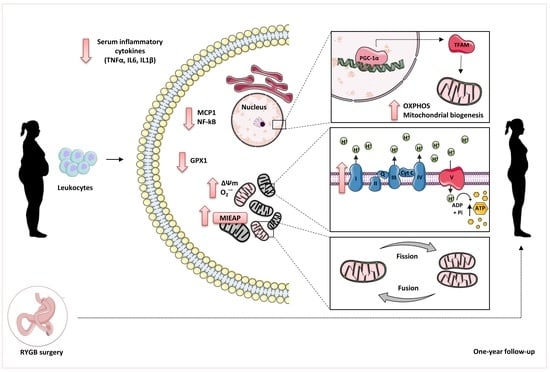
Impact of Roux-en-Y Gastric Bypass on Mitochondrial Biogenesis and Dynamics in Leukocytes of Obese Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection, Anthropometric and Biochemical Determinations
2.3. Isolation of Leukocytes
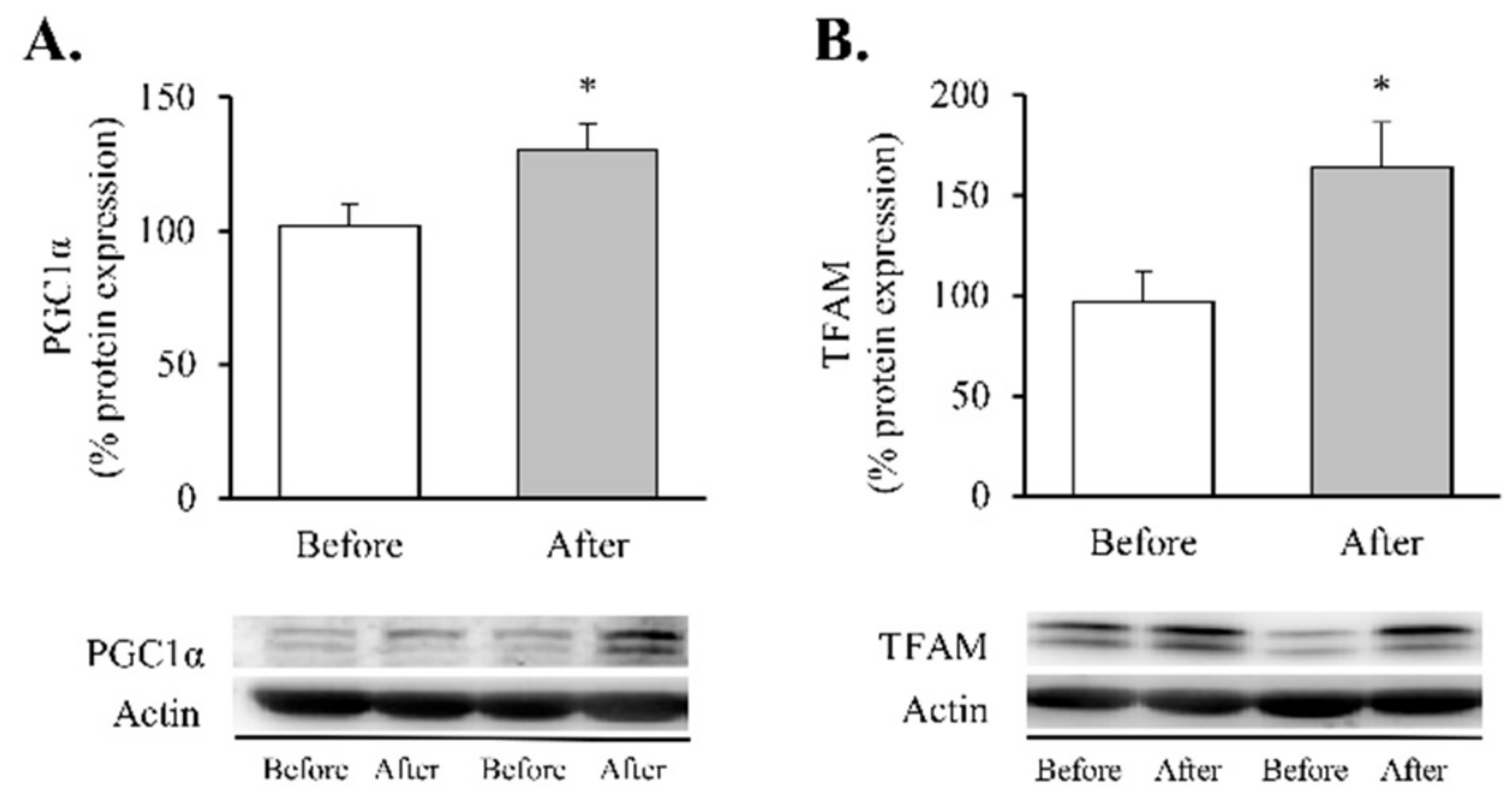
2.4. Protein Expression Analysis
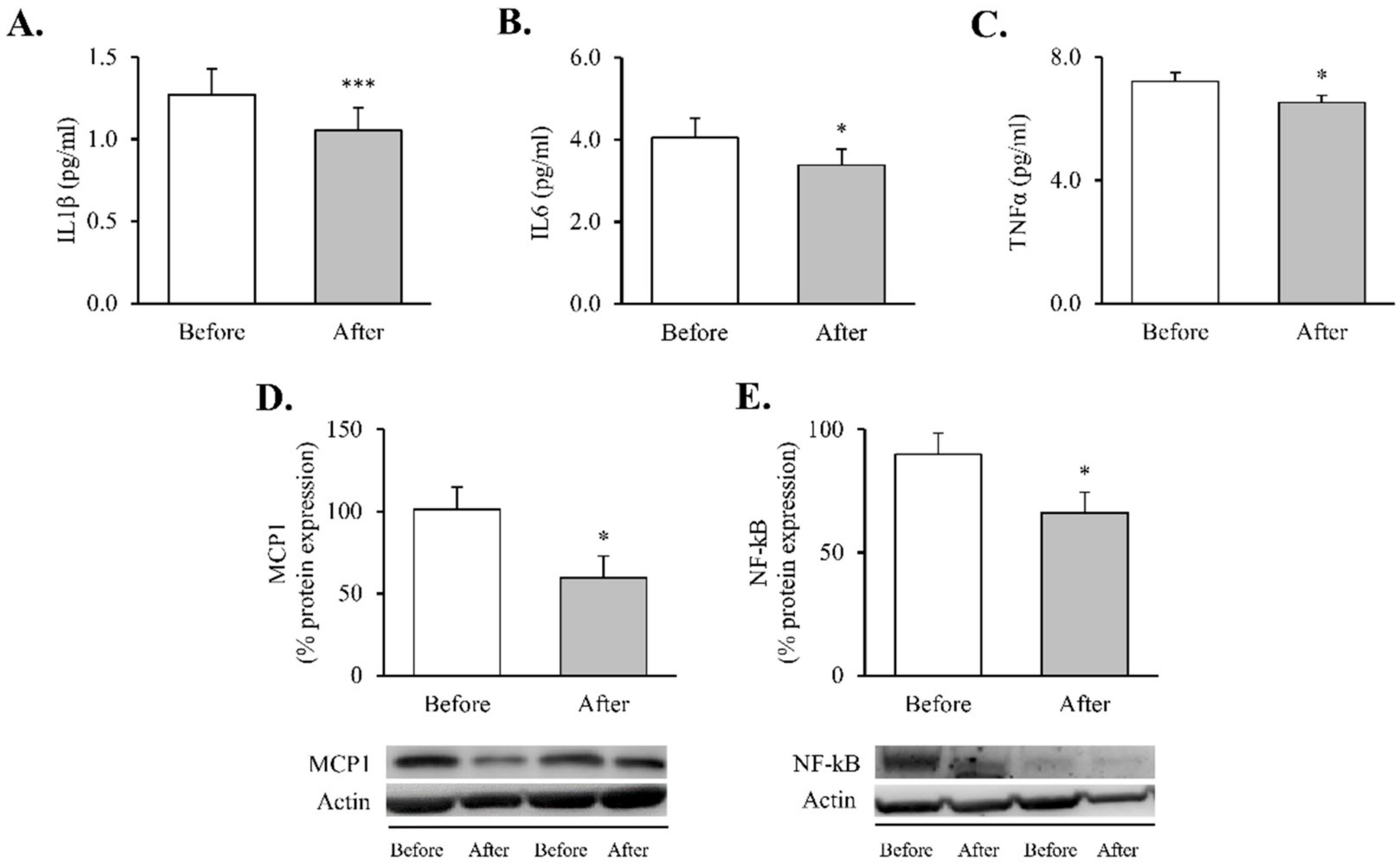
2.5. Evaluation of Systemic Cytokines TNFɑ, IL6 and IL1β
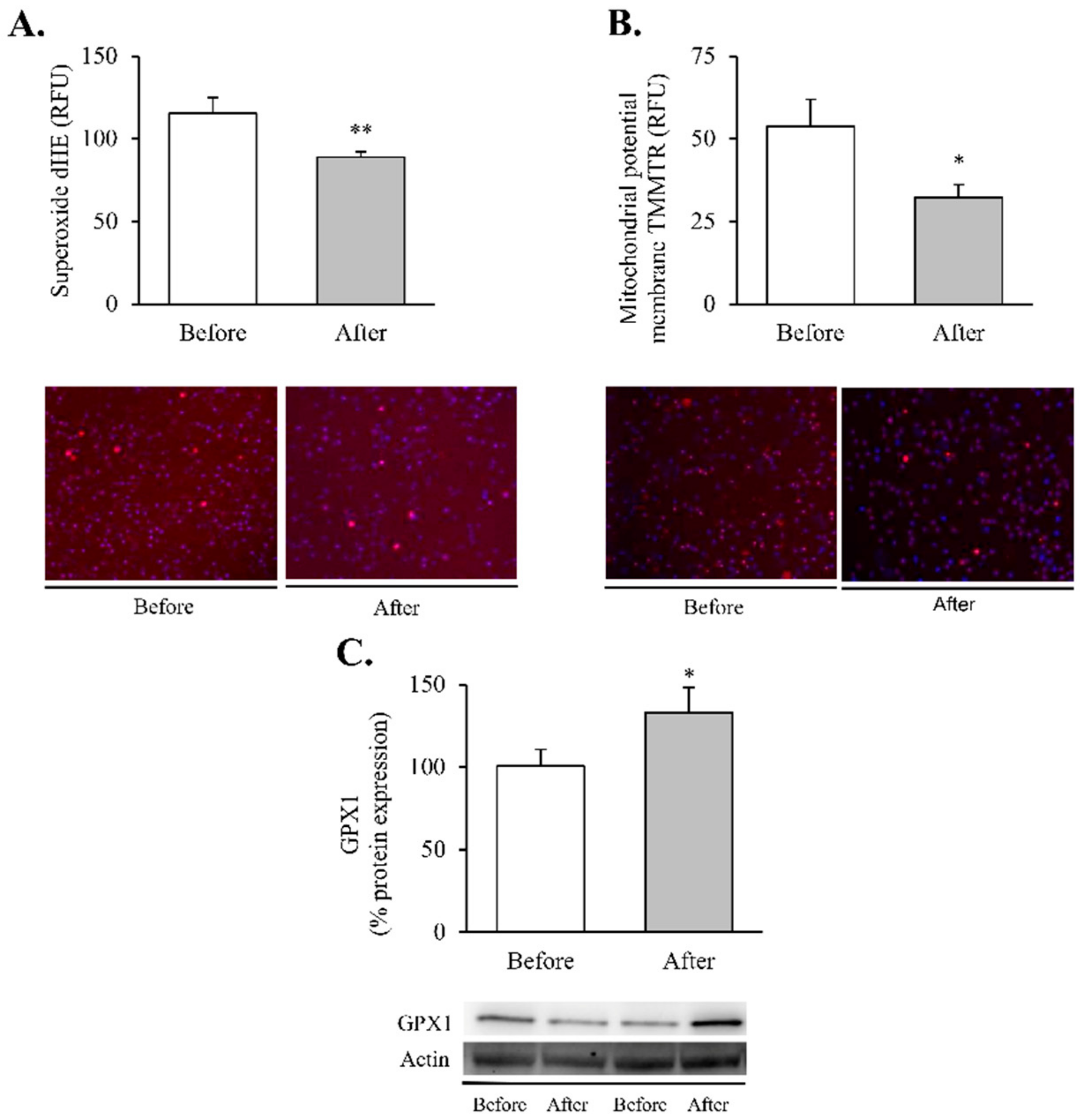
2.6. Superoxide Production and Mitochondrial Membrane Potential
2.7. Statistical Analysis
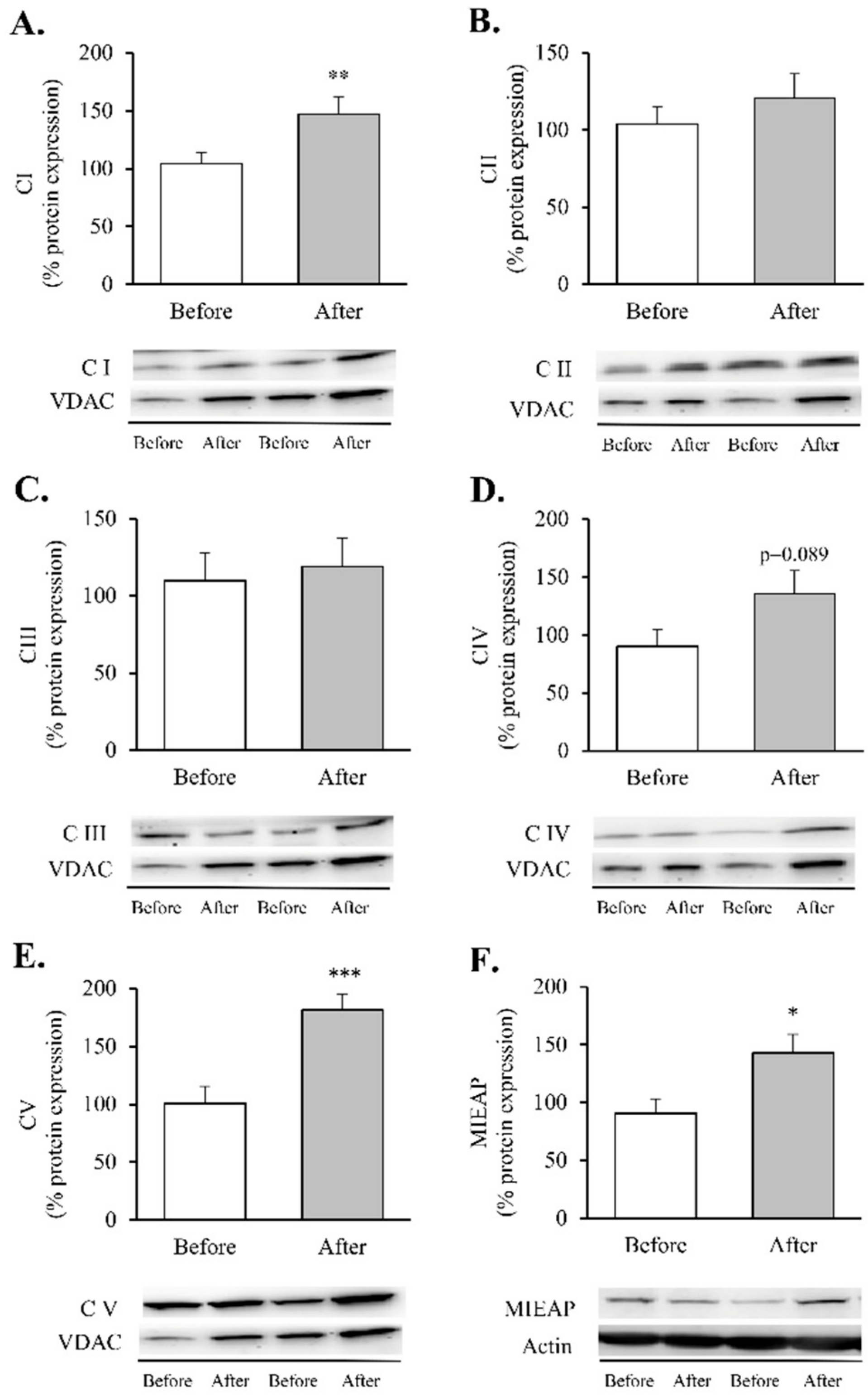
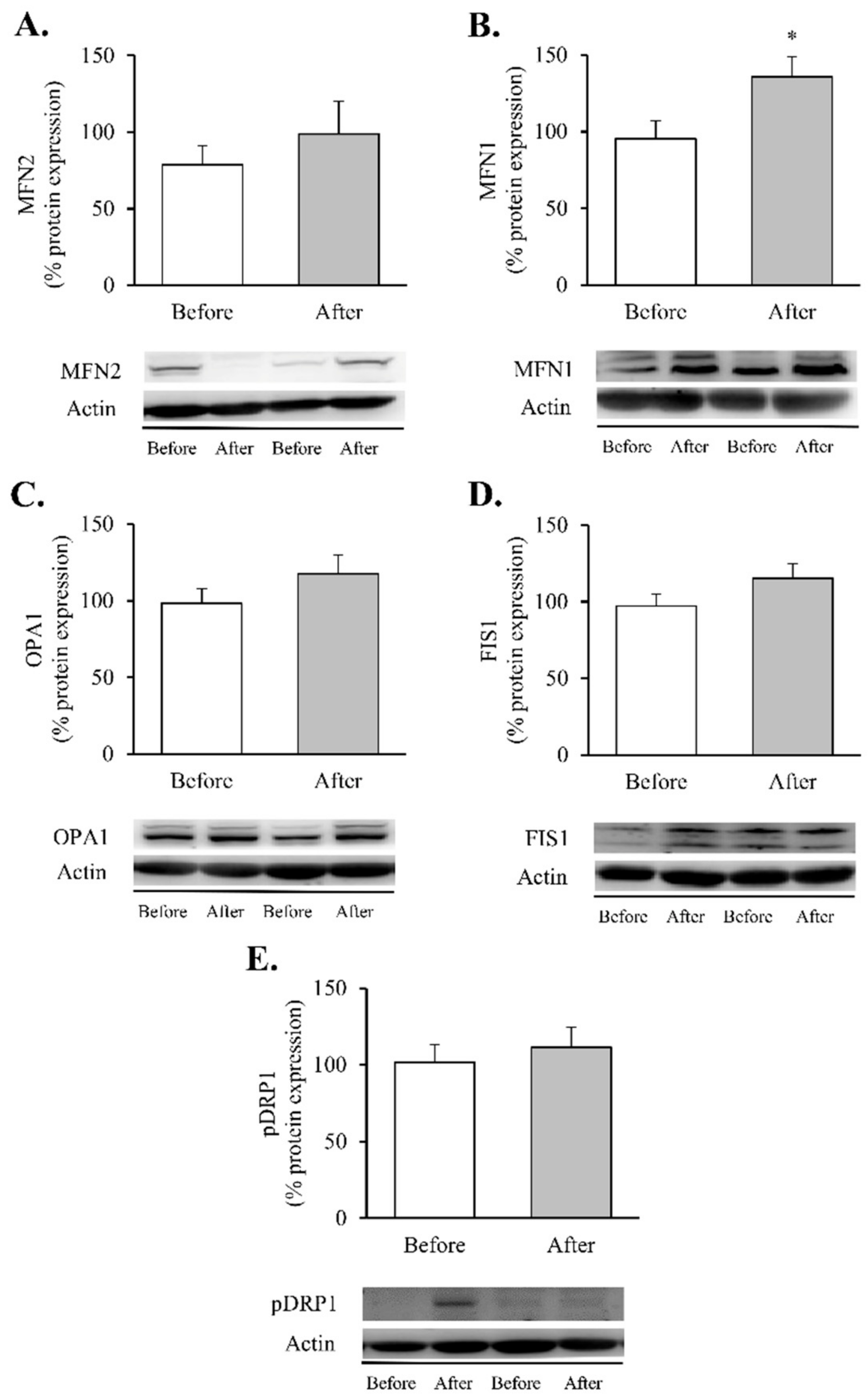
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; McFarlane, S.I.; Jean-Louis, G.; Myers, A.K. Energy imbalance: Obesity, associated comorbidities, prevention, management and public health implications. Adv. Obes. Weight. Manag. Control. 2020, 10, 146. [Google Scholar] [CrossRef]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 2020, 75, 235–251. [Google Scholar] [CrossRef]
- May, M.; Schindler, C.; Engeli, S. Modern pharmacological treatment of obese patients. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018819897527. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Heneghan, H.M. Bariatric Surgery for Obesity. Med. Clin. N. Am. 2018, 102, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Rega-Kaun, G.; Kaun, C.; Jaegersberger, G.; Prager, M.; Hackl, M.; Demyanets, S.; Wojta, J.; Hohensinner, P.J. Roux-en-Y-Bariatric Surgery Reduces Markers of Metabolic Syndrome in Morbidly Obese Patients. Obes. Surg. 2020, 30, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad-Jimenez, Z.; Lopez-Domenech, S.; Gomez-Abril, S.A.; Perianez-Gomez, D.; De Maranon, A.M.; Banuls, C.; Morillas, C.; Victor, V.M.; Rocha, M. Effect of Roux-en-Y Bariatric Bypass Surgery on Subclinical Atherosclerosis and Oxidative Stress Markers in Leukocytes of Obese Patients: A One-Year Follow-Up