Antioxidant Activity and Oxidative Stress-Oriented Apoptosis Pathway in Saccharides Supplemented Cryopreserved Sperm of Pacific Abalone, Haliotis discus hannai
Abstract
1. Introduction
2. Materials and Methods
2.1. Abalone Handling, Management, and Sperm Collection
2.2. Motility Observation of Fresh Sperm
2.3. Experimental Reagents
2.4. Sperm Cryopreservation Protocol
2.5. Detection of PMI and MMP of Saccharide Supplemented Cryopreserved Sperm of Pacific Abalone
2.6. Production of Superoxide Anion (O2•−)
2.7. Comet Assay
2.8. Measurements of Antioxidant Enzyme Activities
2.8.1. Catalase (CAT) Activity
2.8.2. Superoxide Dismutase (SOD) Activity
2.8.3. Glutathione (GSH) Activity
2.9. Lipid Peroxidation (MDA) Assay
2.10. Total RNA (tRNAs) Extraction and cDNA Synthesis of Cryopreserved Sperm
2.11. Quantitative Real-Time PCR (qPCR)
2.12. Apoptosis Pathway of Cryopreserved Pacific Abalone Sperm
2.13. Fertility and Hatchability Test
2.14. Statistical Analysis
3. Results
3.1. Post-Thaw Sperm Quality
3.1.1. Post-Thaw Motility of Saccharide Supplemented Sperm
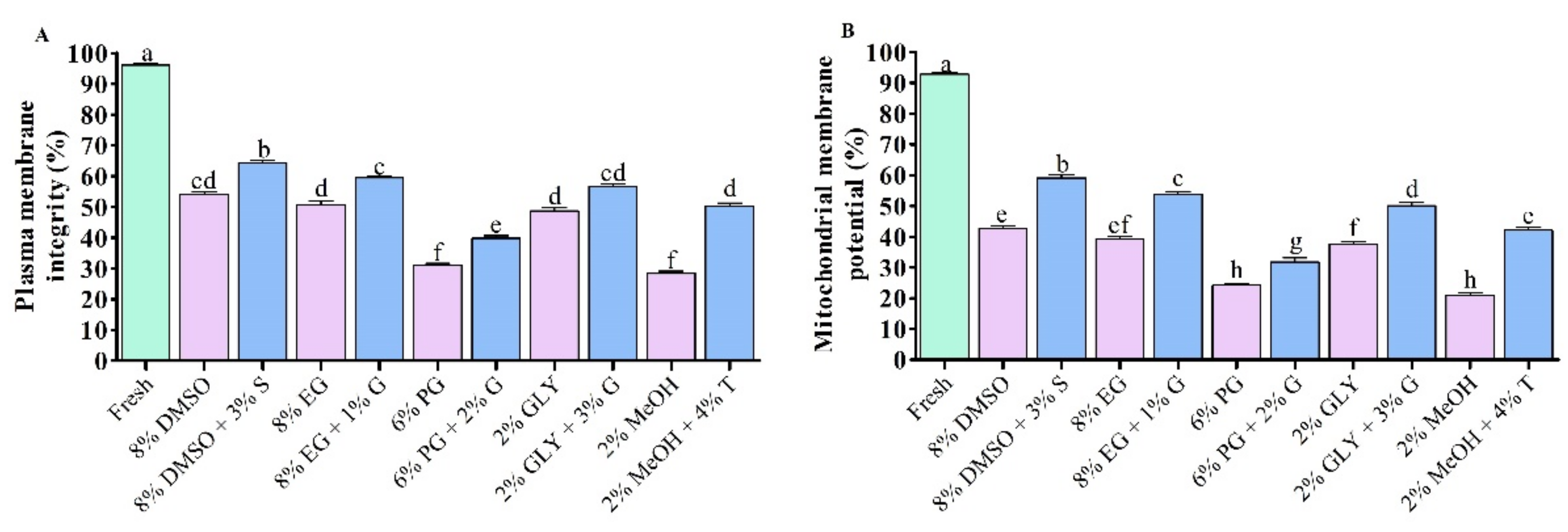
3.1.2. PMI and MMP of Saccharide Supplemented Cryopreserved Sperm
3.2. Production of Superoxide Anion (O2•−)
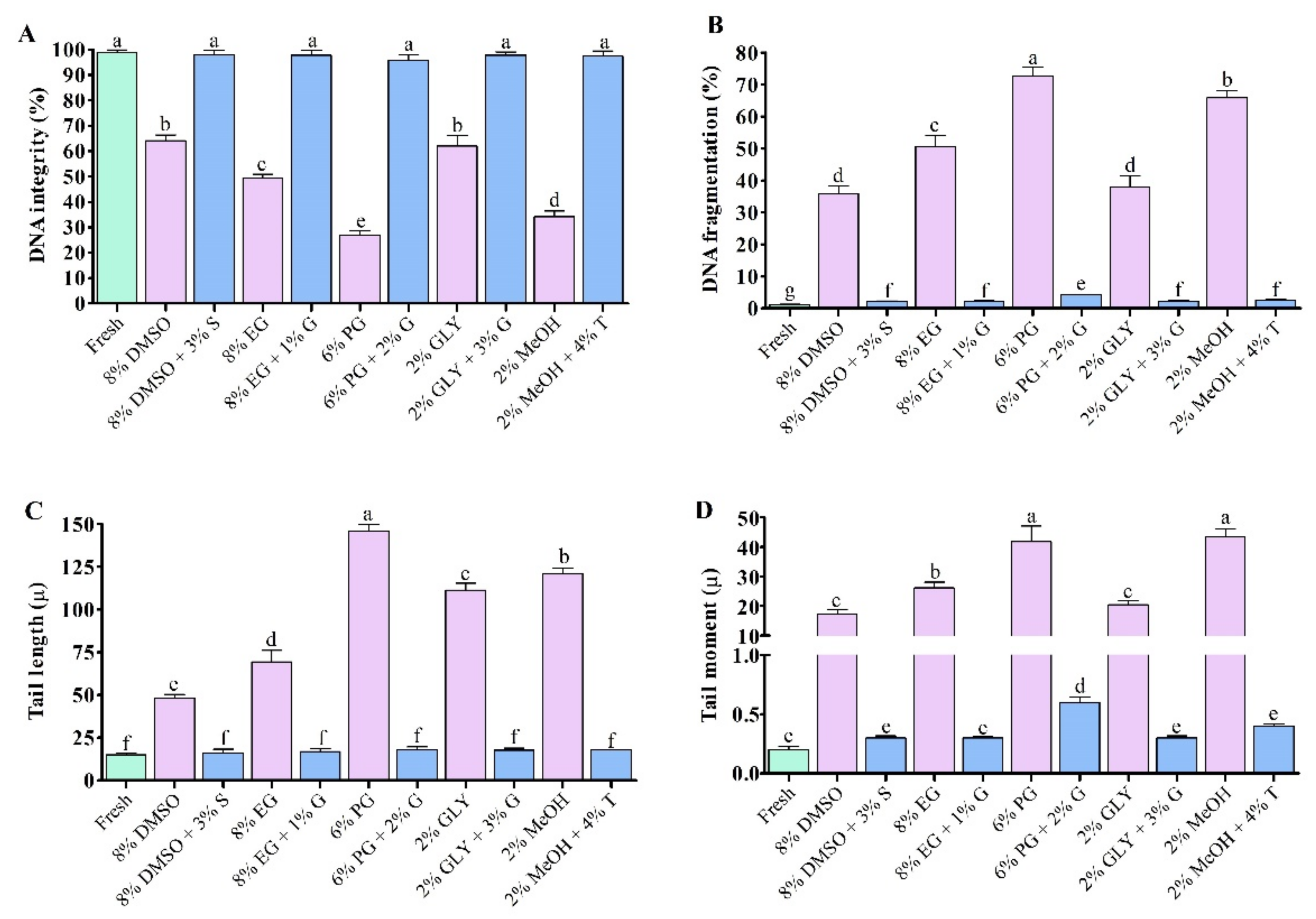
3.3. Sperm DNA Integrity
3.4. Antioxidant Enzyme Activities
3.4.1. Catalase (CAT) Activity
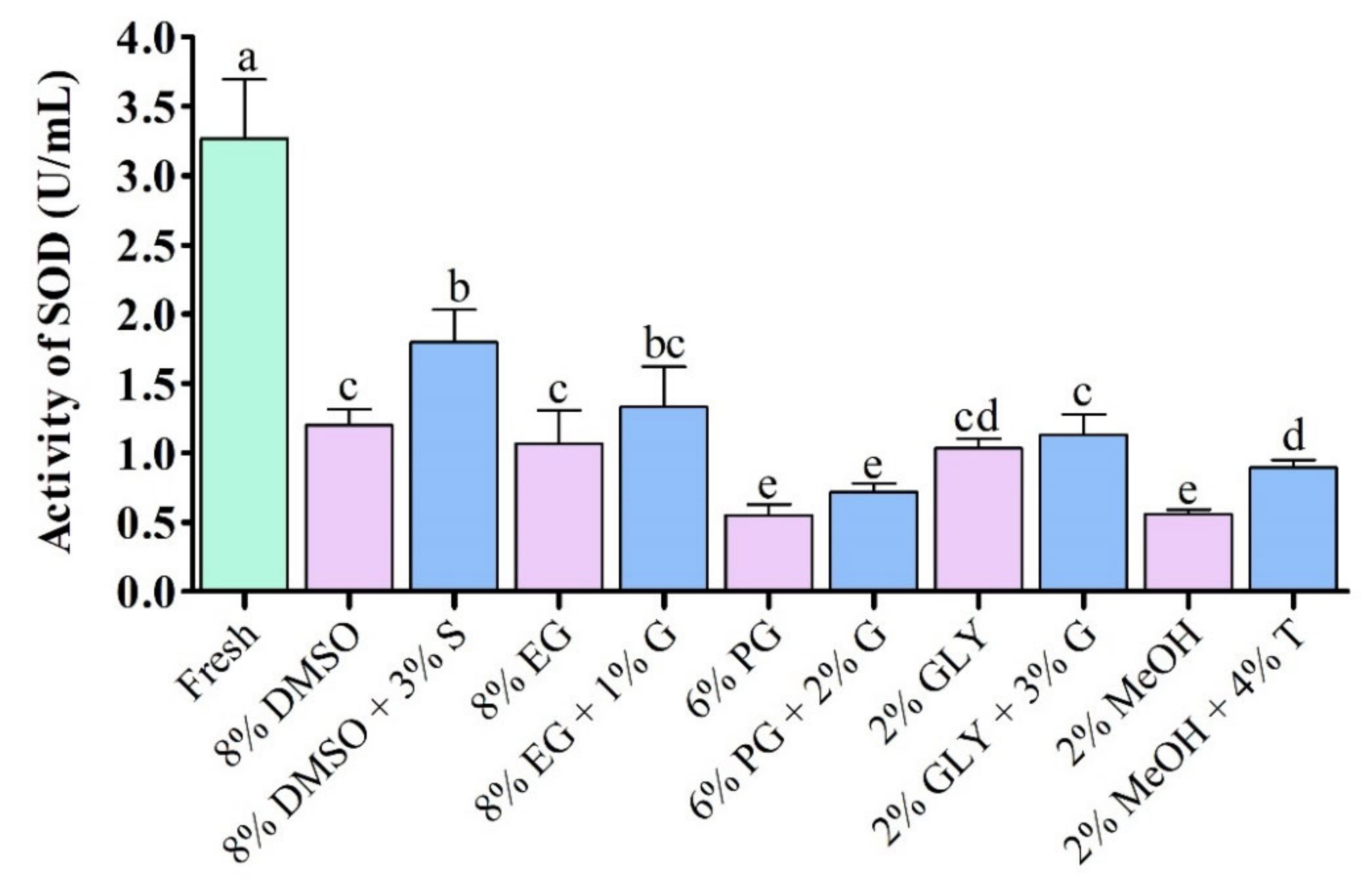
3.4.2. Superoxide Dismutase (SOD) Activity
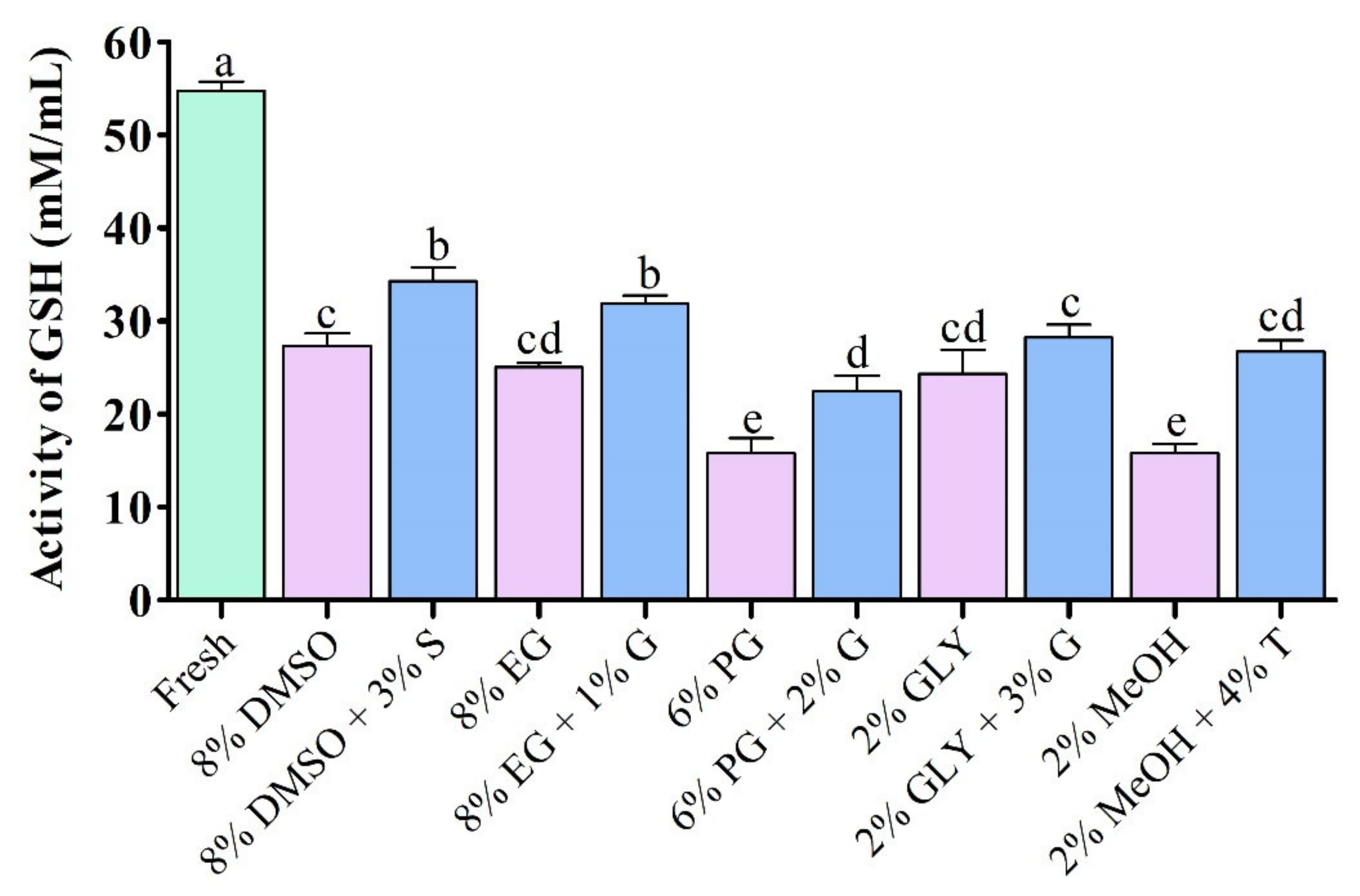
3.4.3. Glutathione (GSH) Activity
3.5. Lipid Peroxidation (MDA) Activity
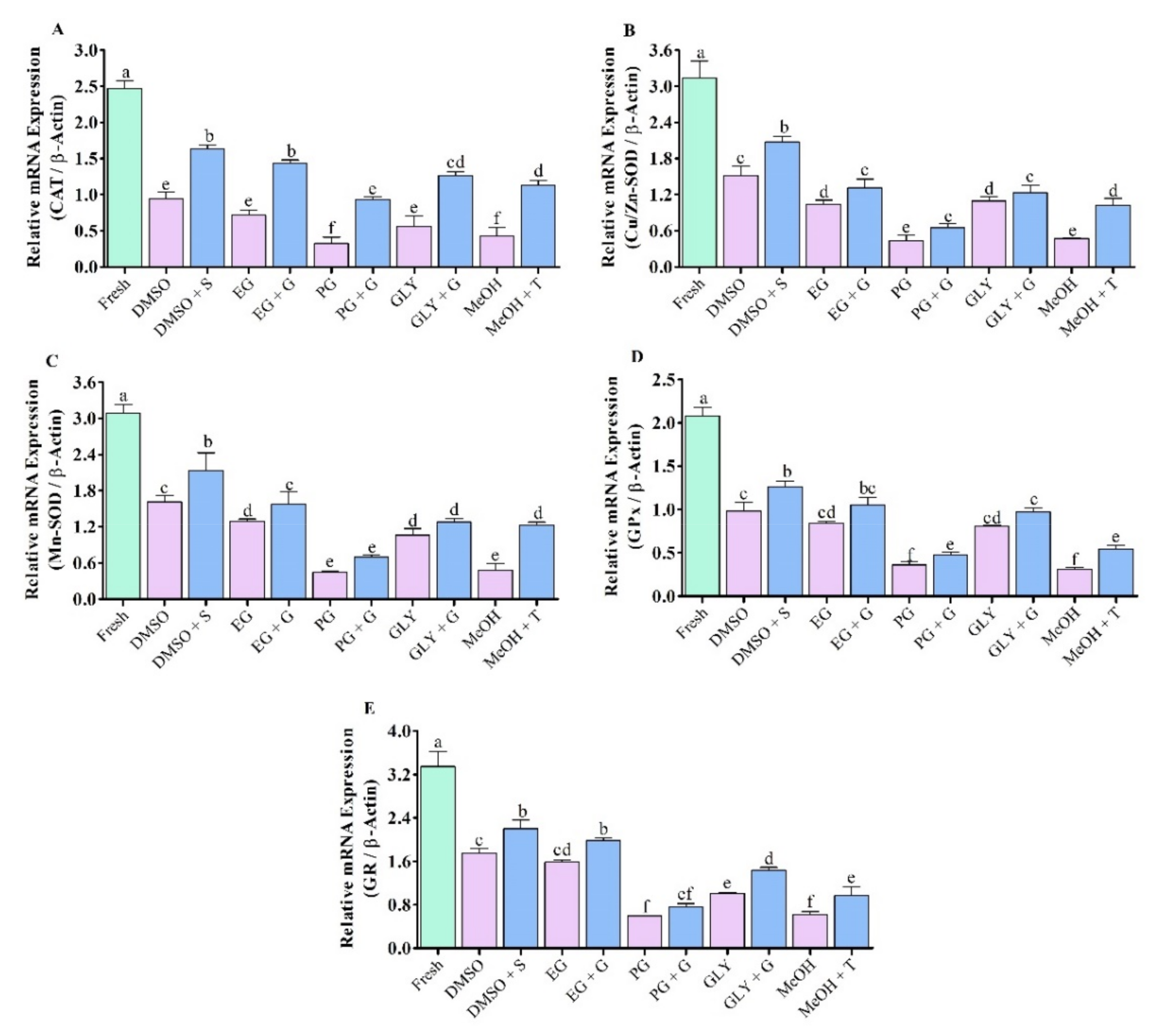
3.6. Expression Analysis of Vital Antioxidant Gene in Saccharide Supplimented Cryopreserved Sperm
3.6.1. Expression Analysis of CAT mRNA Transcript
3.6.2. Expression Analysis of Cu/Zn-SOD mRNA Transcript
3.6.3. Expression Analysis of Mn-SOD mRNA Transcript
3.6.4. Expression Analysis of GPx mRNA Transcript
3.6.5. Expression Analysis of GR mRNA Transcript
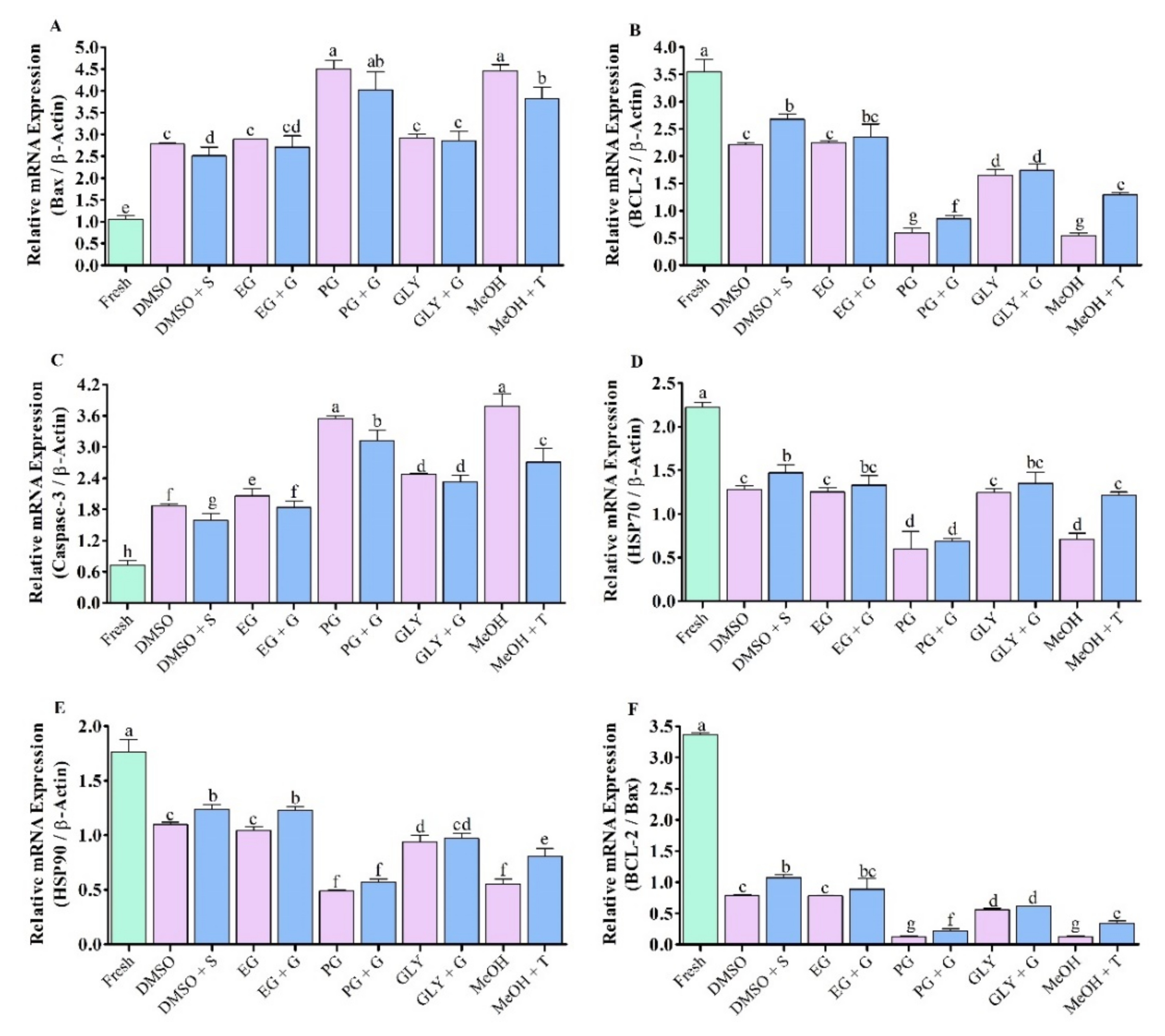
3.7. Expression Analysis of Apopotosis Regulated Gene in Saccharide Supplimented Cryopreserved Sperm
3.7.1. Expression Analysis of Bax mRNA Transcript
3.7.2. Expression Analysis of BCL-2 mRNA Transcript
3.7.3. Expression Analysis of Caspase-3 mRNA Transcript
3.7.4. Expression Analysis of HSP70 mRNA Transcript
