Abstract
Our previous studies in gibel carp (Carassius gibelio) have shown that cadmium (Cd) exposure elicits deleterious effects depending on the genetic background, and thus we hypothesized that mitigation via nutritional intervention may vary between strains. Therefore, two gibel carp strains (the A and F strains) were fed diets supplemented with 0% or 1% taurine for 8 weeks prior to 96 h Cd exposure, and the responses of antioxidant pathways, endoplasmic reticulum (ER) stress, autophagy, and apoptosis were investigated. The results showed that taurine supplementation had no effect on the growth performance of gibel carp. After Cd exposure, histological damage to mitochondria and ER, induction of oxidative stress and antioxidant responses, occurrence of ER stress, and apoptotic signals were observed in the livers. Upon the diet effects, taurine supplementation alleviated the ER-stress-induced autophagy and apoptosis after Cd exposure and stimulated antioxidant pathways. Regarding the difference between strains, taurine played a protective role in alleviating Cd toxicity through the antioxidant response, ER stress, and autophagy in the F strain, whereas such effects were achieved by the attenuation of apoptosis in the A strain. Taken together, our results demonstrate the potential use of taurine in the mitigation of heavy metal toxicity in aquatic organisms.
1. Introduction
Owing to widespread environmental pollution, the diverse hazardous impacts of exposure to toxic heavy metals on living organisms are becoming a global issue of great concern [1]. Cadmium (Cd) is one of the most abundant environmental pollutants in the biosphere, and it can be both toxic and carcinogenic [2,3]. Compared to other animals, aquatic species are vulnerable to Cd toxicity via the dietborne as well as the waterborne routes [4,5]. Therefore, aquatic toxicological evaluation of the effects of Cd has been widely investigated in teleosts under chronic or acute exposure in species such as gilthead sea bream, tilapia, yellow perch, and gibel carp (Carassius gibelio) [6,7,8,9].
Cadmium is reported to elicit deleterious effects via neurotoxicity, immunotoxicity, induction of oxidative stress, damage to organ structure, and cellular dysfunction [2,10]. Much effort has been made to investigate the mechanism of Cd toxicity and to develop a safe therapeutic approach to mitigating the toxic effects [1]. Some chemopreventive agents such as garlic extract containing specific organosulfur compounds have been used to protect against the toxic effects of Cd in both animal models and cell lines [11,12]. Cd exposure disrupts the cellular oxidative homeostasis [13] that is regulated by various enzymatic or non-enzymatic antioxidants. Importantly, oxidative stress and glutathione (GSH) depletion are crucial components of Cd toxicity in aquatic organisms [14]. Therefore, nutrients with antioxidant properties have been applied to ameliorate the hepatotoxicity by modulating antioxidant pathways, such nutrients include vitamin C, vitamin E, carotenoids, and selenium [1,15].
Taurine (TAU, 2-amino ethanesulfonic acid), as a semi-essential amino acid, is a derivative of a sulfur-containing amino acid that has multiple functions in fish physiology [16]. Taurine is usually supplemented as an additive in the diet of aquatic animals for the promotion of growth as well as boosting the reproduction system, immune functions, and antioxidant effects [17]. The mechanism of the antioxidant activity of taurine was reported to be associated with enhanced mitochondrial function that protects the mitochondria from excessive superoxide [18]. In addition, taurine has been considered as a promising candidate for the improvement of liver function, and it has been reported as possessing tissue protective effects in treating oxidant-induced injury [19,20]. In mammalian models, taurine has been reported to alleviate the toxic effects of copper, lead, aluminum, and cadmium [21,22,23]. Similarly, administration of taurine affected hepatic metabolism and reduced Cd contamination in red sea bream and catfish [16,24]. Nevertheless, the mechanisms underlying the ameliorative effects of taurine against Cd poisoning in teleost fish are still not fully elaborated.
Previous studies have shown that exposure to Cd caused different toxic effects in gibel carp (Carassius gibelio) A strain (CAS III) and F strain (CAS V), regardless of whether via the dietborne or waterborne routes [9,25]. Specifically, these two strains of gibel carp showed genetically based metabolic strategies in response to Cd toxicity, verifying the fact that differences in genetic background may be an important cause of metabolic differences between fish strains. To ascertain the potential of taurine in the prevention of Cd poisoning and to explore whether these effects would vary between the two strains, experiments were performed with taurine supplementation via the diet route in the present study. We assessed the liver functions of the two strains, because the liver is the center of intermediary metabolism and plays vital roles in detoxifying processes [26,27].
2. Materials and Methods
2.1. Experimental Procedures
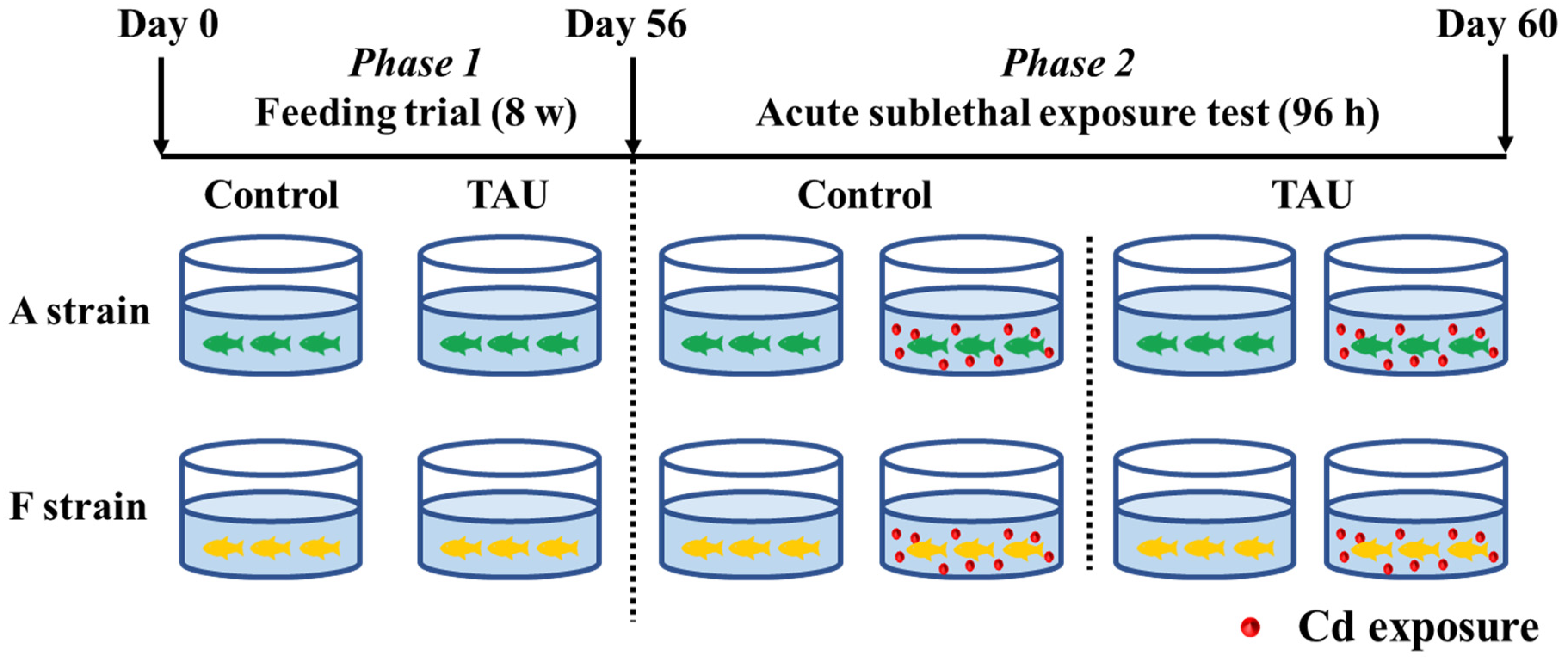
The experimental scheme is illustrated in Figure 1. Gibel carp used in this trial were obtained from the hatchery of the Institute of Hydrobiology, the Chinese Academy of Sciences, Wuhan, Hubei, China. The healthy and uniformly sized gibel carp A (4.61 ± 0.03 g) and F (4.58 ± 0.04 g) strains were fed diets supplemented with 0% (Control) or 1% TAU for 8 weeks (Figure 1, Phase 1). Diets were formulated in the laboratory according to the procedures described by Li et al. [28]. The diet formulation and approximate composition are shown in Table 1. Therefore, four groups of fish were obtained: the A strain fed the control diet (A0), the A strain fed a 1% TAU supplemented diet (A1), the F strain fed the control diet (F0), and the F strain fed a 1% TAU supplemented diet (F1).
