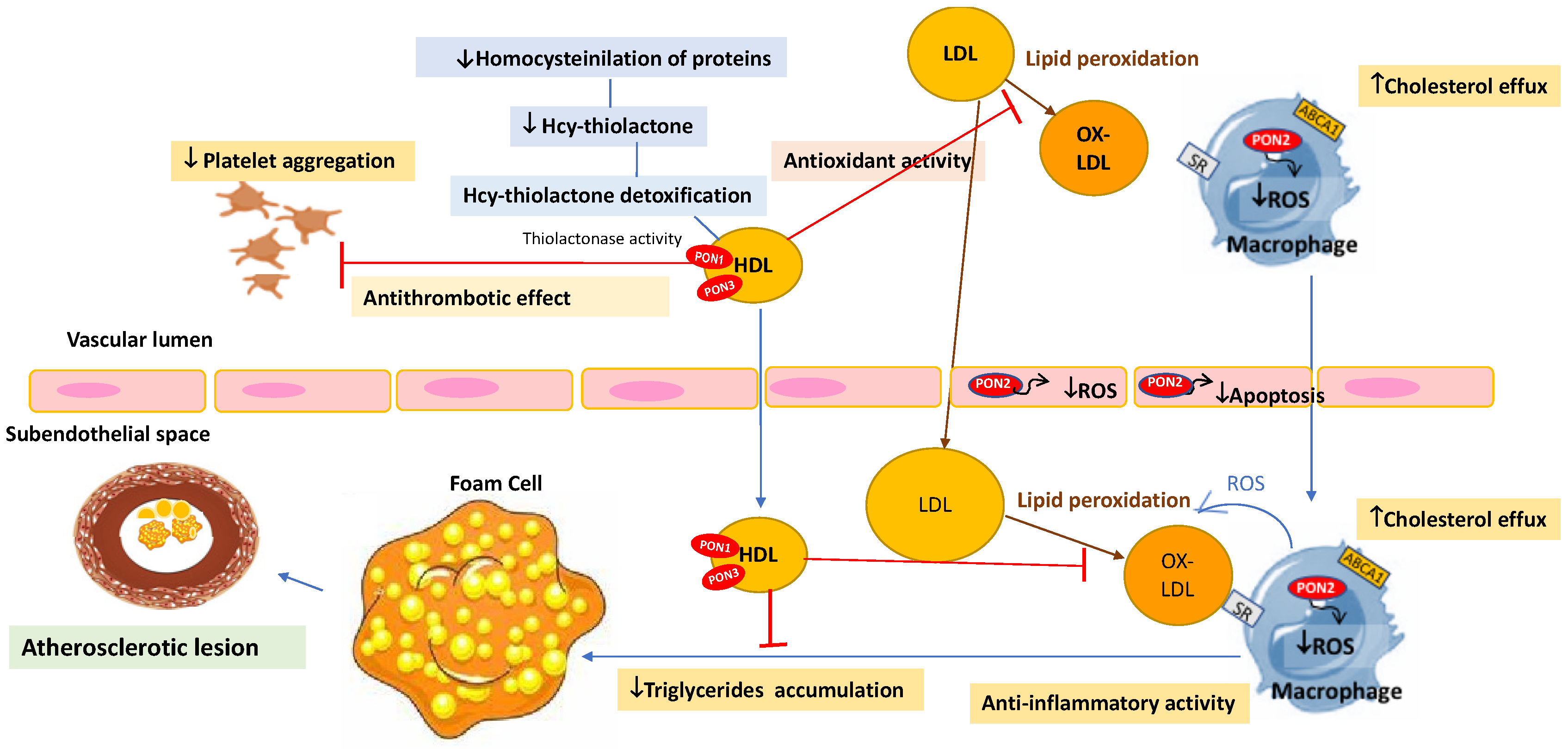
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 2017, 132, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. The paraoxonase gene family and coronary heart disease. Curr. Opin. Lipidol. 2002, 13, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M. A review of paraoxonase 1 properties and diagnostic applications. Pol. J. Vet. Sci. 2016, 19, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Sorenson, R.C.; Bisgaier, C.L.; Aviram, M.; Hsu, C.; Billecke, S.; La Du, B.N. Human serum paraoxonase/arylesterase’s retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: Apolipoprotein AI stabilizes activity. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2214–2225. [Google Scholar] [CrossRef] [Green Version]
- James, R.W.; Deakin, S.P. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic. Biol. Med. 2004, 37, 1986–1994. [Google Scholar] [CrossRef]
- Mazur, A. An enzyme in animal tissues capable of hydrolyzing the phosphorus-fluorine bond of alkyl fluorophosphates. J. Biol. Chem. 1946, 164, 271–289. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Teiber, J.F.; Draganov, D.I.; La Du, B.N. Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem. Pharmacol. 2003, 66, 887–896. [Google Scholar] [CrossRef]
- Tougou, K.; Nakamura, A.; Watanabe, S.; Okuyama, Y.; Morino, A. Paraoxonase has a major role in the hydrolysis of prulifloxacin (NM441), a prodrug of a new antibacterial agent. Drug Metab. Dispos. 1998, 26, 355–359. [Google Scholar]
- Khersonsky, O.; Tawfik, D.S. Structure− reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef] [PubMed]
- Parada-Turska, J.; Wójcicka, G.; Beltowski, J. Paraoxonase 1 Phenotype and Protein N-Homocysteinylation in Patients with Rheumatoid Arthritis: Implications for Cardiovascular Disease. Antioxidants 2020, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Zielaskowska, J. The polymorphism of paraoxonase and its effects in physiological and pathological processes. Adv. Clin. Exp. Med. 2006, 15, 1073–1078. [Google Scholar]
- Grdic Rajkovic, M.; Rumora, L.; Barisic, K. The paraoxonase 1, 2 and 3 in humans. Biochem. Med. 2011, 21, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Socha, E.; Milnerowicz, H. The role of paraoxonase in cardiovascular diseases. Ann. Clin. Lab. Sci. 2015, 45, 226–233. [Google Scholar]
- Camps, J.; García-Heredia, A.; Rull, A.; Alonso-Villaverde, C.; Aragones, G.; Beltrán-Debón, R.; Rodríguez-Gallego, E.; Joven, J. PPARs in regulation of paraoxonases: Control of oxidative stress and inflammation pathways. PPAR Res. 2012, 2012, 616371. [Google Scholar] [CrossRef]
- Park, S.; Mathis, K.; Lee, I. The physiological roles of apolipoprotein J/clusterin in metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014, 15, 45–53. [Google Scholar] [CrossRef]
- Seo, J.A.; Kang, M.-C.; Ciaraldi, T.P.; Kim, S.S.; Park, K.S.; Choe, C.; Hwang, W.M.; Lim, D.M.; Farr, O.; Mantzoros, C. Circulating ApoJ is closely associated with insulin resistance in human subjects. Metabolism 2018, 78, 155–166. [Google Scholar] [CrossRef]