Study. Antioxidants 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.; Hachey, K.; Pernar, L.I. Gender disparities in weight loss surgery. Mini Invasive Surg. 2020, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.L.; Juvanhol, L.L.; Licursi de Oliveira, L.; Clemente, R.C.; Bressan, J. Changes in oxidative stress markers and cardiometabolic risk factors among Roux-en-Y gastric bypass patients after 3- and 12-months postsurgery follow-up. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 1738–1745. [Google Scholar] [CrossRef]
- Vafai, S.B.; Mootha, V.K. Mitochondrial disorders as windows into an ancient organelle. Nature 2012, 491, 374–383. [Google Scholar] [CrossRef]
- Gao, A.W.; Canto, C.; Houtkooper, R.H. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol. Med. 2014, 6, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Bach, D.; Naon, D.; Pich, S.; Soriano, F.X.; Vega, N.; Rieusset, J.; Laville, M.; Guillet, C.; Boirie, Y.; Wallberg-Henriksson, H.; et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: Effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 2005, 54, 2685–2693. [Google Scholar] [CrossRef] [Green Version]
- Montaigne, D.; Marechal, X.; Coisne, A.; Debry, N.; Modine, T.; Fayad, G.; Potelle, C.; El Arid, J.M.; Mouton, S.; Sebti, Y.; et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 2014, 130, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Bakkman, L.; Fernstrom, M.; Loogna, P.; Rooyackers, O.; Brandt, L.; Lagerros, Y.T. Reduced respiratory capacity in muscle mitochondria of obese subjects. Obes. Facts 2010, 3, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374.e1376–1389.e1376. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. TEM 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011, 13, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Molina, A.J.; Wikstrom, J.D.; Stiles, L.; Las, G.; Mohamed, H.; Elorza, A.; Walzer, G.; Twig, G.; Katz, S.; Corkey, B.E.; et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 