HBOC-301 in Porcine Kidney Normothermic Machine Perfusion and the Effect of Vitamin C on Methemoglobin Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of pRBCs
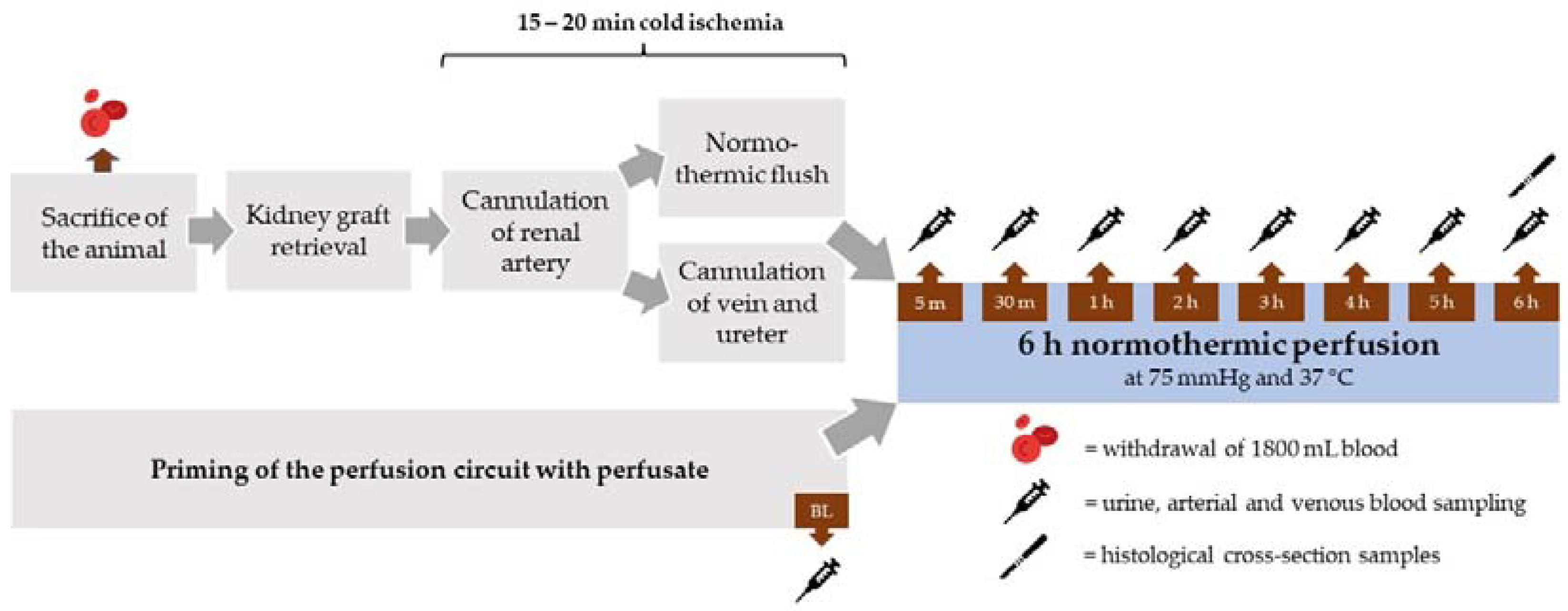
2.2. Kidney Retrieval and Cannulation
2.3. Perfusate
2.4. Normothermic Machine Perfusion System
2.5. Sampling and Laboratory Analysis
2.6. Histology
2.7. Electron Microscopy
2.8. Statistical Analysis
3. Results
3.1. Kidney Weights
3.2. Perfusion Parameters
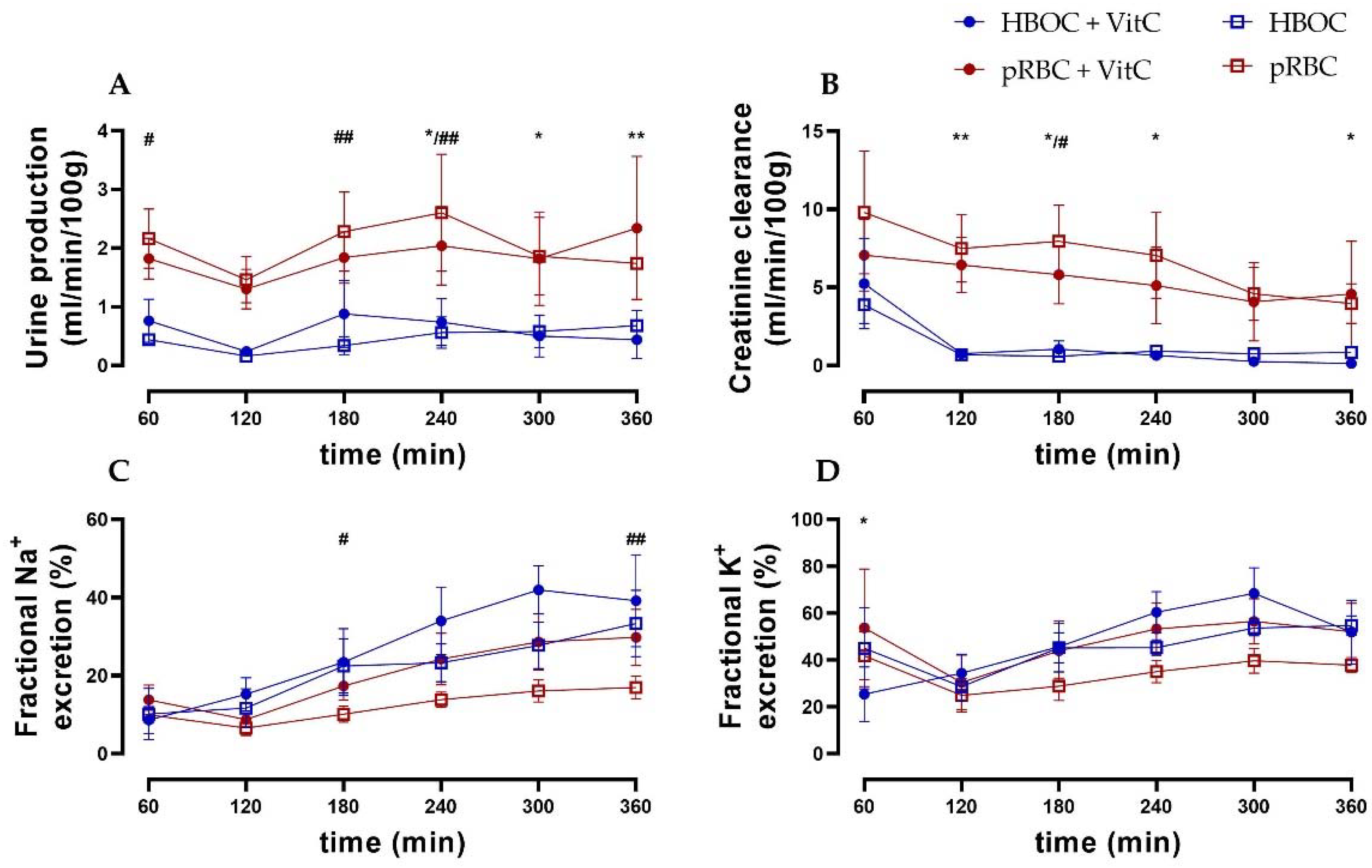
3.3. Renal Function
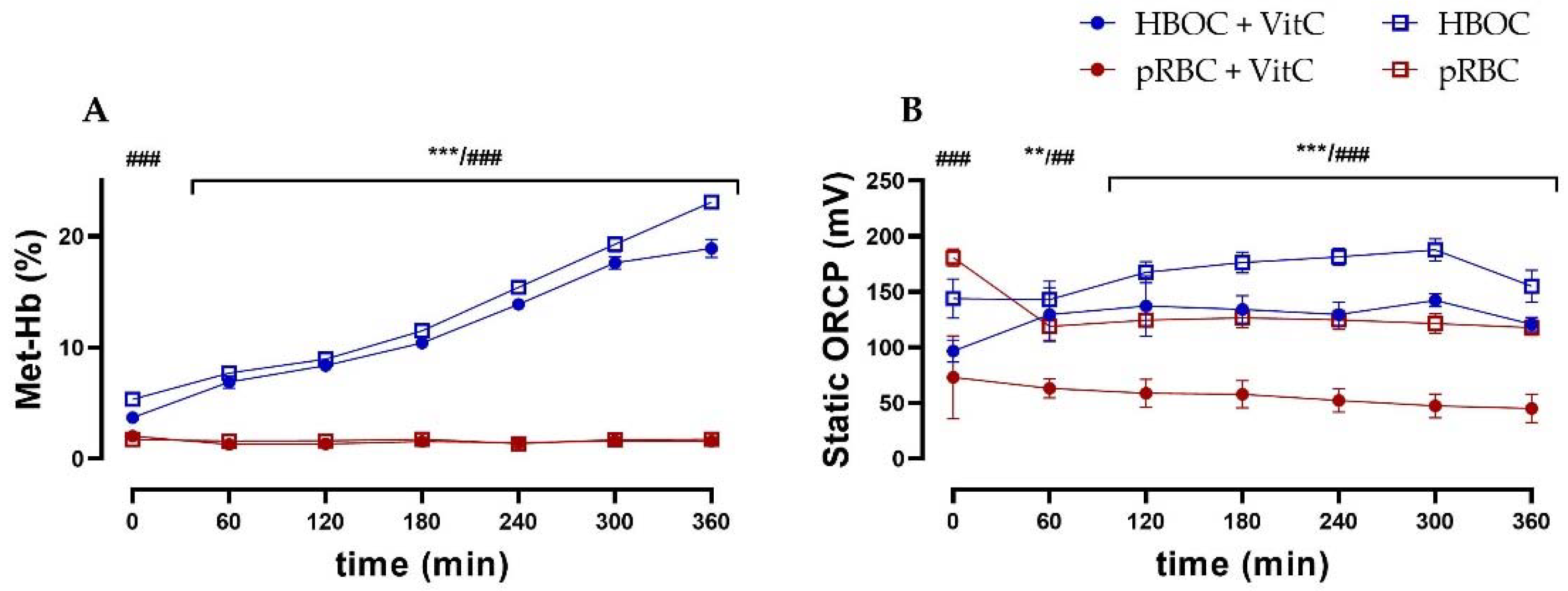
3.4. Blood-Gas Analysis
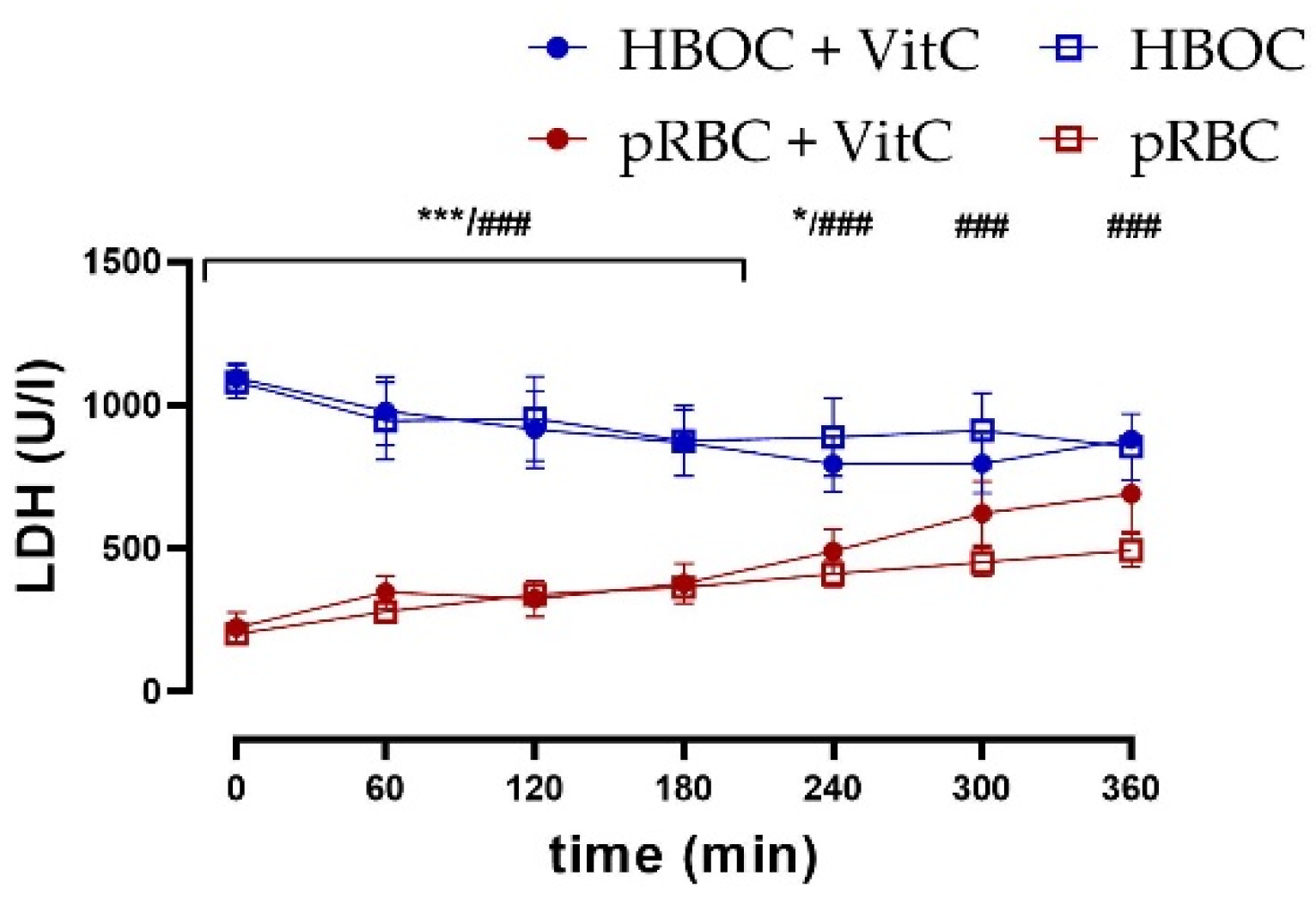
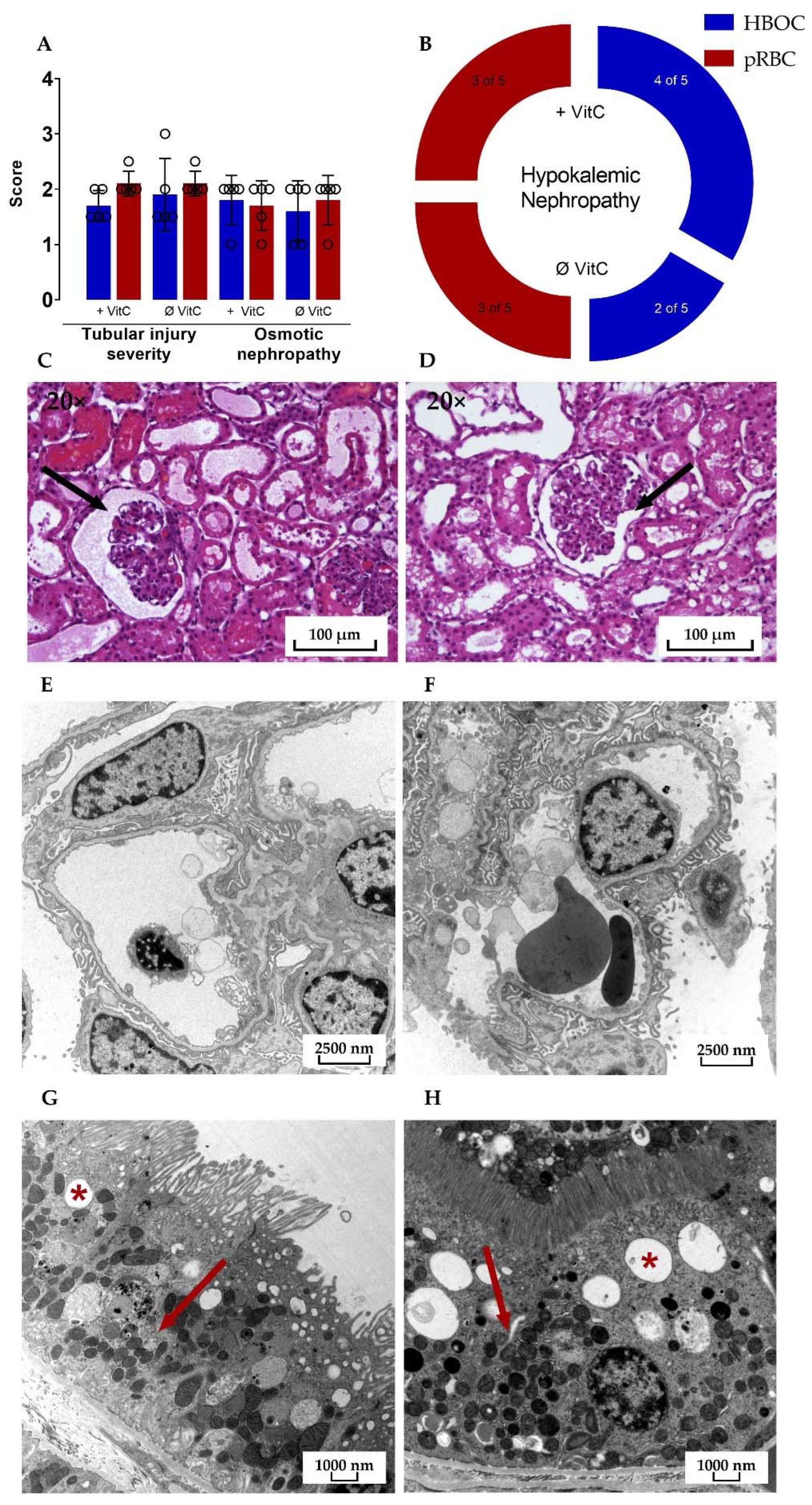
3.5. Kidney Damage
3.6. Oxidative Stress
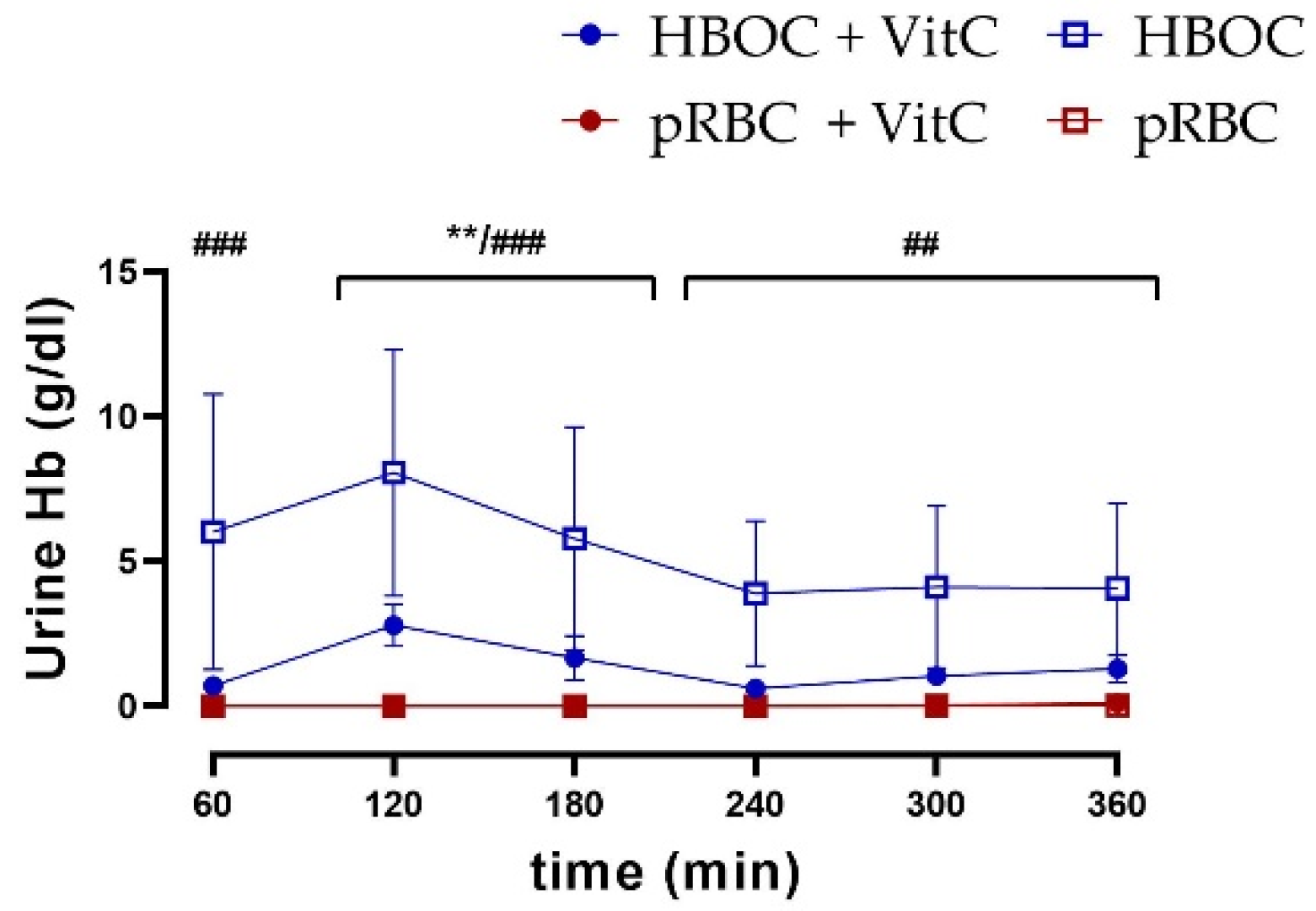
3.7. Hemoglobin Fragmentation
3.8. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamar, M.; Selzner, M. Ex-Vivo machine perfusion for kidney preservation. Curr. Opin. Organ Transplant. 2018, 23, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. 2021, 21, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Dufour, N.; Radjou, A.; Thuong, M. Hemolysis and Plasma Free Hemoglobin During Extracorporeal Membrane Oxygenation Support: From Clinical Implications to Laboratory Details. ASAIO J. 2020, 66, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hokka, M.; Egi, M.; Kubota, K.; Mizobuchi, S. Perioperative Serum Free Hemoglobin and Haptoglobin Levels in Valvular and Aortic Surgery with Cardiopulmonary Bypass: Their Associations With Postoperative Kidney Injury. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3207–3214. [Google Scholar] [CrossRef]
- Omar, H.R.; Mirsaeidi, M.; Socias, S.; Sprenker, C.; Caldeira, C.; Camporesi, E.M.; Mangar, D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE 2015, 10, e0124034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaghan, C.J.; Phillips, B.L.; Foukaneli, T.; Robinson, S.; Watson, C.J.E. The use of third-party packed red blood cells during ex situ normothermic machine perfusion of organs for transplantation: Underappreciated complexities? Am. J. Transplant. 2021, 21, 1376–1381. [Google Scholar] [CrossRef]
- Gautam, R.; Oh, J.Y.; Marques, M.B.; Dluhy, R.A.; Patel, R.P. Characterization of Storage-Induced Red Blood Cell Hemolysis Using Raman Spectroscopy. Lab. Med. 2018, 49, 298–310. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Vaara, S.T.; Pettila, V.; Bellomo, R.; Tuimala, J.; Cooper, D.J.; Krusius, T.; Kuitunen, A.; Reinikainen, M.; Koskenkari, J.; et al. Age of red blood cells and outcome in acute kidney injury. Crit. Care 2013, 17, R222. [Google Scholar] [CrossRef] [Green Version]