3.7.5. Expression Analysis of HSP90 mRNA Transcript
3.8. Ratio of BCL-2 to Bax mRNA Expression
3.9. Correlations of Post-Thaw Motility with Oxidative Stress Associated Parameters
3.10. Apoptosis Pathway of Saccharide Supplemented Cryopreserved Sperm of Pacific Abalone
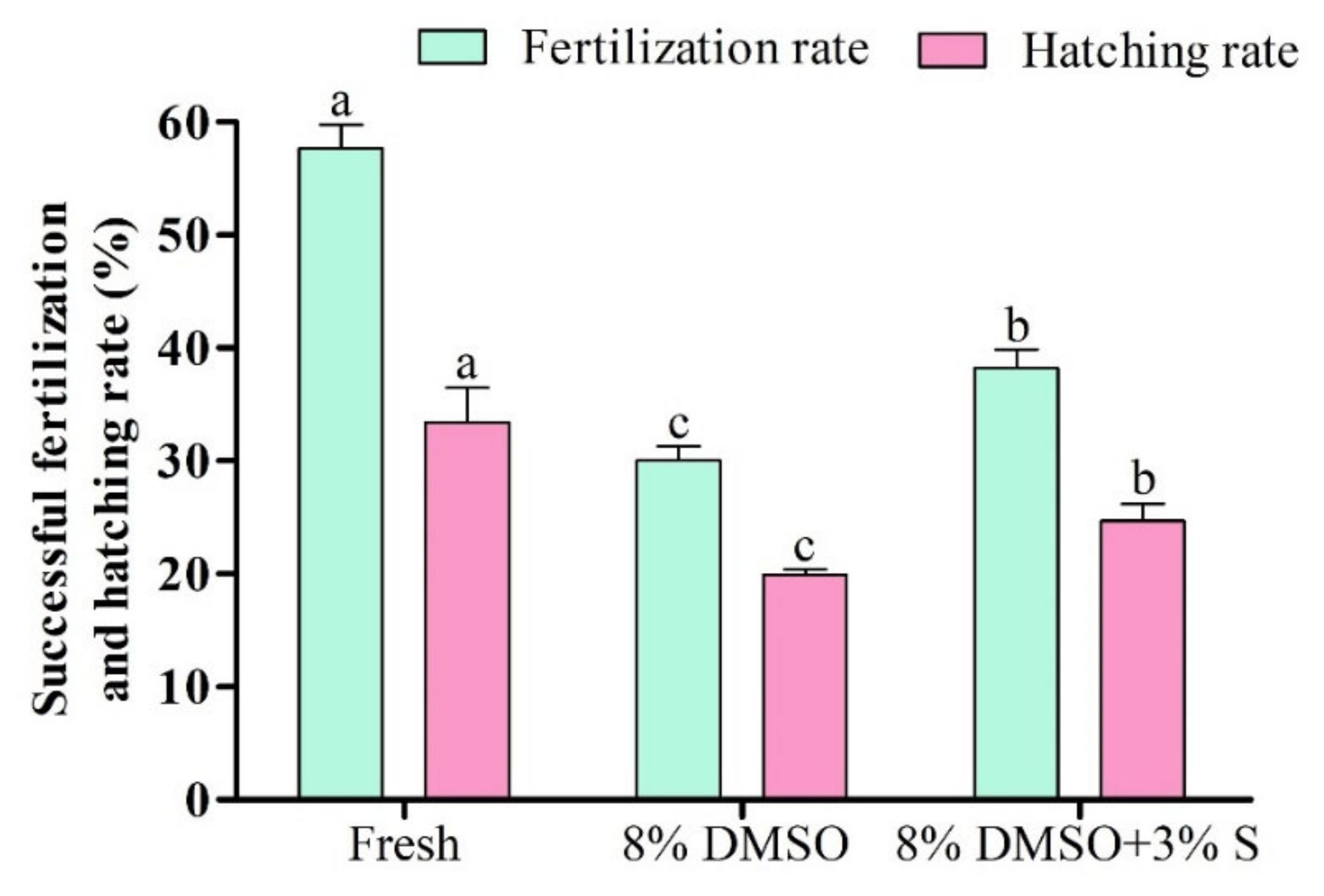
3.11. Fertilization and Hatching Rate of Saccharides Supplemented Cryopreserved Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suleria, H.A.R.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Sukhan, Z.P.; Cho, Y.; Kho, K.H. Effects of cryopreservation on gene expression and post thaw sperm quality of Pacific abalone, Haliotis discus hannai. Front. Mar. Sci. 2021, 8, 652390. [Google Scholar] [CrossRef]
- Kim, S.C.; Hossen, S.; Kho, K.H. Cryopreservation of sperm from farmed Pacific abalone, Haliotis discus hannai. Cryobiology 2020, 94, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Li, H.; Yang, W.; Fu, Q.; Gong, C.; Wang, L.; Song, L. The effects of protein kinase a catalytic subunit on sperm motility regulation in Pacific abalone Haliotis discus hannai. Aquac. Res. 2020, 51, 2525–2534. [Google Scholar] [CrossRef]
- Zidni, I.; Lee, Y.H.; Park, J.Y.; Lee, H.B.; Hur, J.W.; Lim, H.K. Effects of cryoprotective medium composition, dilution ratio, and freezing rates on spotted halibut (Verasper variegatus) sperm cryopreservation. Animals 2020, 10, 2153. [Google Scholar] [CrossRef]
- Kim, S.C.; Hossen, S.; Kho, K.H. Effects of 3 years of cryopreservation on sperm quality of seven-band grouper, Epinephelus septemfasciatus. Aquac. Res. 2020, 51, 3050–3053. [Google Scholar] [CrossRef]
- Hossen, S.; Kim, S.C.; Cho, Y.; Kho, K.H. Vital analysis of cryopreserved sperm of marbled flounder, Pseudopleuronectes yokohamae. Front. Physiol. 2021, 12, 696737. [Google Scholar] [CrossRef]
- Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, I.; Governini, L.; et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants 2021, 11, 10. [Google Scholar] [CrossRef]
- Sooksawat, T.; Tongnunui, S.; Nimrat, S.; Vuthiphandchai, V. Influences of contamination of Aeromonas hydrophila, on quality, oxidative damage, and ultrastructure in cryopreserved sperm of the silver barb, Barbonymus gonionotus. Aquaculture 2022, 547, 737440. [Google Scholar] [CrossRef]