2009, 58, 2303–2315. [Google Scholar] [CrossRef] [Green Version]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.H.; Park, J.Y.; Park, H.S.; Jeon, M.J.; Ryu, J.W.; Kim, M.; Kim, S.Y.; Kim, M.S.; Kim, S.W.; Park, I.S.; et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 2007, 56, 2973–2981. [Google Scholar] [CrossRef] [Green Version]
- Heilbronn, L.K.; Gan, S.K.; Turner, N.; Campbell, L.V.; Chisholm, D.J. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J. Clin. Endocrinol. Metab. 2007, 92, 1467–1473. [Google Scholar] [CrossRef] [Green Version]
- Sacks, J.; Mulya, A.; Fealy, C.E.; Huang, H.; Mosinski, J.D.; Pagadala, M.R.; Shimizu, H.; Batayyah, E.; Schauer, P.R.; Brethauer, S.A.; et al. Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol. Rep. 2018, 6, e13600. [Google Scholar] [CrossRef]
- Ferraz-Bannitz, R.; Welendorf, C.R.; Coelho, P.O.; Salgado, W., Jr.; Nonino, C.B.; Beraldo, R.A.; Foss-Freitas, M.C. Bariatric surgery can acutely modulate ER-stress and inflammation on subcutaneous adipose tissue in non-diabetic patients with obesity. Diabetol. Metab. Syndr. 2021, 13, 19. [Google Scholar] [CrossRef]
- Gastaldi, G.; Russell, A.; Golay, A.; Giacobino, J.P.; Habicht, F.; Barthassat, V.; Muzzin, P.; Bobbioni-Harsch, E. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure. Diabetologia 2007, 50, 2348–2355. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Zamora-Mendoza, R.; Rosas-Vargas, H.; Ramos-Cervantes, M.T.; Garcia-Zuniga, P.; Perez-Lorenzana, H.; Mendoza-Lorenzo, P.; Perez-Ortiz, A.C.; Estrada-Mena, F.J.; Miliar-Garcia, A.; Lara-Padilla, E.; et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int. J. Obes. 2018, 42, 618–624. [Google Scholar] [CrossRef]
- Kugler, B.A.; Gundersen, A.E.; Li, J.; Deng, W.; Eugene, N.; Gona, P.N.; Houmard, J.A.; Zou, K. Roux-en-Y gastric bypass surgery restores insulin-mediated glucose partitioning and mitochondrial dynamics in primary myotubes from severely obese humans. Int. J. Obes. 2020, 44, 684–696. [Google Scholar] [CrossRef]