- Draganov, D.I.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef] [Green Version]
- Koren-Gluzer, M.; Aviram, M.; Hayek, T. Paraoxonase1 (PON1) reduces insulin resistance in mice fed a high-fat diet, and promotes GLUT4 overexpression in myocytes, via the IRS-1/Akt pathway. Atherosclerosis 2013, 229, 71–78. [Google Scholar] [CrossRef] [PubMed]
- García-Heredia, A.; Kensicki, E.; Mohney, R.P.; Rull, A.; Triguero, I.; Marsillach, J.; Tormos, C.; Mackness, B.; Mackness, M.; Shih, D.M. Paraoxonase-1 deficiency is associated with severe liver steatosis in mice fed a high-fat high-cholesterol diet: A metabolomic approach. J. Proteome Res. 2013, 12, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Viktorinova, A.; Jurkovicova, I.; Fabryova, L.; Kinova, S.; Koren, M.; Stecova, A.; Svitekova, K. Abnormalities in the relationship of paraoxonase 1 with HDL and apolipoprotein A1 and their possible connection to HDL dysfunctionality in type 2 diabetes. Diabetes Res. Clin. Pr. 2018, 140, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Memon, R.A.; Moser, A.H.; Grunfeld, C. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 1998, 139, 307–315. [Google Scholar] [CrossRef]
- Paikra, B.K. Phytochemistry and pharmacology of Moringa oleifera Lam. J. Pharmacopunct. 2017, 20, 194. [Google Scholar]
- Jin, X.; Wang, K.; Liu, H.; Hu, F.; Zhao, F.; Liu, J. Protection of Bovine Mammary Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Cell Damage by Resveratrol. Oxid. Med. Cell. Longev. 2016, 2016, 2572175. [Google Scholar] [CrossRef]
- Liu, J.; Ma, G.; Wang, Y.; Zhang, Y. Moringa oleifera leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative stress in vitro. Reprod. Domest. Anim. 2020, 55, 711–719. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Pannangpetch, P.; Tangsucharit, P. Moringa oleifera leaf extract induces vasorelaxation via endothelium-dependent hyperpolarization and calcium channel blockade in mesenteric arterial beds isolated from L-NAME hypertensive rats. Clin. Exp. Hypertens. 2020, 42, 490–501. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Alizadeh-Fanalou, S.; Nourian, A.; Yarahmadi, S.; Farahmandian, N.; Nabi-Afjadi, M.; Alipourfard, I.; Bahreini, E. Evaluation of testicular glycogen storage, FGF21 and LDH expression and physiological parameters of sperm in hyperglycemic rats treated with hydroalcoholic extract of Securigera securidaca seeds, and Glibenclamide. Reprod. Biol. Endocrinol. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marefati, N.; Eftekhar, N.; Kaveh, M.; Boskabadi, J.; Beheshti, F.; Boskabady, M. The effect of Allium cepa extract on lung oxidant, antioxidant, and immunological biomarkers in ovalbumin-sensitized rats. Med. Princ. Pract. 2018, 27, 122–128. [Google Scholar] [CrossRef]
- Boaventura, B.C.B.; Di Pietro, P.F.; Stefanuto, A.; Klein, G.A.; de Morais, E.C.; de Andrade, F.; Wazlawik, E.; da Silva, E.L. Association of mate tea (Ilex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutrition 2012, 28, 657–664. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Vitalone, A.; Cole, T.B.; Furlong, C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005, 69, 541–550. [Google Scholar] [CrossRef]