- Aburawi, M.M.; Fontan, F.M.; Karimian, N.; Eymard, C.; Cronin, S.; Pendexter, C.; Nagpal, S.; Banik, P.; Ozer, S.; Mahboub, P.; et al. Synthetic hemoglobin-based oxygen carriers are an acceptable alternative for packed red blood cells in normothermic kidney perfusion. Am. J. Transplant. 2019, 19, 2814–2824. [Google Scholar] [CrossRef]
- Mahboub, P.; Aburawi, M.; Karimian, N.; Lin, F.; Karabacak, M.; Fontan, F.; Tessier, S.N.; Markmann, J.; Yeh, H.; Uygun, K. The efficacy of HBOC-201 in ex situ gradual rewarming kidney perfusion in a rat model. Artif. Organs 2020, 44, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, R.N.; Patel, S.V.B.; Sun, Q.; Jiang, L.; Richard-Mohamed, M.; Ruthirakanthan, A.; Aquil, S.; Al-Ogaili, R.; Juriasingani, S.; Sener, A.; et al. Renal Protection Against Ischemia Reperfusion Injury: Hemoglobin-based Oxygen Carrier-201 Versus Blood as an Oxygen Carrier in Ex Vivo Subnormothermic Machine Perfusion. Transplantation 2020, 104, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, J.T.; Wilkerson, R.G.; Nappe, T.M. Methemoglobinemia. In StatPearls; Stat Pearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cooper, C.E.; Silaghi-Dumitrescu, R.; Rukengwa, M.; Alayash, A.I.; Buehler, P.W. Peroxidase