- Nijhawan, S.; Richards, W.; O’Hea, M.F.; Audia, J.P.; Alvarez, D.F. Bariatric surgery rapidly improves mitochondrial respiration in morbidly obese patients. Surg. Endosc. 2013, 27, 4569–4573. [Google Scholar] [CrossRef]
- Abad-Jiménez, Z.; López-Domènech, S.; García-Gargallo, C.; Vezza, T.; Gómez-Abril, S.Á.; Morillas, C.; Díaz-Pozo, P.; Falcón, R.; Bañuls, C.; Víctor, V.M. Roux-en-Y Gastric Bypass Modulates AMPK, Autophagy and Inflammatory Response in Leukocytes of Obese Patients. Biomedicines 2022, 10, 430. [Google Scholar] [CrossRef]
- Asghar, A.; Sheikh, N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell. Immunol. 2017, 315, 18–26. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial–A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Douros, J.D.; Tong, J.; D’Alessio, D.A. The Effects of Bariatric Surgery on Islet Function, Insulin Secretion, and Glucose Control. Endocr. Rev. 2019, 40, 1394–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Calderon, J.R.; Castillo, E.C.; Cuellar-Tamez, R.X.; Garcia-Garza, M.; Elizondo-Montemayor, L.; Garcia-Rivas, G. Reduced Th1 response is associated with lower glycolytic activity in activated peripheral blood mononuclear cells after metabolic and bariatric surgery. J. Endocrinol. Investig. 2021, 44, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Domenech, S.; Banuls, C.; Diaz-Morales, N.; Escribano-Lopez, I.; Morillas, C.; Veses, S.; Orden, S.; Alvarez, A.; Victor, V.M.; Hernandez-Mijares, A.; et al. Obesity impairs leukocyte-endothelium cell interactions and oxidative stress in humans. Eur. J. Clin. Investig. 2018, 48, e12985. [Google Scholar] [CrossRef] [PubMed]