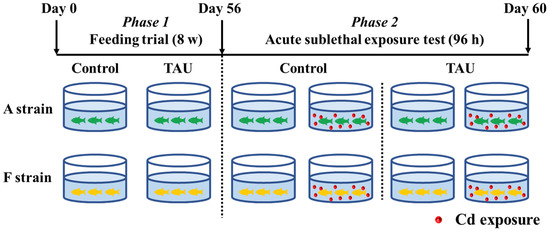
Figure 1.
Experimental design. Control: control diet; TAU: diet supplemented with taurine.

Table 1.
Ingredients and proximate composition of the experimental diets (g/kg).
After the 8-week feeding trial, a challenge test was conducted with fish from each of the four groups by exposing the fish to acute waterborne Cd (11.9 mg/L) (Figure 1, Phase 2). Cd exposure was performed in a static aquarium system with continuous aeration for 96 h, with 10 fish per tank and triplicate replicates for each tank. The concentration of Cd was set based on the value shown by a preliminary experiment that identified the 96 h median lethal concentration (LC50) [25]. CdCl2·2.5 H2O was added to the water by diluting a stock solution according to methods described by Li et al. [25]. During the acute exposure experiment, water in the system was refreshed daily. This experiment was implemented following the guiding principles for the care and use of laboratory animals and was approved by the Institute of Hydrobiology, Chinese Academy of Sciences.
2.2. Sample Collection
At the end of the 96 h Cd waterborne experiment, fish were anesthetized with MS-222 solution (Aminobenzoate methanesulfonate, 0.06 g/L, Sigma, St. Louis, MO, USA). The livers of two fish from each tank were dissected immediately on ice, with one part frozen in liquid nitrogen and then stored at −80 °C, and one part fixed in 2.5% glutaraldehyde solution and 4% paraformaldehyde.
2.3. Transmission Electron Microscopy (TEM) Observation
The liver samples of the two strains were dissected into 1 mm3 cubes and then fixed immediately in 2.5% glutaraldehyde solution. The samples were then rinsed with 0.1 M phosphate buffer solution (pH = 7.4) three times (15 min each time). Postfixation was conducted with 1% osmium tetroxide for 2 h, and then the fixed samples were washed three times with 0.1 M phosphate buffer solution (pH = 7.4). The dehydration was performed in a graded ethanol series followed by acetone. After that, samples were infiltrated with acetone:SPI-Pon 812 resin (1:1) followed by acetone:SPI-Pon 812 resin (1:2) and SPI-Pon 812 resin. Subsequently, the samples were embedded in SPI-Pon 812 resin for 48 h at 60 °C. Ultra-thin sections (80–100 nm) were stained with uranyl acetate and lead citrate. Finally, observations were conducted using a transmission electron microscope (Tecnai G2 20 TWIN, FEI, Hillsboro, OR, USA).
2.4. TUNEL Analysis
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses were performed according to the procedure described by Li et al. [25]. The liver samples were fixed in 4% paraformaldehyde and then embedded in paraffin. After that, the samples were cut into 5 μm sections and deparaffinized in dimethylbenzene. The samples were dehydrated in a graded ethanol series, repaired with proteinase K, and permeabilized with Triton X-100/PBS solutions. DNA fragmentation was determined using TdT and dUTP reagents (1:9) for 2 h incubation followed by staining with 4′,6-diamidino-2-phenylindole (DAPI, 0.3 mmol/L) for 10 min. The samples were examined under a Nikon Eclipse Ti-SR inverted microscope.
2.5. Chemical and Biochemical Analyses
Cd concentrations in water samples were measured in accordance with the National Standards of the Republic of China (GB/T 7475-1987, Water quality determination of copper, zinc, lead and cadmium—atomic absorption spectrometry). In summary, sample digestion was conducted by adding hydrogen peroxide and concentrated nitric acid to the samples. After adding palladium nitrate, the samples were tested via inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer Optima 8000, Waltham, MA, USA).
The activities of total antioxidant capacity (T-AOC), superoxide dismutase (SOD), reduced glutathione (GSH), glutathione peroxidase (GSH-Px), catalase (CAT), and the contents of malondialdehyde (MDA) were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). The activity of caspase 3 (Casp3) in livers was tested using a commercial kit (Caspase 3 Activity Assay Kit, Be-yotime Biotechnology, Shanghai, China).
2.6. qRT-PCR Analysis
Total RNA from liver samples was extracted using TRIzol reagent (Ambion, Life Technologies, Austin, TX, USA) according to the manufacturer’s instructions. The RNA integrity and purity were assessed by agarose gel electrophoresis and NanoDrop spectrophotometer determination, respectively. The cDNA was synthesized by reverse transcription using an M-MLV First-Strand Transcriptase kit (Invitrogen, Carlsbad, CA, USA). All quantitative real-time PCR (qRT-PCR) assays were performed on a LightCycler 480 System (Roche, Jena, Thüringen, Germany). The primers used for quantitative RT-PCR are shown in Table 2. The housekeeping gene tubulin was chosen to normalize the relative quantification of target genes according to the methods described by Pfaffl [29].

Table 2.
Sequences of the primers used for qRT-PCR analysis in gibel carp.