- Mehdipour, M.; Daghigh, K.H.; Najafi, A.; Mohammadi, H.; Álvarez-Rodriguez, M. Effect of crocin and naringenin supplementation in cryopreservation medium on post-thaw rooster sperm quality and expression of apoptosis associated genes. PLoS ONE 2020, 15, e0241105. [Google Scholar] [CrossRef]
- Estudillo, E.; Jiménez, A.; Bustamante-Nieves, P.E.; Palacios-Reyes, C.; Velasco, I.; López-Ornelas, A. Cryopreservation of gametes and embryos and their molecular changes. Int. J. Mol. Sci. 2021, 22, 10864. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Makarova, N.P.; Romanov, Y.A.; Dolgushina, N.V.; Parker, M.M.; Krasnyi, A.M. Comparative analysis of the expression of glutathione peroxidase and glutathione reductase genes in human sperm after cryopreservation. Bull. Exp. Biol. Med. 2018, 165, 166–170. [Google Scholar] [CrossRef]
- Dessouki, S.M.; Ashour, G.; El-Gayar, M.; El-Azzazi, F.E.H.; Kodi, E.; Ghanem, N. Gene expression profile and enzymatic activities of frozen buck sperm supplemented with melatonin in cold and hot temperatures. World’s Vet. J. 2020, 10, 125–136. [Google Scholar] [CrossRef]
- Mirzaei, A.H.; Deldar, H.; Pirsaraei, Z.A.; Shohreh, B. Royal jelly may improve sperm characteristics during preservation of rooster semen: Gene expression of antioxidant enzymes. Reprod. Domest. Anim. 2021, 56, 658–666. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R.; Long, J.A. Mitochondrial function and reactive oxygen species action in relation to boar motility. Theriogenology 2008, 70, 1209–1215. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Liu, T.; Han, Y.; Zhou, T.; Zhang, R.; Chen, H.; Chen, S.; Zhao, H. Mechanisms of ROS-induced mitochondria-dependent apoptosis underlying liquid storage of goat spermatozoa. Aging 2019, 11, 7880. [Google Scholar] [CrossRef]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004, 71, 28–37. [Google Scholar] [CrossRef]
- Karabulut, S.; Demiroğlu-Zergeroğlu, A.; Yılmaz, E.; Kutlu, P.; Keskin, İ. Effects of human sperm cryopreservation on apoptotic markers in normozoospermic and non-normozoospermic patients. Zygote 2018, 26, 308–313. [Google Scholar] [CrossRef]
- Vardiyan, R.; Ezati, D.; Anvari, M.; Ghasemi, N.; Talebi, A. Effect of L-carnitine on the expression of the apoptotic genes Bcl-2 and Bax. Clin. Exp. Reprod. Med. 2020, 47, 155. [Google Scholar] [CrossRef]