- Monzo-Beltran, L.; Vazquez-Tarragon, A.; Cerda, C.; Garcia-Perez, P.; Iradi, A.; Sanchez, C.; Climent, B.; Tormos, C.; Vazquez-Prado, A.; Girbes, J.; et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017, 12, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell. Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Ojuka, E.O.; Nolte, L.A.; Holloszy, J.O. Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J. Appl. Physiol. 2000, 88, 1072–1075. [Google Scholar] [CrossRef]
- Winder, W.W.; Holmes, B.F.; Rubink, D.S.; Jensen, E.B.; Chen, M.; Holloszy, J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 2000, 88, 2219–2226. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.C.; Barnes, B.R.; Mahlapuu, M.; Steiler, T.L.; Martinsson, S.; Leng, Y.; Wallberg-Henriksson, H.; Andersson, L.; Zierath, J.R. Role of AMP-activated protein kinase in the coordinated expression of genes controlling glucose and lipid metabolism in mouse white skeletal muscle. Diabetologia 2005, 48, 2354–2364. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Roves, P.M.; Osler, M.E.; Holmstrom, M.H.; Zierath, J.R. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J. Biol. Chem. 2008, 283, 35724–35734. [Google Scholar] [CrossRef] [Green Version]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15983–15987. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, R.; Ren, J.M.; Cadman, K.S.; Moore, I.K.; Perret, P.; Pypaert, M.; Young, L.H.; Semenkovich, C.F.; Shulman, G.I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1340–E1346. [Google Scholar] [CrossRef]
- Rojas, J.; Arraiz, N.; Aguirre, M.; Velasco, M.; Bermudez, V. AMPK as Target for Intervention in Childhood and Adolescent Obesity. J. Obes. 2011, 2011, 252817. [Google Scholar] [CrossRef] [Green Version]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Semple, R.K.; Crowley, V.C.; Sewter, C.P.; Laudes, M.; Christodoulides, C.; Considine, R.V.; Vidal-Puig, A.; O’Rahilly, S. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.H.; Kim, Y.W.; Seo, D.Y.; Sohn, T.S. Mitochondrial TFAM as a Signaling Regulator between Cellular Organelles: A Perspective on Metabolic Diseases. Diabetes Metab. J. 2021, 45, 853–865. [Google Scholar] [CrossRef]