2.7. Statistical Analysis
Results are presented as means ± standard errors. Normality and homoscedasticity of the data were assessed by Shapiro–Wilk and Levene tests. Two-way analysis of variance (ANOVA) was conducted with SPSS 26.0 (Chicago, IL, USA), and p < 0.05 was considered as a significant difference. Independent t-tests were conducted to examine the differences between pre- and post-challenge test groups. Gene expression heatmap of genes related to antioxidation, ER stress, autophagy, and apoptosis were created using heatmapper (http://www.heatmapper.ca/ accessed on 4 July 2022).
3. Results
3.1. Growth Performance
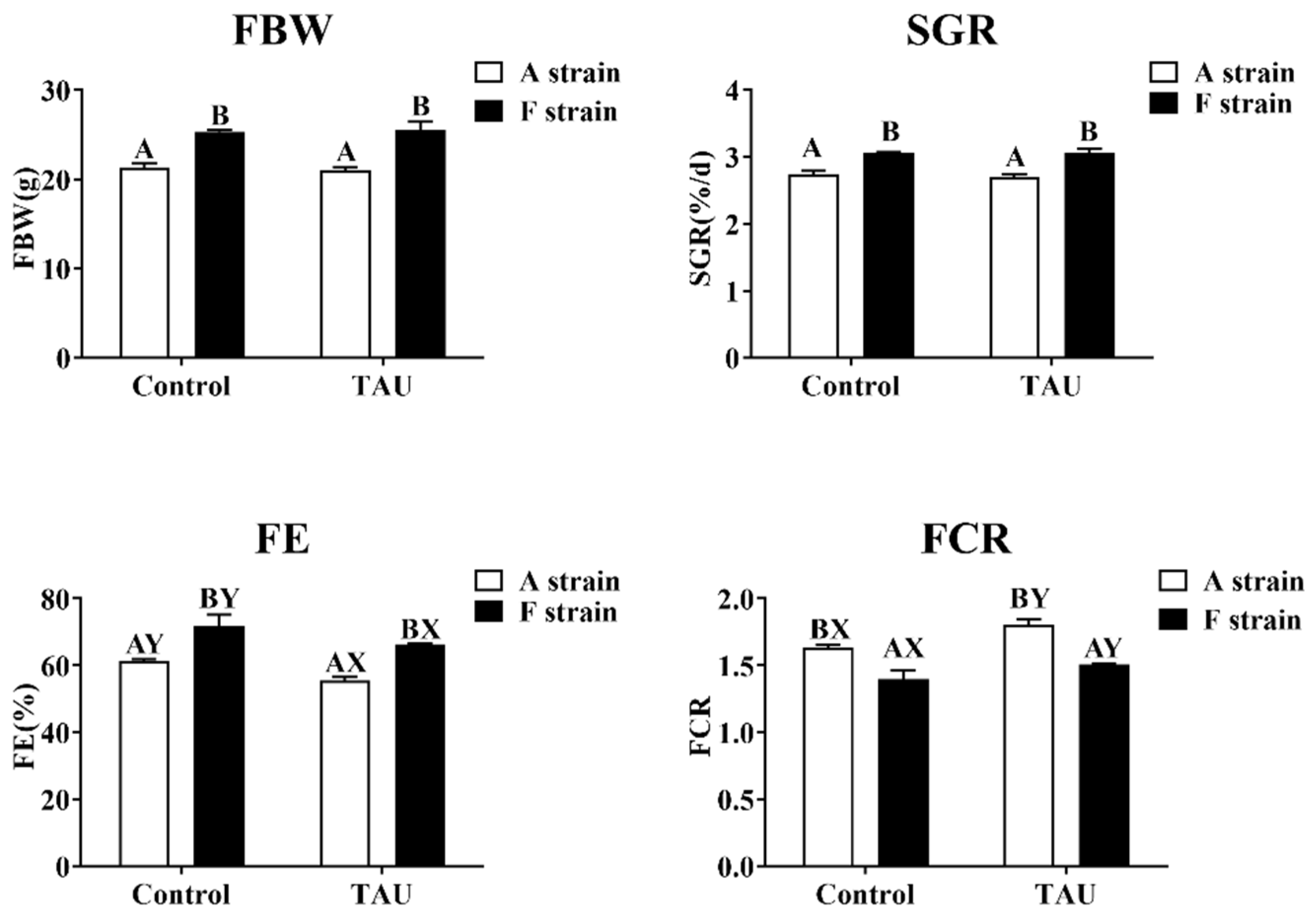
No significant differences in final body weight (FBW) or specific growth rate (SGR) were observed in the two strains of gibel carp fed diets with taurine supplementation (Figure 2). However, dietary taurine supplementation significantly decreased the feed efficiency (FE) and increased the feed conversion ratio (FCR) in both strains. The F strain presented significantly higher FBW, SGR, and FE and lower FCR than the A strain (p < 0.05). During the experiment, the survival rate of fish was 100%.
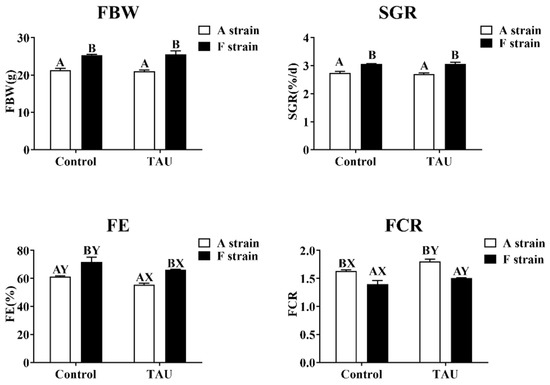
Figure 2.
Specific growth rate and feed efficiency of two gibel carp strains fed the control diet and diet supplemented with taurine. Control: control diet, white bars; TAU: diet supplemented with taurine, black bars. FBW: final body weight; SGR: specific growth rate (%/d) = 100 × [ln (final weight) − ln (initial weight)]/day; FE: feeding efficiency (%) = (100 × body weight gain)/dry feed intake; FCR: feed conversion ratio = (100 × dry feed intake)/body weight gain. Bars with different uppercase letters (A, B) represent significant differences between the A and F strains (p < 0.05). Bars with different upper-case letters (X, Y) represent significant differences between the control diet group and the taurine diet group (p < 0.05).