- Nori-Garavand, R.; Hormozi, M.; Narimani, L.; Beigi Boroujeni, N.; Rajabzadeh, A.; Zarei, L.; Boroujeni, M.B.; Boroujeni, M.B. Effect of Selenium on Expression of Apoptosis-Related Genes in Cryomedia of Mice Ovary after Vitrification. Biomed Res. Int. 2020, 2020, 5389731. [Google Scholar] [CrossRef]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Esmailpoor, A.; Ghasemian, A.; Dehnavi, E.; Peidayesh, H.; Teimouri, M. Physalis alkekengi hydroalcoholic extract enhances the apoptosis in mouse model of breast cancer cells. Gene Rep. 2019, 15, 100366. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Lartigue, L.; Perkins, G. Targeting Mcl-1 and other Bcl-2 family member proteins in cancer therapy. Pharmacol. Ther. 2019, 195, 13–20. [Google Scholar] [CrossRef]
- Hossen, S.; Sharker, M.; Cho, Y.; Sukhan, Z.P.; Kho, K.H. Effects of antifreeze protein III on sperm cryopreservation of Pacific abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2021, 22, 3917. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.; Robinson, N.; Qin, J.; Li, X. Cryopreservation of sperm in farmed Australian greenlip abalone Haliotis laevigata. Cryobiology 2014, 68, 185–193. [Google Scholar] [CrossRef]
- Sandoval-Vargas, L.; Dumorne, K.; Contreras, P.; Farias, J.G.; Figueroa, E.; Risopatron, J.; Valdebenito, I. Cryopreservation of coho salmon sperm (Oncorhynchus kisutch): Effect on sperm function, oxidative stress and fertilizing capacity. Aquaculture 2021, 533, 736151. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Xu, T.; Robinson, N.; Qin, J. Improvement in non-programmable sperm cryopreservation technique in farmed greenlip abalone Haliotis laevigata. Aquaculture 2014, 434, 362–366. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Liu, S.; Xu, T.; Liu, Q.; Li, X. Cryopreservation of strip spawned sperm using non-programmable freezing technique in the blue mussel Mytilus galloprovincialis. Aquac. Res. 2016, 47, 3888–3898. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Liu, B.; Qin, J.; Xu, T.; Li, X. Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis. J. Oceanol. Limnol. 2018, 36, 2351–2357. [Google Scholar] [CrossRef]