- Schottl, T.; Kappler, L.; Fromme, T.; Klingenspor, M. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol. Metab. 2015, 4, 631–642. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef] [Green Version]
- Antoun, G.; McMurray, F.; Thrush, A.B.; Patten, D.A.; Peixoto, A.C.; Slack, R.S.; McPherson, R.; Dent, R.; Harper, M.E. Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 2015, 58, 2861–2866. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, N.; Nakamura, Y.; Miyamoto, Y.; Miyamoto, T.; Kabu, K.; Yoshida, M.; Futamura, M.; Ichinose, S.; Arakawa, H. Mieap, a p53-inducible protein, controls mitochondrial quality by repairing or eliminating unhealthy mitochondria. PLoS ONE 2011, 6, e16060. [Google Scholar] [CrossRef]
- Ghosh, S.; Dent, R.; Harper, M.E.; Stuart, J.; McPherson, R. Blood gene expression reveal pathway differences between diet-sensitive and resistant obese subjects prior to caloric restriction. Obesity 2011, 19, 457–463. [Google Scholar] [CrossRef]
- Hansen, M.; Lund, M.T.; Gregers, E.; Kraunsøe, R.; Van Hall, G.; Helge, J.W.; Dela, F. Adipose tissue mitochondrial respiration and lipolysis before and after a weight loss by diet and RYGB. Obesity 2015, 23, 2022–2029. [Google Scholar] [CrossRef]
- Coen, P.M.; Menshikova, E.V.; Distefano, G.; Zheng, D.; Tanner, C.J.; Standley, R.A.; Helbling, N.L.; Dubis, G.S.; Ritov, V.B.; Xie, H.; et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes 2015, 64, 3737–3750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezquerro, S.; Becerril, S.; Tuero, C.; Mendez-Gimenez, L.; Mocha, F.; Moncada, R.; Valenti, V.; Cienfuegos, J.A.; Catalan, V.; Gomez-Ambrosi, J.; et al. Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats. Int. J. Obes. 2020, 44, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [Google Scholar] [CrossRef] [PubMed]