activity of hemoglobin towards ascorbate and urate: A synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC). Biochim. Biophys. Acta 2008, 1784, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Bleilevens, C.; Doorschodt, B.M.; Fechter, T.; Grzanna, T.; Theissen, A.; Liehn, E.A.; Breuer, T.; Tolba, R.H.; Rossaint, R.; Stoppe, C.; et al. Influence of Vitamin C on Antioxidant Capacity of In Vitro Perfused Porcine Kidneys. Nutrients 2019, 11, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gifford, S.C.; Strachan, B.C.; Xia, H.; Voros, E.; Torabian, K.; Tomasino, T.A.; Griffin, G.D.; Lichtiger, B.; Aung, F.M.; Shevkoplyas, S.S. A portable system for processing donated whole blood into high quality components without centrifugation. PLoS ONE 2018, 13, e0190827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabry, G.; Doorschodt, B.M.; Grzanna, T.; Boor, P.; Elliott, A.; Stollenwerk, A.; Tolba, R.H.; Rossaint, R.; Bleilevens, C. Cold Preflush of Porcine Kidney Grafts Prior to Normothermic Machine Perfusion Aggravates Ischemia Reperfusion Injury. Sci. Rep. 2019, 9, 13897. [Google Scholar] [CrossRef] [Green Version]
- Hosgood, S.; Harper, S.; Kay, M.; Bagul, A.; Waller, H.; Nicholson, M.L. Effects of arterial pressure in an experimental isolated haemoperfused porcine kidney preservation system. Br. J. Surg. 2006, 93, 879–884. [Google Scholar] [CrossRef]