- Anjos, C.; Santos, A.L.; Duarte, D.; Matias, D.; Cabrita, E. Effect of trehalose and sucrose in post-thaw quality of Crassostrea angulata sperm. Front. Physiol. 2021, 12, 749735. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Park, J.Y.; Lim, H.K. Effects of different diluents, cryoprotective agents, and freezing rates on sperm cryopreservation in Epinephelus akaara. Cryobiology 2018, 83, 60–64. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, X.; Pan, Y.; Lu, Y.; Zeng, W. Trehalose improves rabbit sperm quality during cryopreservation. Cryobiology 2017, 75, 45–51. [Google Scholar] [CrossRef]
- Ariyan, F.; Farshad, A.; Rostamzadeh, J. Protective effects of Tribulus terrestris and Cinnamomum zeylanicum extracts and trehalose added to diluents on goat epididymal sperm freezability. Cryobiology 2021, 98, 172–180. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef]
- Abdillah, D.A.; Setyawan, E.M.; Oh, H.J.; Ra, K.; Lee, S.H.; Kim, M.J.; Lee, B.C. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. J. Vet. Sci. 2019, 20, 79–86. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef]
- Ball, B.A. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 2008, 107, 257–267. [Google Scholar] [CrossRef]
- Cabrita, E.; Ma, S.; Diogo, P.; Martínez-Páramo, S.; Sarasquete, C.; Dinis, M.T. The influence of certain aminoacids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim. Reprod. Sci. 2011, 125, 189–195. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.H.; Dzyuba, B.; Hulak, M.; Rodina, M.; Linhart, O. Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio, L.) sperm caused by cryopreservation techniques. Biol. Reprod. 2010, 83, 852–858. [Google Scholar] [CrossRef]
- Figueroa, E.; Farias, J.G.; Lee-Estevez, M.; Valdebenito, I.; Risopatrón, J.; Magnotti, C.; Romero, J.; Watanabe, I.; Oliveira, R.P.S. Sperm cryopreservation with supplementation of α-tocopherol and ascorbic acid in freezing media increase sperm function and fertility rate in Atlantic salmon (Salmo salar). Aquaculture 2018, 493, 1–8. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From past to present: The link between reactive oxygen species in sperm and male infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Nashtaei, M.S.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Banerjee, R.; Becker, D.; Dickman, M.; Gladyshev, V.; Ragsdale, S. (Eds.) Redox Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Riesco, M.F.; Oliveira, C.; Soares, F.; Gavaia, P.J.; Dinis, M.T.; Cabrita, E. Solea senegalensis sperm cryopreservation: New insights on sperm quality. PLoS ONE 2017, 12, e0186542. [Google Scholar] [CrossRef]