3.2. Histological Observation
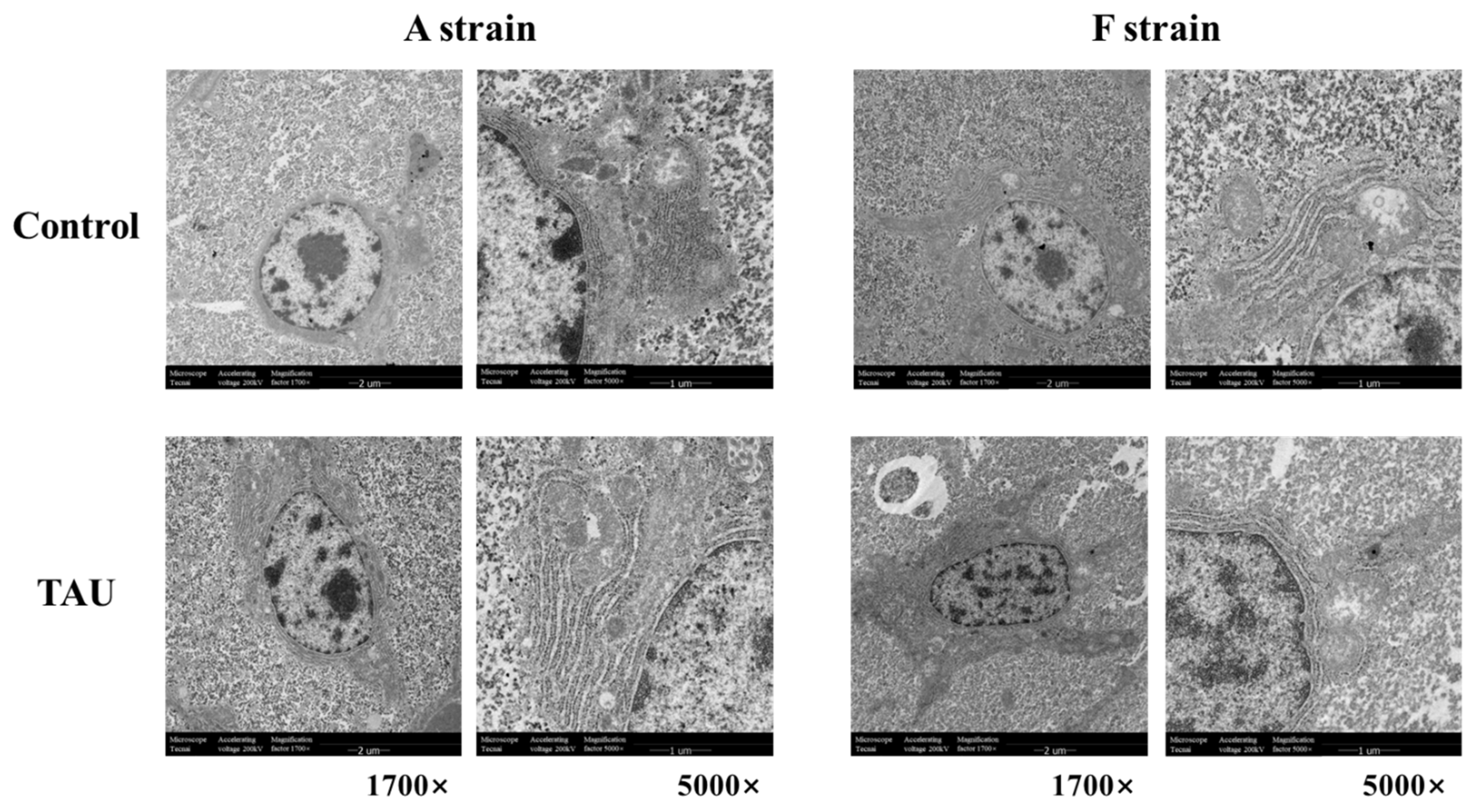
Ultrastructural images of the liver in the two gibel carp strains exposed to Cd are shown in Figure 3. Cd exposure induced ultrastructural alterations in the two gibel carp strains, as shown by the degenerated cristae and swelling of mitochondria. Meanwhile, irregular parallel stacked endoplasmic reticulum and plaque accumulation within hepatocytes were detected by transmission electron microscopy.
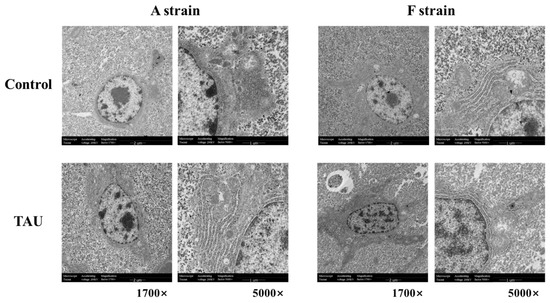
Figure 3.
Representative histological transmission electron microscopy (TEM) images of gibel carp (A and F strains) after 96 h cadmium exposure. Control: control diet; TAU: diet supplemented with taurine.
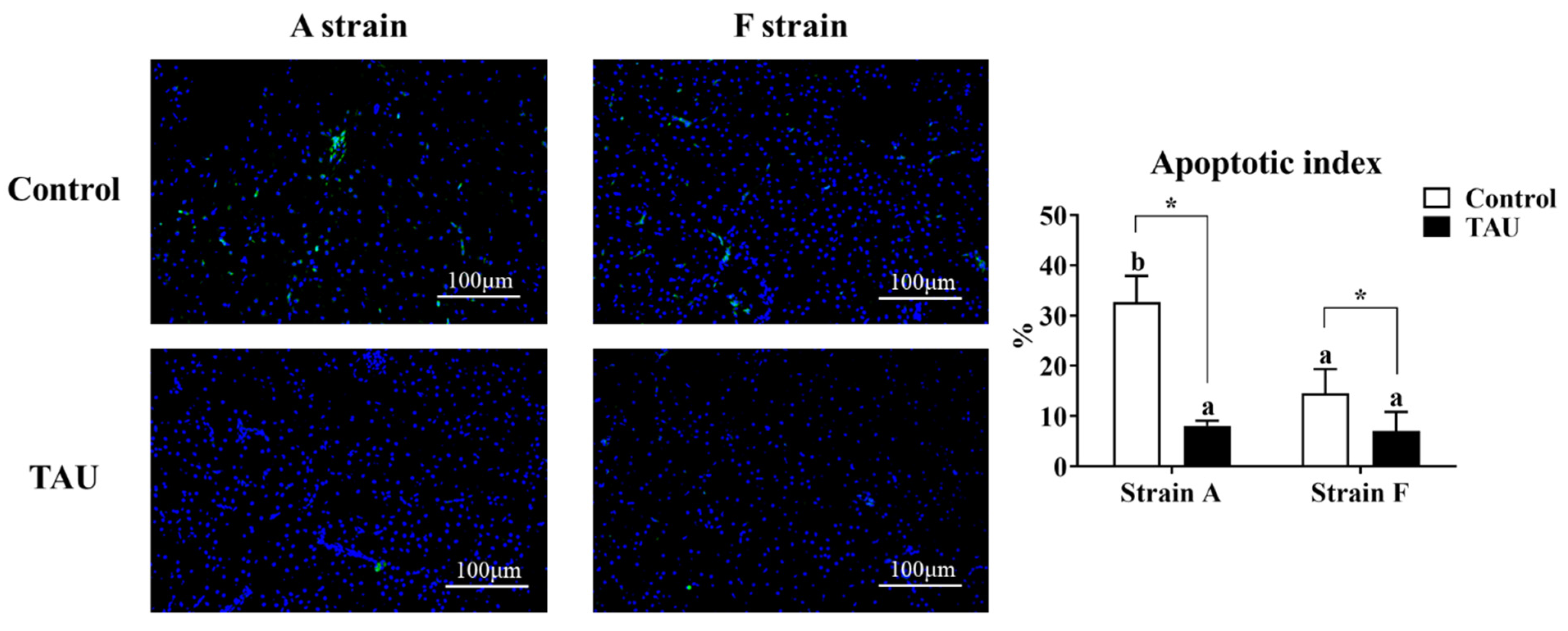
A terminal deoxynucleotidyl transferase dUTP nick end labeling assay (TUNEL) was conducted to assess the apoptosis index in the livers of gibel carp (Figure 4). The results showed that apoptosis signals increased significantly after Cd exposure (p < 0.05), while dietary taurine supplementation decreased the apoptosis index compared with the control group. Moreover, the apoptosis index in the A strain fed the control diet was the highest (p < 0.05).