- Patel, M.; Hosgood, S.; Nicholson, M.L. The effects of arterial pressure during normothermic kidney perfusion. J. Surg. Res. 2014, 191, 463–468. [Google Scholar] [CrossRef]
- Panigrahi, M.K.; Kaliaperumal, V.; Akella, A.; Venugopal, G.; Ramadass, B. Mapping microbiome-redox spectrum and evaluating Microbial-Redox Index in chronic gastritis. Sci. Rep. 2022, 12, 8450. [Google Scholar] [CrossRef]
- Rael, L.T.; Bar-Or, R.; Salottolo, K.; Mains, C.W.; Slone, D.S.; Offner, P.J.; Bar-Or, D. Injury severity and serum amyloid A correlate with plasma oxidation-reduction potential in multi-trauma patients: A retrospective analysis. Scand. J. Trauma Resusc. Emerg. Med. 2009, 17, 57. [Google Scholar] [CrossRef] [Green Version]
- Kalenski, J.; Mancina, E.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Tothova, L.; Boor, P.; Doorschodt, B.M.; Tolba, R.H. Improved preservation of warm ischemia-damaged porcine kidneys after cold storage in Ecosol, a novel preservation solution. Ann. Transplant. 2015, 20, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Mancina, E.; Kalenski, J.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Boor, P.; Doorschodt, B.M.; Tolba, R.H. Determination of the preferred conditions for the isolated perfusion of porcine kidneys. Eur. Surg. Res. 2015, 54, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Kalenski, J.; Mancina, E.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Tothova, L.; Boor, P.; Gross, D.; Tolba, R.H.; Doorschodt, B.M. Comparison of Aerobic Preservation by Venous Systemic Oxygen Persufflation or Oxygenated Machine Perfusion of Warm-Ischemia-Damaged Porcine Kidneys. Eur. Surg. Res. 2016, 57, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Ferenz, K.B. Künstliche Sauerstoffträger—Wie lange müssen wir noch warten? Drk-Hämotherapie 2015, 25, 27–36. [Google Scholar]
- Faggiano, S.; Ronda, L.; Bruno, S.; Abbruzzetti, S.; Viappiani, C.; Bettati, S.; Mozzarelli, A. From hemoglobin allostery to hemoglobin-based oxygen carriers. Mol. Asp. Med. 2022, 84, 101050. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, Y.; He, H.; Yue, R.; Pan, L.; Hu, H.; Ren, Y.; Qin, Q.; Yi, X.; Yin, T.; et al. New Applications of HBOC-201: A 25-Year Review of the Literature. Front. Med. 2021, 8, 794561. [Google Scholar] [CrossRef]
- Linberg, R.; Conover, C.D.; Shum, K.L.; Shorr, R.G. Hemoglobin based oxygen carriers: How much methemoglobin is too much? Artif. Cells Blood Substit. Immobil. Biotechnol. 1998, 26, 133–148. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 2014, 5, 500. [Google Scholar] [CrossRef] [Green Version]