- Gundogdu, M. Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. fruits. Adv. Environ. Biol. 2013, 7, 344–348. [Google Scholar]
- Su, X.; He, Y.; Yang, W.; Wang, Y.; Zhang, W.; Wang, Y. Effect of Dan Hong injection on PON1, SOD activity and MDA levels in elderly patients with coronary heart disease. Int. J. Clin. Exp. Med. 2014, 7, 5886. [Google Scholar]
- Basu, A.; Izuora, K.; Betts, N.M.; Kinney, J.W.; Salazar, A.M.; Ebersole, J.L.; Scofield, R.H. Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial. Nutrients 2021, 13, 1421. [Google Scholar] [CrossRef]
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, M.; Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Vidaković, M. The Influence of Plant Extracts and Phytoconstituents on Antioxidant Enzymes Activity and Gene Expression in the Prevention and Treatment of Impaired Glucose Homeostasis and Diabetes Complications. Antioxidants 2021, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Nowak, M.; de Graft-Johnson, J.; Padula, G.; Bialasiewicz, P.; Markowski, J. Consumption of strawberries on a daily basis increases the non-urate 2, 2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of fasting plasma in healthy subjects. J. Clin. Biochem. Nutr. 2014, 55, 13–93. [Google Scholar] [CrossRef] [Green Version]
- Alsamri, H.; Athamneh, K.; Pintus, G.; Eid, A.H.; Iratni, R. Pharmacological and antioxidant activities of Rhus coriaria L. (Sumac). Antioxidants 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Schaich, K. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef]
- Baliga, M.S.; Baliga, B.R.V.; Kandathil, S.M.; Bhat, H.P.; Vayalil, P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 2011, 44, 1812–1822. [Google Scholar] [CrossRef]
- Amiri, M.; Raeisi-Dehkordi, H.; Sarrafzadegan, N.; Forbes, S.C.; Salehi-Abargouei, A. The effects of Canola oil on cardiovascular risk factors: A systematic review and meta-analysis with dose-response analysis of controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2133–2145. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Tain, Y.-L.; Yu, H.-R.; Huang, L.-T. The effects of resveratrol in the treatment of metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef] [Green Version]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Wong, N.D.; Muntner, P.; Graham, I.M.; Mikhailidis, D.P.; Rizzo, M.; Rysz, J.; Sperling, L.S.; et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors—Results from a systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015, 189, 47–55. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- FarhMortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghanei, M.; Bashiri, S.; Hajihashemi, A.; Sahebkar, A. Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res. 2014, 65, 567–573. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic potential of curcumin in diabetic complications. Pharmacol. Res. 2018, 136, 181–193. [Google Scholar] [CrossRef]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Afshari, A.R.; Jalili-Nik, M.; Abbasinezhad-Moud, F.; Javid, H.; Karimi, M.; Mollazadeh, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. Anti-tumor effects of curcuminoids in glioblastoma multiforme: An updated literature review. Curr. Med. Chem. 2021, 28, 8116–8138. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. The effect of curcumin on the differentiation of mesenchymal stem cells into mesodermal lineage. Molecules 2019, 24, 4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; McClure, D.; Jimenez, L.A.; Megson, I.L.; Rahman, I. Curcumin induces glutathione biosynthesis and inhibits NF-κB activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005, 7, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Botchway, B.O.A.; Liu, X. Curcumin Can Activate the Nrf2/HO-1 Signaling Pathway and Scavenge Free Radicals in Spinal Cord Injury Treatment. Neurorehabilit. Neural Repair 2021, 35, 576–584. [Google Scholar] [CrossRef]
- Lima, T.F.O.; Costa, M.C.; Figueiredo, I.D.; Inácio, M.D.; Rodrigues, M.R.; Assis, R.P.; Baviera, A.M.; Brunetti, I.L. Curcumin, alone or in combination with aminoguanidine, increases antioxidant defenses and glycation product detoxification in streptozotocin-diabetic rats: A therapeutic strategy to mitigate glycoxidative stress. Oxidative Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef]

| Cell Type | Phytochemical Treatment | Intervention | Treatment Duration | Results | Ref. | |
|---|---|---|---|---|---|---|
| Case | Control | |||||
| Human hepatoma cell line (HuH7) | Resveratrol (RSV) | RSV (10 µmol/L) | Ethanol 0.1% | 48 h | ↑ PON1 gene promoter activity through aryl hydrocarbon receptor (AhR) and an unconventional AhR responsive element ↑ PON1 mRNA levels | [17] |
| Human hepatoma cell line (HuH7) | Pomegranate juice (PJ)/ Punicalagin/ Gallic acid/ or Ellagic acid | 0.36 mmol/L | untreated cell | 24 h | ↑ PON1 expression and activity via PPAR-γ/PKA/cAMP pathway ↓ levels of TBARS in LDL and HDL oxidized by copper ions | [18] |
| HepG2 cells | Graptopetalum paraguayense (GP) | GP in water (30, 100, 300 µg/mL) GP in ethanol 50% (30, 100, 300 µg/mL) GP in ethanol 95% (30, 100, 300 µg/mL) | untreated cell | 48 h | ↑ enzymatic activities of secreted PON1 ↑ IKKα/β ↓ IKBα ↑ PON1 mRNA expression via Akt/NF-kB pathway (GP in ethanol 50% with 100 and 300 µg/mL concentration) | [19] |
| Human hepatoma cell line (HuH7) | Curcumin | Curcumin (1, 5, 10, 15, 20 µmol/L) | RSV (25 µmol/L) | 48 h | ↑ PON1 transactivation (10 µmol/L and higher concentration of curcumin) | [20] |
| Human hepatoma cell line (HuH7) | Quercetin | 10, 20 µmol/L | DMSO 0.1% | 48 h | ↑ PON1 level and activity ↑ PON gene transcription via binding SREBP2 to SRE-like sequence in the PON1 promoter | [21] |
| Mouse Strain | Treatment | Disease | Intervention | Number of Animals | Treatment Duration | Results | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | ||||||
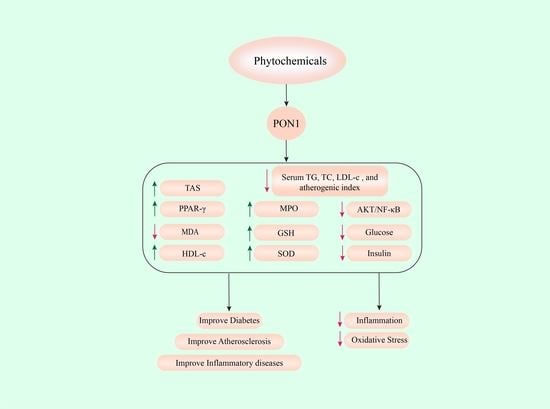