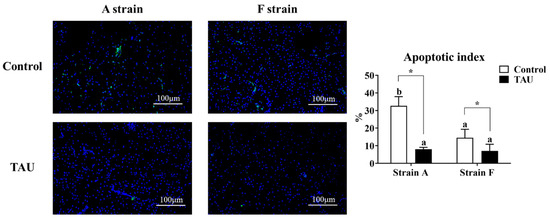
Figure 4.
Representative DAPI and TUNEL double staining and image quantification results from the livers of gibel carp (A and F strains) after 96 h cadmium exposure. Positive apoptotic cells appear in green, and normal nuclei appear in blue. Control: control diet, white bars; TAU: diet supplemented with taurine, black bars. Bars with different lowercase letters (a, b) indicate the interaction effect and represent significant differences among groups (p < 0.05). Bars with * indicate significant changes between diets in the same strain (p < 0.05). The magnification factor is 200×, and the scale bar is 100 μm.
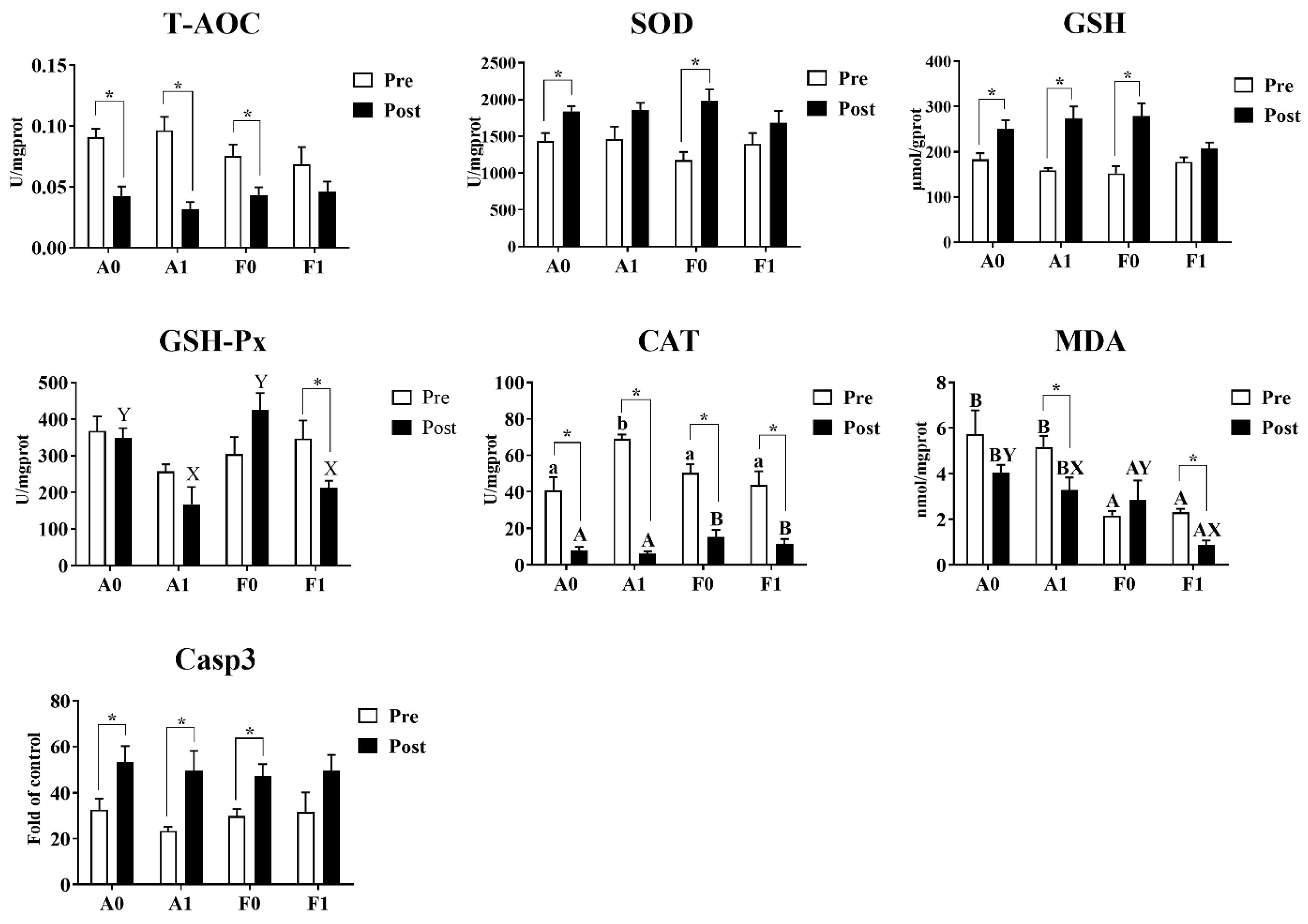
3.3. Activities of the Antioxidant and Caspase Enzymes
Before Cd exposure, the enzyme activities of T-AOC, SOD, GSH-Px, Casp3, and contents of GSH showed no significant variation among treatments (Figure 5). Dietary taurine supplementation significantly enhanced the activities of CAT in the A strain compared to other groups (p < 0.05), while MDA contents were significantly lower in the F strain than in the A strain (p < 0.05). After Cd exposure, no significant differences were found among groups in the activities of T-AOC, SOD, or Casp3 or in GSH content. The A strain had significantly higher GSH-Px activities and MDA contents than the F strain (p < 0.05). For the diet effects, dietary taurine supplementation elevated the CAT activities, whereas taurine decreased the MDA content after Cd exposure in both strains.
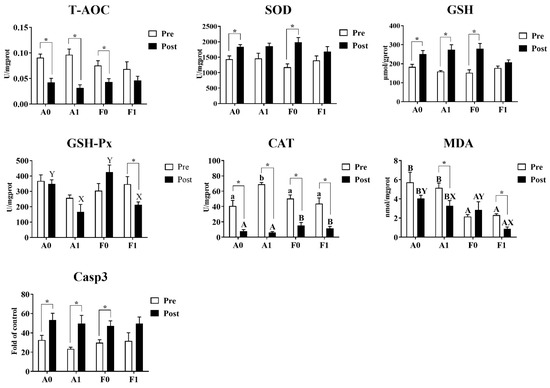
Figure 5.