- Dunne, J.; Caron, A.; Menu, P.; Alayash, A.I.; Buehler, P.W.; Wilson, M.T.; Silaghi-Dumitrescu, R.; Faivre, B.; Cooper, C.E. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem. J. 2006, 399, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Kasil, A.; Giraud, S.; Couturier, P.; Amiri, A.; Danion, J.; Donatini, G.; Matillon, X.; Hauet, T.; Badet, L. Individual and Combined Impact of Oxygen and Oxygen Transporter Supplementation during Kidney Machine Preservation in a Porcine Preclinical Kidney Transplantation Model. Int. J. Mol. Sci. 2019, 20, 1992. [Google Scholar] [CrossRef] [Green Version]


| Group 1: HBOC + VitC | Group 3: pRBC + VitC |
| 250 mL HBOC-301 (Oxyglobin®) | 250 mL pig pRBC |
| 250 mL perfusion buffer | 250 mL perfusion buffer |
| Initial bolus of 330 μmol ascorbic acid | Initial bolus of 330 μmol ascorbic acid |
| Infusion of 330 μmol ascorbic acid/h | Infusion of 330 μmol ascorbic acid/h |
| Group 2: HBOC | Group 4: pRBC |
| 250 mL HBOC-301 (Oxyglobin®) | 250 mL pig pRBC |