| CD1 Mice | Punica granatum (Pomegranate) | HFD-induced obesity | HFD; HFD + APJ (200 µL, o.g.); HFD + EPJ (200 µL, o.g.); HFD + APJ (200 µL, o.g.) + EPJ (200 µL, o.g.); | Normal diet | n = 5/group | 6 month | ↑ AREactivity ↓ serum TG,TC,LDL-c, and atherogenic index ↑ HDL-c ↑ GSH levels ↓ serum TBARS levels ↑ mouse peritoneal macrophages (MPM)PON2 activity ↓ MPM mediated oxidation of LDL ↓ MPM uptake of ox-LDL | [22] | |
| Balb/c Mice | Quercetin | HFD-induced obesity | HFD + vehicle ((DDW + 2% Tween 80), s.c. implanted minipump); HFD + punicalagin (140 µg/100 µL, s.c. implanted minipump); HFD + quercetin (42 µg/100 µL, s.c. implanted minipump); HFD + atorvastatin (15 mg/100 µL, s.c. implanted minipump); | Normal diet | n = 18/group | 12 weeks | Punicalagin: ↑ PON1 lactonase activity ↑ HDL anti-inflammatory activity evaluated by dichlorofluorescein cell-free assay ↓ glucose levels | [23] | |
| Female Rats Fischer 344 | Euterpe oleracea Mart (Açai) (EO) | Non-alcoholic fatty liver disease (NAFLD) | HFD; EO (2 g/day/single dose, o.g.); HFD + EO (2 g/day/single dose, o.g.); | AIN-93M (control diet) | n = 8/group | 6 weeks | ↑ PON1 and apoA-1 expression in the liver ↑ serum/hepatic PON1 activity ↓ serum ox-LDL levels ↓ hepatic injury markers (ALT) ↓ hepatic TG levels | [24] | |
| Male Wistar Rats | Hydroalcoholic extract of Securigera securidaca seeds (HESS) | STZ-induced Diabetes mellitus (DM) | HESS (100, 200, 400 mg/kg, p.o.); HESS (100, 200, 400 mg/kg, p.o.) + Glibenclamide (Gb) (5 mg/kg, p.o.); Gb (5 mg/kg, p.o.). | No treatment | n = 6/group | 5 weeks | 200 and 400 mg/kg HESS alone and in combination with GB: ↓ cardiovascular risk lipid indices ↑ serum PON1 activity ↓ serum MDA levels | [25] | |
| Male Wistar Rats | Beta-sitosterol (BS) | Gamma irradiation-induced oxidative stress | BS (40 mg/kg/day, oral); Irradiation + Saline (0.5 mL/day, oral) Irradiation + BS (40 mg/kg/day, oral); | Saline (0.5 mL/day, oral) | n = 6/group | 10 days | ↑ serum and hepatic PON1 activity ↓ TG, TC, and LDL-c ↑ HDL-c ↓ MDA ↑ PPAR-γ gene expression ↑ SOD, CAT activity | [26] | |
| Female B6C3F1 Mice | Curcumin | - | Normal diet + Curcumin (500 mg/kg, p.o.) | Normal diet | n = 8/group | 2 weeks | no effect | [20] | |
| Male Wistar Rats | Kaempferol/Galangin/Apigenin | - | Kaempferol (10 mg/kg/day, p.o.); Kaempferol (20 mg/kg/day, p.o.); Galangin (10 mg/kg/day, p.o.); Galangin (20 mg/kg/day, p.o.); Apigenin (10 mg/kg/day, p.o.); Apigenin (20 mg/kg/day, p.o.); Vehicle (Ethanol 10%, p.o.) | Normal diet | n = 5/group | 2 months | ↑ serum PON1 activity ↓ MDA production Kaempferol > Galangin > Apigenin | [27] | |
| Male NMRI Rats | Methanolic date seed extract (DSE) | Hypercholesterolemia (HC) | HC + DSE (250 mg/kg/day, p.o.); HC + DSE (500 mg/kg/day, p.o.); HC + DSE (1000 mg/kg/day, p.o.); HC; DSE (1000 mg/kg/day, p.o.); HC + atorvastatin (10/ mg/day, p.o.); Atorvastatin (10/ mg/day, p.o.); | Normal diet | not mentioned | 4 weeks | ↑ serum PON1 and ARE activities | [28] | |
| Male Sprague-Dawley (SD) Rats | Resveratrol(RSV) | STZ-induced DM | RSV (0.1 and 1 µg/mL/day, intravitreal); | Vehicle (PBS, 5 µL, intravitreal); | n = 5/group | 24 h post-injection | ↑ PON1 mRNA levels in retina ↑ VEGF and bFGF mRNA levels in retina ↓ blood LDL and ↑ HDL (1 µg/mL/day) | [29] | |