Antioxidant indices and Casp3 activity in the livers of gibel carp (A and F strains) before cadmium exposure (white bars) and after cadmium exposure (black bars). A0: A strain fed the control diet; A1: A strain fed a diet supplemented with taurine; F0: A strain fed the control diet; F1: A strain fed a diet supplemented with taurine. Bars with different uppercase letters (A, B) represent significant differences between the A and F strains (p < 0.05). Bars with different uppercase letters (X, Y) represent significant differences between the control diet group and the taurine diet group (p < 0.05). Bars with different lowercase letters (a, b) indicate the interaction effect and represent significant differences among all groups (p < 0.05). Bars with * indicate significant changes between before and after cadmium exposure (p < 0.05).
Cd exposure significantly inhibited the activities of CAT in both strains of gibel carp and reduced the content of MDA in the F strain. For both gibel carp strains, Cd exposure suppressed the activity of T-AOC, whereas the activity of Casp3 and the contents of GSH were elevated. However, the F strain fed the diet with taurine supplementation did not show significant differences in the activities of T-AOC or Casp3 or in GSH content. Among all groups, only the F strain given dietary taurine supplementation showed significant reduction in the activity of GSH-Px (p < 0.05).
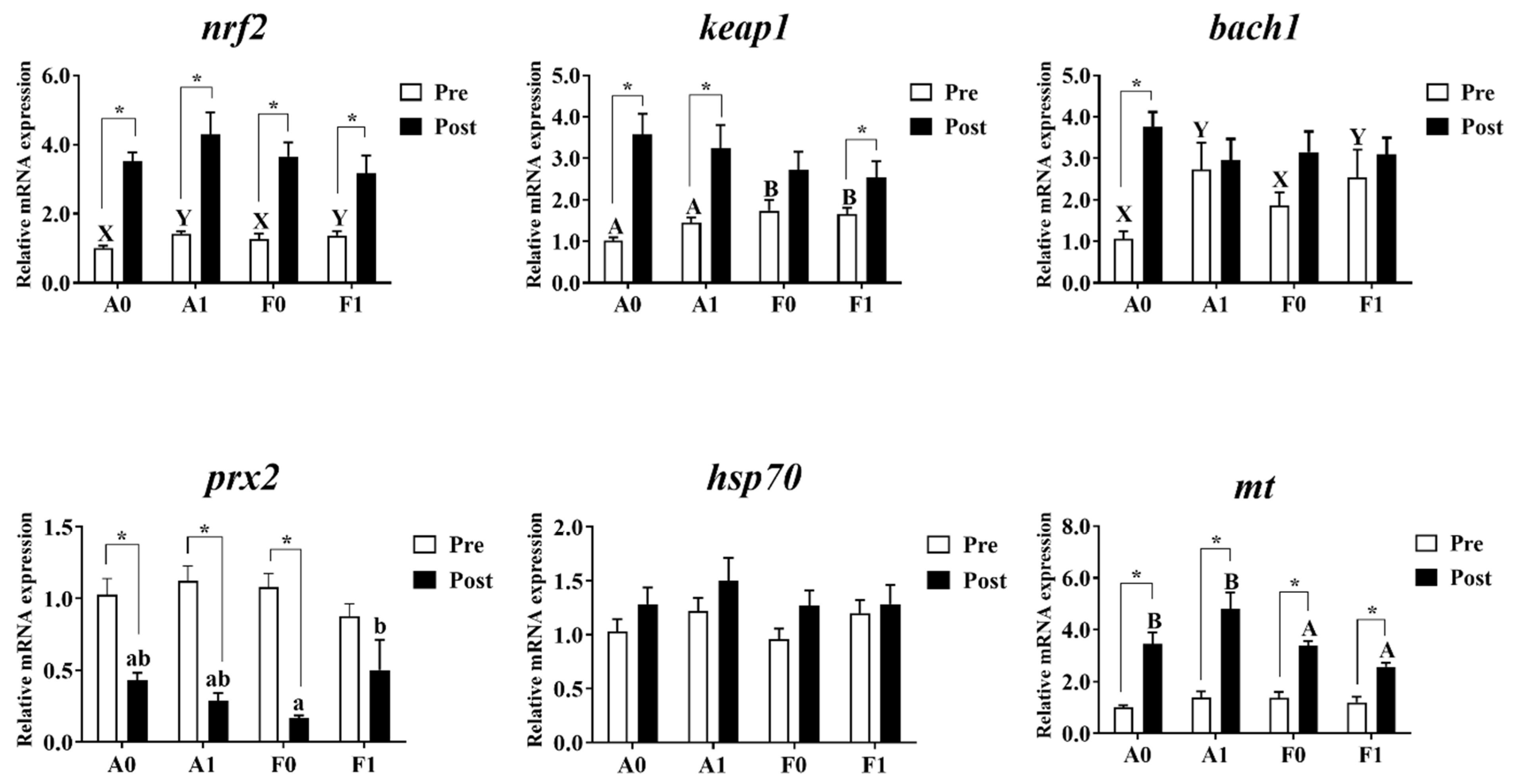
3.4. Antioxidant Pathways and Metallothionein Levels
The expression levels of antioxidant genes and metallothionein are shown in Figure 6. Prior to Cd exposure, the gene expression levels of prx2, hsp70, and mt showed no significant differences among groups (p > 0.05). Dietary taurine supplementation significantly upregulated the expression of bach1 and nrf2 in both strains compared to the control group (p < 0.05). The F strain showed significantly higher mRNA levels of keap1 than the A strain (p < 0.05).
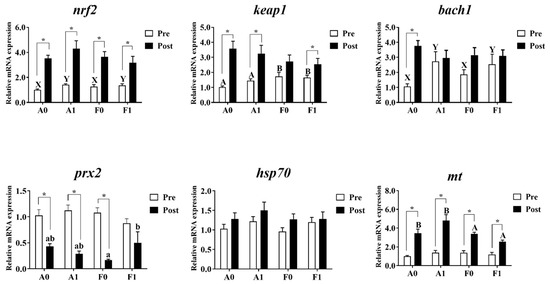
Figure 6.
Expression levels of genes related to antioxidation and metallothionein (mt) in the livers of gibel carp (A and F strains) before cadmium exposure (white bars) and after cadmium exposure (black bars). A0: A strain fed the control diet; A1: A strain fed a diet supplemented with taurine; F0: A strain fed the control diet; F1: A strain fed a diet supplemented with taurine. Bars with different uppercase letters (A, B) represent significant differences between the A and F strains (p < 0.05). Bars with different uppercase letters (X, Y) represent significant differences between the control diet group and the taurine diet groups (p < 0.05). Bars with different lowercase letters (a, b) indicate a significant interaction effect and represent the differences among all the groups (p < 0.05). Bars with * indicate significant changes between before and after cadmium exposure (p < 0.05).