- He, W.H.; Zhai, X.H.; Duan, X.J.; Di, H.S. Effect of resveratrol treatment on apoptosis and apoptotic pathways during boar semen freezing. J. Zhejiang Univ. Sci. B 2020, 21, 485–494. [Google Scholar] [CrossRef]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirão, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function—In sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Jeuthe, H.; Palaiokostas, C.; Johannisson, A. DNA fragmentation and membrane integrity in sperm of farmed Arctic charr (Salvelinus alpinus). Aquaculture 2022, 547, 737537. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Bucak, M.N.; Canturk, F.; Özdamar, S.; Yay, A.; Tuncer, P.B.; Özcan, S.; Sorgucu, N.; Caner, Y. The effects of different sugars on motility, morphology and DNA damage during the liquid storage of rat epididymal sperm at 4 °C. Cryobiology 2012, 65, 93–97. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.Y.; Cai, M.D.; Li, X.X.; Li, Y.H.; Lei, Y.; Yu, X.L. Effect of vitrification temperature and cryoprotectant concentrations on the mRNA transcriptome of bovine mature oocytes after vitrifying at immature stage. Theriogenology 2020, 148, 225–235. [Google Scholar] [CrossRef]
- Takayama, S.; Reed, J.C.; Homma, S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003, 22, 9041–9047. [Google Scholar] [CrossRef]
- Antonsson, B.; Martinou, J.C. The Bcl-2 protein family. Exp. Cell Res. 2000, 256, 50–57. [Google Scholar] [CrossRef]
- Kim, M.R.; Tilly, J.L. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1644, 205–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, P.S.P.; Kaushik, K.; Johnson, P.; Krishna, K.; Nandi, S.; Mondal, S.; Tej, J.N.K.; Somoskoi, B.; Cseh, S. Effect of different vitrification protocols on post thaw viability and gene expression of ovine preantral follicles. Theriogenology 2022, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vogeler, S.; Carboni, S.; Li, X.; Joyce, A. Phylogenetic analysis of the caspase family in bivalves: Implications for programmed cell death, immune response and development. BMC Genom. 