| RSV (5, 10,50 µg/kg/day, TVI) | Vehicle (PBS, equal volume, TVI); | 12 weeks | RSV 10 μg/kg and 50 μg/kg/day: ↑ plasma PON1 activity ↓ blod glucose and insulin levels ↑ retinal PON1 mRNA levels ↓ retinal AGEs levels and apotosis ↓ retinal caspase3 activation ↓ plasma ox-LDL ↓ retinal inflammatory factors (IL-1β, IL-6, TNF-α, VEGF, IFN-γ and MCP-1) | ||||||
| Male Wistar Rats | Allium cepa | STZ-induced DM | DM + Normal diet; DM + diet including 5% onion powder (dried at −76 °C in lyophilizator); DM + diet including 5% onion powder (dried at +80 °C in furnace); | Normal diet | n = 8/group | 8 weeks | Lyophilized onion powder: ↑ PON1 activity ↑ Total Antioxidant Capacity ↓ Total Oxidant Status | [30] | |
| Male Wistar Rats | Moringa oleifera leaves extract(MOLE) | Alloxan-induced DM | DM; DM + MOLE (200 mg/kg/day, o.g.); | Distilled water | n = 5/group | 3 weeks | ↑ serum PON1 lactonase activity ↑ serum CAT activity | [31] | |
| Apo E −/− Mice | Canola oil | Atherosclerosis | PS-CO, soybean sterols esterified to fatty acids from Canola oil (2.5 mg/day, oral); CO, Canola oil; | PBS | n = 5/group | 10 weeks | PS-CO treatment: No effect on PON1 activity ↓ TC and TG ↑ TAS ↓ Ox-LDL retention from MPM | [32] | |
| Male Wistar Rats | Curcumin | Chronic liver disease induced by bile duct ligation (BDL) | BDL + Curcumin (100 mg/kg/day, o.g.); Curcumin (100 mg/kg/day, o.g.); BDL (vehicle (CMC), o.g.); | No treatment | n = 8/group | 4 weeks | ↑ PON1 activity ↑ PON1 expression ↑ expression of Sp-1, JNK, AhR, SREBP2, PKC-α | [33] | |
| Female Wistar Rats | Curcumin | Allergic rhinitis (AR) | AR + azelastine HCl (intranasal, from day 21 to 28, twice a day); AR + Curcumin (200 mg/mL, 20 µL/nostril, intranasal, from day 21 to 28, twice a day) | No treatment | n = 8–10/group | 4 weeks | ↑ serum PON1 activity ↑ serum SOD activity ↑ tissue GSH level, ↑ Serum GSH-Px activity ↓ Tissue MDA levels | [34] | |
| Female Wistar-Furth rats | Curcumin | Ethanol-induced hepatosteatosis | LFO + ethanol (35% of dietary calories derived from ethanol); HFO + ethanol (35% of dietary calories derived from ethanol); HFO + ethanol (35% of dietary calories derived from ethanol) + Curcumin (150 mg/kg/day, oral); LFO + ethanol (35% of dietary calories derived from ethanol) + Curcumin (150 mg/kg/day, oral); | LFO; HFO; | n = 4/group | 8 weeks | ↑ PON1 mRNA ↑ serum PON1 activity ↑ PON1 homocysteine thiolactonase activity | [35] | |
| Female Balb/c Mice | Curcumin | DSS-induced ulcerative colitis | Ulcerative colitis (5% DSS + water, o.g.); Ulcerative colitis + Sulfasalazine (dissolved in olive oil, o.g.); Ulcerative colitis + Curcumin (dissolved in olive oil, o.g.); | (Water + olive oil, o.g.) | n = 7/group | 1 week | ↑ serum PON1 activity ↑ MPO activity ↓ Weight loss ↑ Colon lengths | [36] | |
| Male Wistar Rats | Curcumin | Ethanol-induced hepatotoxicity | Ethanol-curcumin (50 mg/kg, i.p., 5 times per week); Ethanol- swimming training (5 times per week); Ethanol- swimming training (5 times per week) + curcumin (50 mg/kg, i.p., 5 times per week); | Dextrose-control; Ethanol-control; Ethanol-saline; Ethanol- DMSO; | n = 8/group | 2 weeks | ↑ PON1 gene expression ↓ NF-κB gene expression | [37] | |