| 250 mL perfusion buffer | 250 mL perfusion buffer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edgworth, E.; Ernst, L.; Czigany, Z.; Saritas, T.; Zarnitz, L.S.; Wiartalla, M.; Boor, P.; Buhl, E.M.; Rossaint, R.; Tolba, R.H.; et al. HBOC-301 in Porcine Kidney Normothermic Machine Perfusion and the Effect of Vitamin C on Methemoglobin Formation. Antioxidants 2022, 11, 1329. https://doi.org/10.3390/antiox11071329
Edgworth E, Ernst L, Czigany Z, Saritas T, Zarnitz LS, Wiartalla M, Boor P, Buhl EM, Rossaint R, Tolba RH, et al. HBOC-301 in Porcine Kidney Normothermic Machine Perfusion and the Effect of Vitamin C on Methemoglobin Formation. Antioxidants. 2022; 11(7):1329. https://doi.org/10.3390/antiox11071329
Chicago/Turabian StyleEdgworth, Eileen, Lisa Ernst, Zoltan Czigany, Turgay Saritas, Laura Sophie Zarnitz, Marc Wiartalla, Peter Boor, Eva Miriam Buhl, Rolf Rossaint, René H. Tolba, and et al. 2022. "HBOC-301 in Porcine Kidney Normothermic Machine Perfusion and the Effect of Vitamin C on Methemoglobin Formation" Antioxidants 11, no. 7: 1329. https://doi.org/10.3390/antiox11071329
APA StyleEdgworth, E., Ernst, L., Czigany, Z., Saritas, T., Zarnitz, L. S., Wiartalla, M., Boor, P., Buhl, E. M., Rossaint, R., Tolba, R. H., Doorschodt, B., Fabry, G., & Bleilevens, C. (2022). HBOC-301 in Porcine Kidney Normothermic Machine Perfusion and the Effect of Vitamin C on Methemoglobin Formation. Antioxidants, 11(7), 1329. https://doi.org/10.3390/antiox11071329