2021, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Hu, J.R.; Wang, L.Y.; Hu, Y.J.; Tan, F.Q.; Zhou, H.; Shao, J.Z.; Yang, W.X. The apoptotic function analysis of p53, apaf1, caspase3 and caspase7 during the spermatogenesis of the chinese fire-bellied newt Cynops orientalis. PLoS ONE 2012, 7, e39920. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Grunewald, S.; Sharma, R.; Paasch, U.; Glander, H.J.; Agarwal, A. Impact of caspase activation in human spermatozoa. Microsc. Res. Tech. 2009, 72, 878–888. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Zhou, Y.; Wang, Y.; Wang, S.; Zhang, W. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. 2012, 321, 137–143. [Google Scholar] [CrossRef]
- Pandey, P.; Saleh, A.; Nakazawa, A.; Kumar, S.; Srinivasula, S.M.; Kumar, V.; Weichselbaum, R.; Nalin, C.; Alnemri, E.S.; Kufe, D.; et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000, 19, 4310–4322. [Google Scholar] [CrossRef]
- Lone, S.A.; Prasad, J.K.; Ghosh, S.K.; Das, G.K.; Balamurugan, B.; Verma, M.R. Study on correlation of sperm quality parameters with antioxidant and oxidant status of buffalo bull semen during various stages of cryopreservation. Andrologia 2018, 50, e12970. [Google Scholar] [CrossRef]




 in the apoptosis pathway where appropriate.
in the apoptosis pathway where appropriate.
 in the apoptosis pathway where appropriate.
in the apoptosis pathway where appropriate.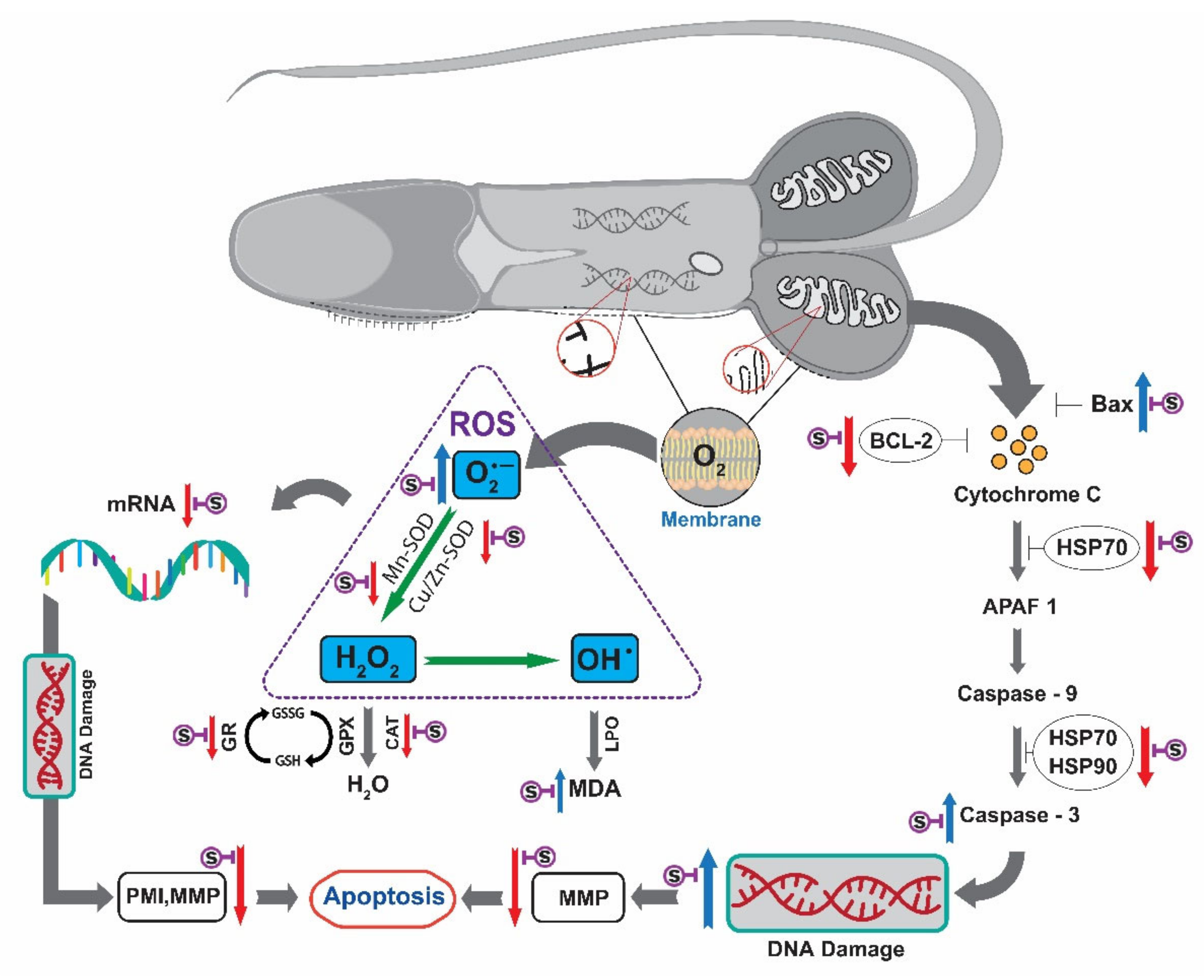
| Gene | Primer | Sequence (5′---3′) | Amplicon Length (bp) | GenBank Accession |
|---|---|---|---|---|
| β-Actin | Sense | CCGTGAAAAGATGACCCAGA | 204 | AY380809.1 |
| Antisense | TACGACCGGAAGCGTACAGA | |||
| CAT | Sense | CTGAGAGAGTCGTACATGC | 153 | OK042347.1 |
| Antisense | CCTTCTCACCACCTACAGTT | |||
| Cu/Zn-SOD | Sense | GCTGAGAGGTGATTCGGAAG | 158 | KX302627.1 |
| Antisense | CACTGGTACAGCCATTGGTG | |||
| Mn-SOD | Sense | TCTCGAGCCCTACATCTCG | 208 | KX302628.1 |
| Antisense | GCTAAGCACCTCCCAGAAG | |||
| GPx | Sense | ACAGAAAGTCGCGTGTCAAG | 200 | GU254066.1 |
| Antisense | GTCCAGCATAGTCGGTCATG | |||
| GR | Sense | CGTTCGATTACCTGGTGATC | 198 | OL944707.1 |
| Antisense | TCACGAACACAAGGAGTACG | |||
| Caspase-3 | Sense | ACTTCCTCGTCGCATATGC | 171 | MT506591.1 |
| Antisense | CAGGATAATCATGGGCGACT | |||