After Cd exposure, no significant variation was observed in the mRNA levels of bach1, keap1, nrf2, or hsp70 among all groups (p > 0.05). The A strain showed significantly higher mt mRNA levels than the F strain (p < 0.05). Interactions were identified in the expression of prx2, with the highest levels found in the F strain given dietary taurine supplementation (p < 0.05). Cd exposure enhanced the expression levels of nrf2 and mt in both strains, while the A strain showed significant elevation of the mRNA level of bach1 (p < 0.05). The expression of prx2 was significantly upregulated after Cd exposure (p < 0.05), while no significant differences were observed in the F strains (p > 0.05). The expression of keap1 was significantly higher after Cd exposure (p < 0.05), except for the F strain fed with the control diet.
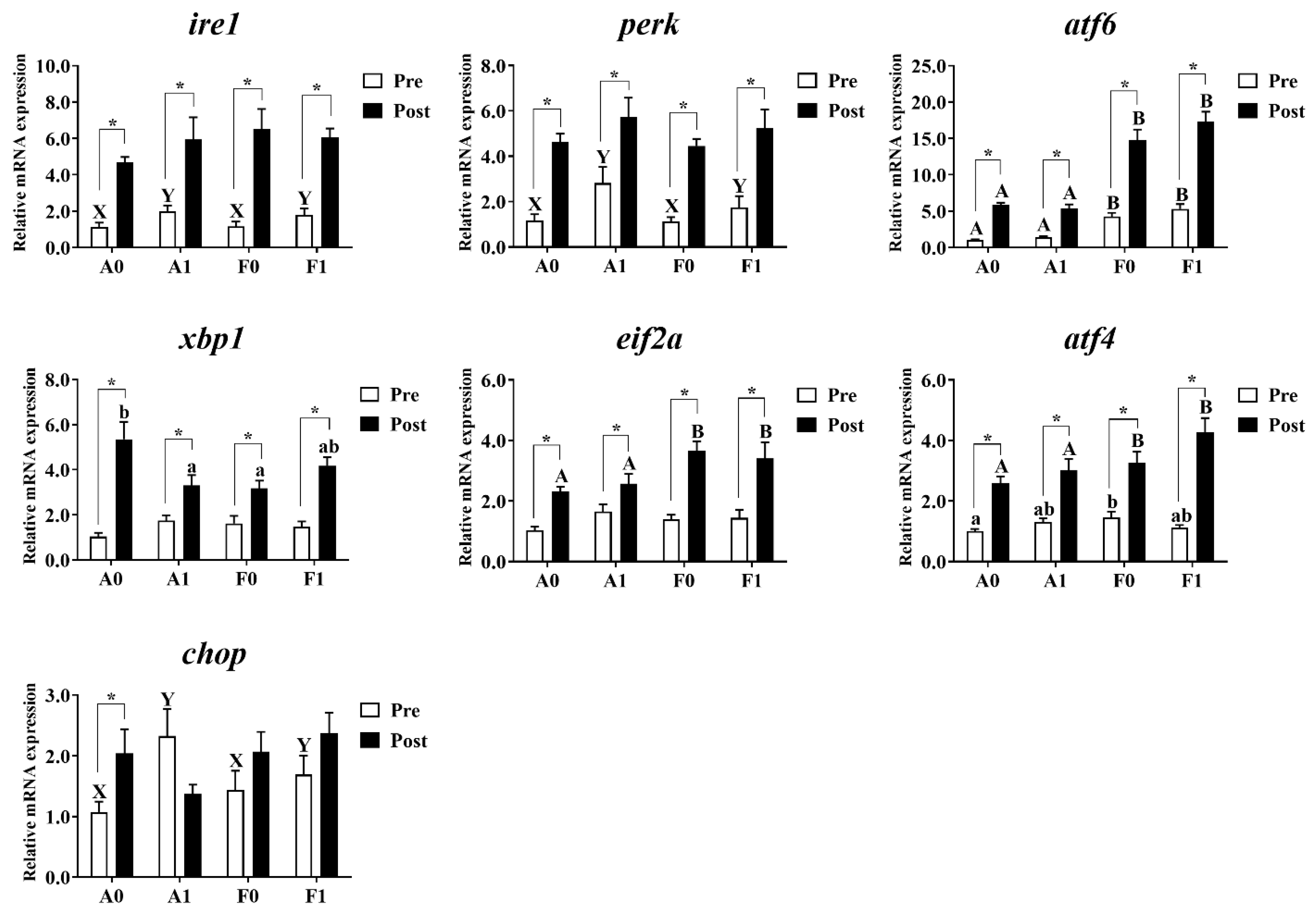
3.5. ER Stress
As shown in Figure 7, no significant differences were observed in the expression of xbp1 or eif2a among all groups before Cd exposure (p > 0.05). Dietary taurine supplementation significantly upregulated the mRNA levels of ire1, perk, and chop in the livers of both strains compared to the control group. The F strain had markedly higher levels of atf6 than the A strain (p < 0.05). Diets interacted with strains to affect the expression of atf4 in the livers of gibel carp, with the F strain showed the highest levels among all groups (p < 0.05).
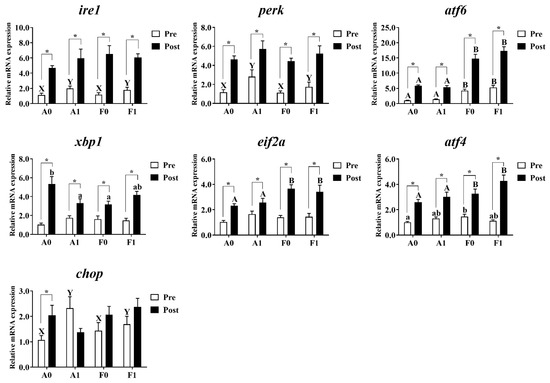
Figure 7.
Expression levels of genes related to ER stress in the livers of gibel carp (A and F strains) before cadmium exposure (white bars) and after cadmium exposure (black bars). A0: A strain fed the control diet; A1: A strain fed a diet supplemented with taurine; F0: A strain fed the control diet; F1: A strain fed a diet supplemented with taurine. Bars with different uppercase letters (A, B) represent significant differences between the A and F strains (p < 0.05). Bars with different uppercase letters (X, Y) represent significant differences between the control diet and taurine diet groups (p < 0.05). Bars with different lowercase letters (a, b) indicate the interaction effect and represent the differences among all groups (p < 0.05). Bars with * indicate significant changes between before and after cadmium exposure (p < 0.05).
After Cd exposure, no significant variation was observed in the expression of ire1, perk, or chop among all groups (p > 0.05). The F strain showed significantly higher expression levels of atf6, eif2a, and atf4 than the A strain (p < 0.05). Interactions were observed in the mRNA level of xbp1, with the highest level found in the A strain fed the control diet (p < 0.05). Cd exposure significantly induced higher mRNA levels of ire1, perk, atf6, xbp1, eif2a, and atf4 in the livers of both strains, whereas the A strain fed the control diet had the higher chop mRNA levels (p < 0.05).