- Elgass, K.; Pakay, J.; Ryan, M.T.; Palmer, C.S. Recent advances into the understanding of mitochondrial fission. Biochim. Et Biophys. Acta 2013, 1833, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Zorzano, A.; Liesa, M.; Palacin, M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch. Physiol. Biochem. 2009, 115, 1–12. [Google Scholar] [CrossRef]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet impact on mitochondrial bioenergetics and dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Bach, D.; Pich, S.; Soriano, F.X.; Vega, N.; Baumgartner, B.; Oriola, J.; Daugaard, J.R.; Lloberas, J.; Camps, M.; Zierath, J.R.; et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 2003, 278, 17190–17197. [Google Scholar] [CrossRef] [Green Version]
- Mingrone, G.; Manco, M.; Calvani, M.; Castagneto, M.; Naon, D.; Zorzano, A. Could the low level of expression of the gene encoding skeletal muscle mitofusin-2 account for the metabolic inflexibility of obesity? Diabetologia 2005, 48, 2108–2114. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.D.; Petersen, S.M.; Moller, K.E.; Lund, M.T.; Hansen, M.; Hansen, C.N.; Courraud, J.; Helge, J.W.; Dela, F.; Prats, C. Obesity leads to impairments in the morphology and organization of human skeletal muscle lipid droplets and mitochondrial networks, which are resolved with gastric bypass surgery-induced improvements in insulin sensitivity. Acta Physiol. 2018, 224, e13100. [Google Scholar] [CrossRef]
- Peng, Y.; Murr, M.M. Roux-en-Y gastric bypass improves hepatic mitochondrial function in obese rats. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2013, 9, 429–435. [Google Scholar] [CrossRef]
- Verbeek, J.; Lannoo, M.; Pirinen, E.; Ryu, D.; Spincemaille, P.; Vander Elst, I.; Windmolders, P.; Thevissen, K.; Cammue, B.P.; Van Pelt, J.; et al. Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut 2015, 64, 673–683. [Google Scholar] [CrossRef]
| Parameters | Before | After |
|---|---|---|
| n (females %) | 47 (100) | |
| Age (years) | 45.5 ± 10.2 | |
| Weight (kg) | 107.1 ± 15.6 | 76.3 ± 12.0 *** |