| Male Wistar Rats | Curcumin | STZ-induced DM | DM treated with yoghurt; DM treated with yoghurt + Curcumin (90 mg/kg/day); DM treated with yoghurt + Metformin (250 mg/kg/day); DM treated with 4 U/day insulin | Normal rat treated with yoghurt | n = 10/group | 1 month | ↑ plasma PON1 activity ↓ Plasma levels of glucose ↓ TG, TC, TBARS ↓ fluorescent advanced glycation end products (AGEs) | [38] | |
| Male SD Rats | Grape Seed Extract (GSE) | STZ-induced DM | GSE (100 mg/kg/day); DM; DM + GSE (100 mg/kg/day); | No treatment | n = 6–10/group | 6 weeks | ↑ serum PON1 activity | [39] | |
| Male SD Rats | Allium cepa Quercetin Catechin | HgCl2-induced oxidative stress | Onion extract (10 mL/kg/day, o.g.); Quercetin (20 mg/kg/day, o.g.); Catechin (20 mg/kg/day, o.g.); HgCl2 (5 mg/kg, i.p.); Onion extract (10 mL/kg/day, o.g., 10 days before HgCl2 (5 mg/kg, i.p.)); Catechin (20 mg/kg/day, o.g., 10 days before HgCl2 (5 mg/kg, i.p.)); Quercetin (20 mg/kg/day, o.g., 10 days before HgCl2 (5 mg/kg, i.p.)); | No treatment | n = 6/group | 4 weeks | ↑ PON1 activity ↑ plasma radical scavenging activity ↓ plasma ox-LDL ↓ plasma MDA | [40] | |
| CBS +/− Mice | Red wine polyphenolic extract | Hyperhomocysteinemia | Methionine diet; Methionine diet + Low polyphenolic extract (LPE) (which contains 25 μg of catechin, and 12 mg of polyphenols); Methionine diet + High polyphenolic extract (HPE) (which contains 100 μg of catechin | No treatment | n = 6/group | 4 weeks | ↑ Plasma and hepatic PON1 activity ↑ PON1 expression ↑ CBS activity ↓ plasma MDA ↓ Plasma homocysteine ↓ ox-LDL | [41] | |
| Male Wistar Rats | Quercetin Catechin Epicatechin | Ethylene glycol-induced renal failure (EG) | EG (water, oral); EG + Quercetin (100 mg/L, 5.5 mg/kg, oral); EG + Catechin (100 mg/L, 5.5 mg/kg, oral); EG + Epicatechin (100 mg/L, 5.5 mg/kg, oral); EG + a folk herbal extract Fagolitos (7 ml/L, 0.4 ml/kg, oral); | No treatment | n = 9/group | 16 days | ↑ PON1 and ARE activities↑ Citrate synthase and SOD activity ↑ PON1/apoA-1 ratio ↓ oxidative damages (SOD and Citrate synthase activities were not modified by EG treatment but SOD activity was increased by Catechin, and Citrate synthase activity was increased by Quercetin, Catechin and folk herbal extract Fagolitos) | [42] | |
| Pregnant Rats | Quercetin | Organophosphorus-induced hepatic apoptosis | Quercetin (100 mg/kg, o.g.); Fenitrothion (4.62 mg/kg, o.g.); Quercetin (100 mg/kg, o.g., 2 h before taking Fenitrothion) + Fenitrothion (4.62 mg/kg, o.g.); | Distilled water | n = 10/group | 2 weeks | ↑ PON1 hepatic gene expression ↑ TAS ↑ SOD and CAT activity ↑ GSH level | [43] | |
| Apo E −/− Mice | Quercetin | Atherosclerosis | Quercetin-enriched diets (2 mg per g diet) | Normal diet | n = 8/group | 6 weeks | ↑ PON1 expression and activity via PPAR-γ pathway | [44] | |