| Bax | Sense | AGCAAACAACCAGTGGATCG | 185 | MT501464.1 |
| Antisense | GCTATCTCTGTCGTGTGTTG | |||
| BCL-2 | Sense | AACGAAATGGCTACGTGTGG | 175 | MT482025.1 |
| Antisense | GCATGAAATGTTGGATACGCT | |||
| HSP70 | Sense | CAGAGAACACAATCTTCGATGC | 277 | DQ324856.1 |
| Antisense | CGTTGAGAGTCGTTGAAGTAAG | |||
| HSP90 | Sense | AACAGTACATCTGGGAGTCG | 216 | GU014545.1 |
| Antisense | CCTCCTTGTCTCTTTCCTTCT |
| Motility | PMI | MMP | O2•− | DNA Integrity | CAT | SOD | GSH | |
|---|---|---|---|---|---|---|---|---|
| PMI | 0.898 ** | 1 | ||||||
| MMP | 0.926 ** | 0.920 ** | 1 | |||||
| O2•− | −0.887 ** | −0.885 ** | −0.881 ** | 1 | ||||
| DNA integrity | 0.889 ** | 0.884 ** | 0.845 ** | 0.799 ** | 1 | |||
| CAT | 0.878 ** | 0.895 ** | 0.892 ** | −0.823 ** | 0.851 ** | 1 | ||
| SOD | 0.692 ** | 0.708 ** | 0.713 ** | −0.721 ** | 0.591 ** | 0.693 ** | 1 | |
| GSH | 0.813 ** | 0.875 ** | 0.870 ** | −0.850 ** | 0.756 ** | 0.811 ** | 0.763 ** | 1 |
| MDA | −0.836 ** | −0.875 ** | −0.868 ** | 0.847 ** | −0.843 ** | −0.805 ** | −0.708 ** | −0.854 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, S.; Sukhan, Z.P.; Cho, Y.; Lee, W.K.; Kho, K.H. Antioxidant Activity and Oxidative Stress-Oriented Apoptosis Pathway in Saccharides Supplemented Cryopreserved Sperm of Pacific Abalone, Haliotis discus hannai. Antioxidants 2022, 11, 1303. https://doi.org/10.3390/antiox11071303
Hossen S, Sukhan ZP, Cho Y, Lee WK, Kho KH. Antioxidant Activity and Oxidative Stress-Oriented Apoptosis Pathway in Saccharides Supplemented Cryopreserved Sperm of Pacific Abalone, Haliotis discus hannai. Antioxidants. 2022; 11(7):1303. https://doi.org/10.3390/antiox11071303
Chicago/Turabian StyleHossen, Shaharior, Zahid Parvez Sukhan, Yusin Cho, Won Kyo Lee, and Kang Hee Kho. 2022. "Antioxidant Activity and Oxidative Stress-Oriented Apoptosis Pathway in Saccharides Supplemented Cryopreserved Sperm of Pacific Abalone, Haliotis discus hannai" Antioxidants 11, no. 7: 1303. https://doi.org/10.3390/antiox11071303
APA StyleHossen, S., Sukhan, Z. P., Cho, Y., Lee, W. K., & Kho, K. H. (2022). Antioxidant Activity and Oxidative Stress-Oriented Apoptosis Pathway in Saccharides Supplemented Cryopreserved Sperm of Pacific Abalone, Haliotis discus hannai. Antioxidants, 11(7), 1303. https://doi.org/10.3390/antiox11071303