| BMI (kg/m2) | 40.3 ± 5.3 | 29.0 ± 4.3 *** |
| EWL (%) | 81.1 ± 29.7 | |
| Waist (cm) | 114.3 ± 10.5 | 88.8 ± 12.0 *** |
| SBP (mmHg) | 130.6 ± 16.1 | 121.6 ± 18.3 ** |
| DBP (mmHg) | 80.6 ± 10.2 | 73.5 ± 11.0 ** |
| Glucose (mg/dL) | 96.4 ± 12.5 | 84.0 ± 6.9 *** |
| Insulin (μU/mL) | 14.4 ± 7.6 | 7.0 ± 3.1 *** |
| HOMA-IR | 3.52 ± 2.18 | 1.44 ± 0.72 *** |
| HbA1c (%) | 5.47 ± 0.54 | 5.16 ± 0.35 *** |
| TC (mg/dL) | 190.0 ± 32.6 | 169.8 ± 26.2 *** |
| HDLc (mg/dL) | 48.1 ± 8.1 | 59.5 ± 9.6 *** |
| LDLc (mg/dL) | 125.1 ± 40.2 | 97.2 ± 21.0 *** |
| TG (mg/dL) | 95.5 (73.8, 136.5) | 76.0 (56.0, 100.5) *** |
| hsCRP (mg/L) | 4.69 (2.08, 8.29) | 0.79 (0.28, 1.48) *** |
| Leukocytes (103/μL) | 7.74 ± 2.41 | 6.39 ± 1.98 * |
| Treatment | ||
| Hypertension % (n) | 36.2 (17) | 14.9 (7) |
| Hyperlipidemia % (n) | 21.3 (10) | 10.6 (5) |
| T2D % (n) | 27.7 (13) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abad-Jiménez, Z.; Vezza, T.; López-Domènech, S.; Fernández-Reyes, M.; Canet, F.; Morillas, C.; Gómez-Abril, S.Á.; Bañuls, C.; Víctor, V.M.; Rocha, M. Impact of Roux-en-Y Gastric Bypass on Mitochondrial Biogenesis and Dynamics in Leukocytes of Obese Women. Antioxidants 2022, 11, 1302. https://doi.org/10.3390/antiox11071302
Abad-Jiménez Z, Vezza T, López-Domènech S, Fernández-Reyes M, Canet F, Morillas C, Gómez-Abril SÁ, Bañuls C, Víctor VM, Rocha M. Impact of Roux-en-Y Gastric Bypass on Mitochondrial Biogenesis and Dynamics in Leukocytes of Obese Women. Antioxidants. 2022; 11(7):1302. https://doi.org/10.3390/antiox11071302
Chicago/Turabian StyleAbad-Jiménez, Zaida, Teresa Vezza, Sandra López-Domènech, Meylin Fernández-Reyes, Francisco Canet, Carlos Morillas, Segundo Ángel Gómez-Abril, Celia Bañuls, Víctor M. Víctor, and Milagros Rocha. 2022. "Impact of Roux-en-Y Gastric Bypass on Mitochondrial Biogenesis and Dynamics in Leukocytes of Obese Women" Antioxidants 11, no. 7: 1302. https://doi.org/10.3390/antiox11071302