| LDLR −/− Mice | Quercetin | Atherosclerosis | Atherogenic diet + 18% ethanol calories; Atherogenic diet + 25% ethanol calories; Atherogenic diet + quercetin 12.5 mg/dL; Atherogenic diet + quercetin 18.75 mg/dL; Atherogenic diet + quercetin 25 mg/dL; | Atherogenic diet | n = 6/group | 8 weeks | ↑ PON1 hepatic gene expression ↑ PON1 activity ↓ decreases in aortic lesions | [45] | |
| Male Wistar Rats | Quercetin | LDL oxidation | Quercetin-enriched liquid diets (10 mg/L); | Normal diet | n = 6/group | 4 weeks | ↑ PON1 hepatic gene expression ↑ PON1 activity ↑ serum PON1 homocysteine thiolactonase ↓ LDL oxidation | [46] | |
| Study Design | Phytochemical Type | Disease | Intervention | Number of Patients | Treatment Duration | Results | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | ||||||
| Clinical randomized trial | Red wine (tannin, gallotannin) | - | Red wine (120 mL/day, contain alcohol 12.5 %) | - | 45 | - | 6 weeks | ↑ PON1 and ARE activities | [47] |
| Clinical randomized trial | Fragaria ananassa (Strawberry) | - | Usual diet + strawberry (500 g/day) | Usual diet | 31 | 20 | 1 month | ↓ PON1 activity = TC, TG, LDL levels | [48] |
| Clinical randomized trial | Ilex paraguariensis(IP) | - | 500 mL IP | 500 mL milk, coffee, or nothing | 2 | 2 | 1 day | ↑ PON1 activity | [49] |
| Double-blind, randomized placebo-controlled | Rhus coriaria (Sumac) | Type 2 diabetes (T2D) | Sumac (3 g/day, oral) | Placebo (3 g/day, oral) | 22 | 19 | 3 months | ↑ PON1 activity ↓ MDA ↓ hs-CRP ↓ Insulin | [50] |
| Clinical randomized trial | Berberis vulgaris (Barberry) | T2D | Berberis vulgaris juice (200 mL/day) | No treatment | 23 | 23 | 8 weeks | ↓ Systolic blood pressure ↑ PON1 activity ↓ Fasting blood glucose ↓ TG | [51] |
| Double-blind, randomized placebo-controlled | Resveratrol | T2D | Resveratrol capsule (1000 mg/day) | Placebo capsule (methylcellulose, 1000 mg/day) | 35 | 36 | 8 weeks | ↑ PON1 activity ↓ Asymmetric de-methyl-arginine | [52] |
| Double-blind, randomized placebo-controlled | Vaccinium macrocarpon (Cranberry) | T2D | Cranberry juice (240 mL/day) | Natural mineral water with strawberry flavor (240 mL/day) | 29 | 29 | 12 weeks | ↑ PON1 activity ↑ PON1 activity ↑ apoA-1 levels ↓ apoB levels ↓ glucose levels | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arab, Z.N.; Khayatan, D.; Razavi, S.M.; Zare, K.; Kheradkhah, E.; Momtaz, S.; Ferretti, G.; Bacchetti, T.; Sathyapalan, T.; Emami, S.A.; et al. Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases. Antioxidants 2022, 11, 1273. https://doi.org/10.3390/antiox11071273
Arab ZN, Khayatan D, Razavi SM, Zare K, Kheradkhah E, Momtaz S, Ferretti G, Bacchetti T, Sathyapalan T, Emami SA, et al. Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases. Antioxidants. 2022; 11(7):1273. https://doi.org/10.3390/antiox11071273
Chicago/Turabian StyleArab, Zahra Najafi, Danial Khayatan, Seyed Mehrad Razavi, Kimia Zare, Elnaz Kheradkhah, Saeideh Momtaz, Gianna Ferretti, Tiziana Bacchetti, Thozhukat Sathyapalan, Seyed Ahmad Emami, and et al. 2022. "Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases" Antioxidants 11, no. 7: 1273. https://doi.org/10.3390/antiox11071273