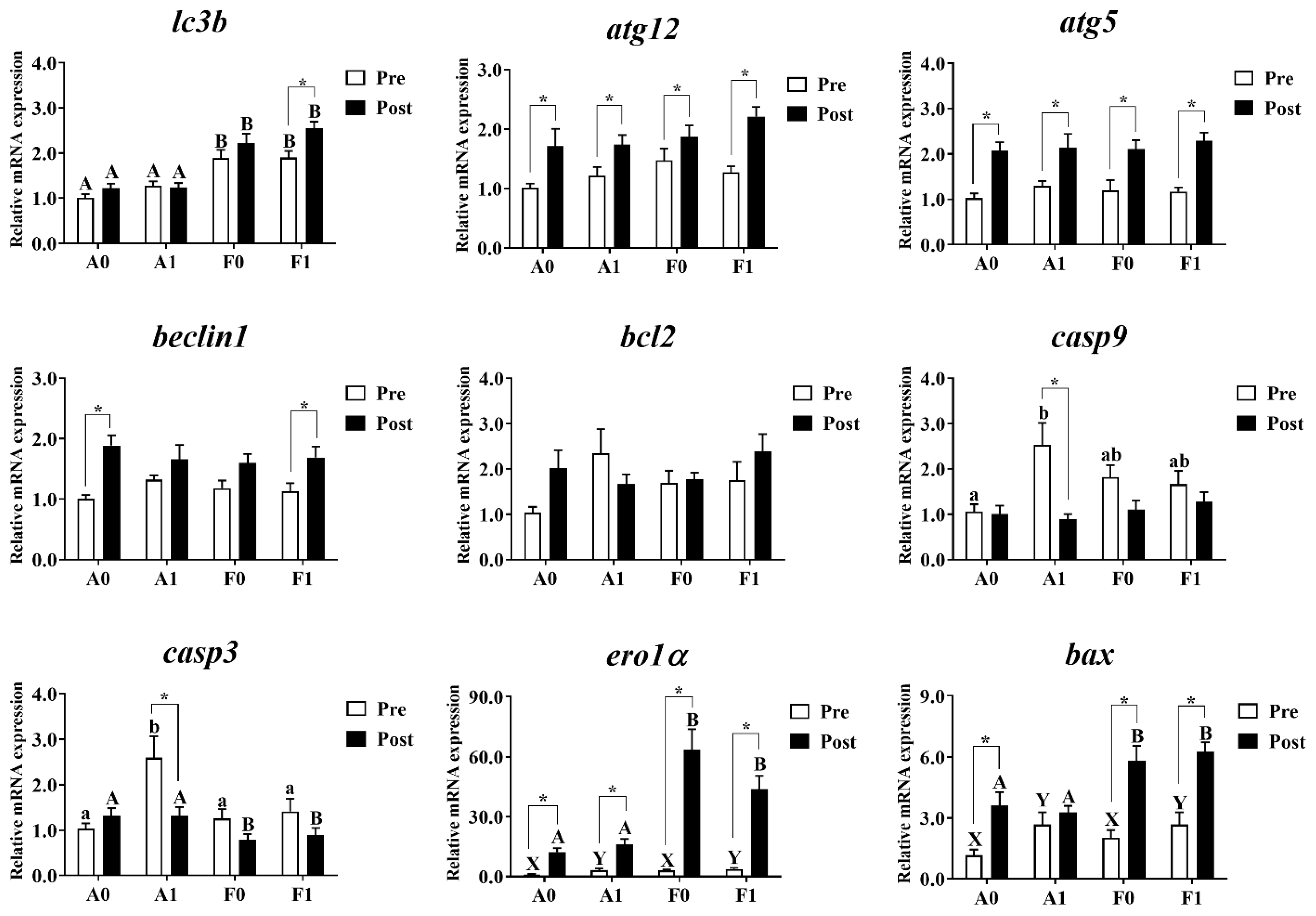
3.6. Autophagy and Apoptosis
Hepatic mRNA levels related to autophagy and apoptosis were investigated in both strains (Figure 8). Prior to Cd exposure, no significant differences were found in the expression of atg12, atg5, beclin1, or bcl2 (p > 0.05). The mRNA levels of lc3b in the A strain were significantly lower than in the F strain among all groups (p < 0.05). Dietary taurine supplementation elevated the expression of ero1α and bax (p < 0.05). The diets and strains interacted to affect the expression of casp9 and casp3, with the highest levels found in the A strain fed the taurine diet (p < 0.05).
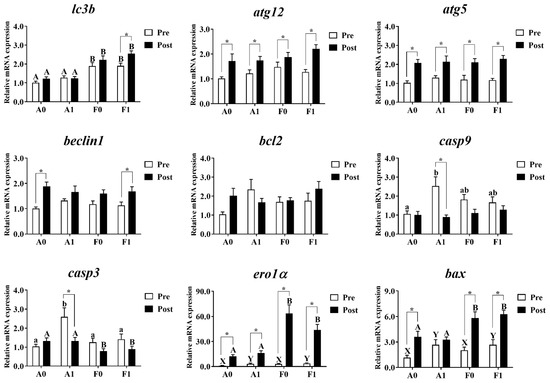
Figure 8.
Expression levels of genes related to autophagy and apoptosis in the livers of gibel carp (A and F strains) before cadmium exposure (white bars) and after cadmium exposure (black bars). A0: A strain fed the control diet; A1: A strain fed a diet supplemented with taurine; F0: A strain fed the control diet; F1: A strain fed a diet supplemented with taurine. Bars with different uppercase letters (A, B) represent significant differences between A and F strains (p < 0.05). Bars with different upper-case letters (X, Y) represent significant differences between the control diet group and the taurine diet group (p < 0.05). Bars with different lowercase letters (a, b) indicate the interaction effect and represent the differences among all groups (p < 0.05). Bars with * mean significant changes between before and after cadmium exposure (p < 0.05).
After Cd exposure, the expression of atg12, atg5, beclin1, bcl2, and casp9 showed no significant differences among all groups (p > 0.05). The A strain showed significantly higher levels of lc3b, ero1α, and bax and significantly lower levels of casp9 than the F strain (p < 0.05). Cd exposure significantly upregulated the mRNA levels of atg12, atg5, and ero1α in the livers of both strains. However, the upregulated levels of lc3b were only found in the F strain fed the taurine diet. The increased expression of beclin1 was found in the A strain fed the control diet and the F strain fed the taurine diet (p < 0.05). The A strain subjected to the taurine diet had significantly higher hepatic mRNA levels of casp3 and casp9. Cd exposure induced significant upregulation of the expression of bax among all groups (p < 0.05) except for the A strain fed the diet with taurine supplementation (p > 0.05).
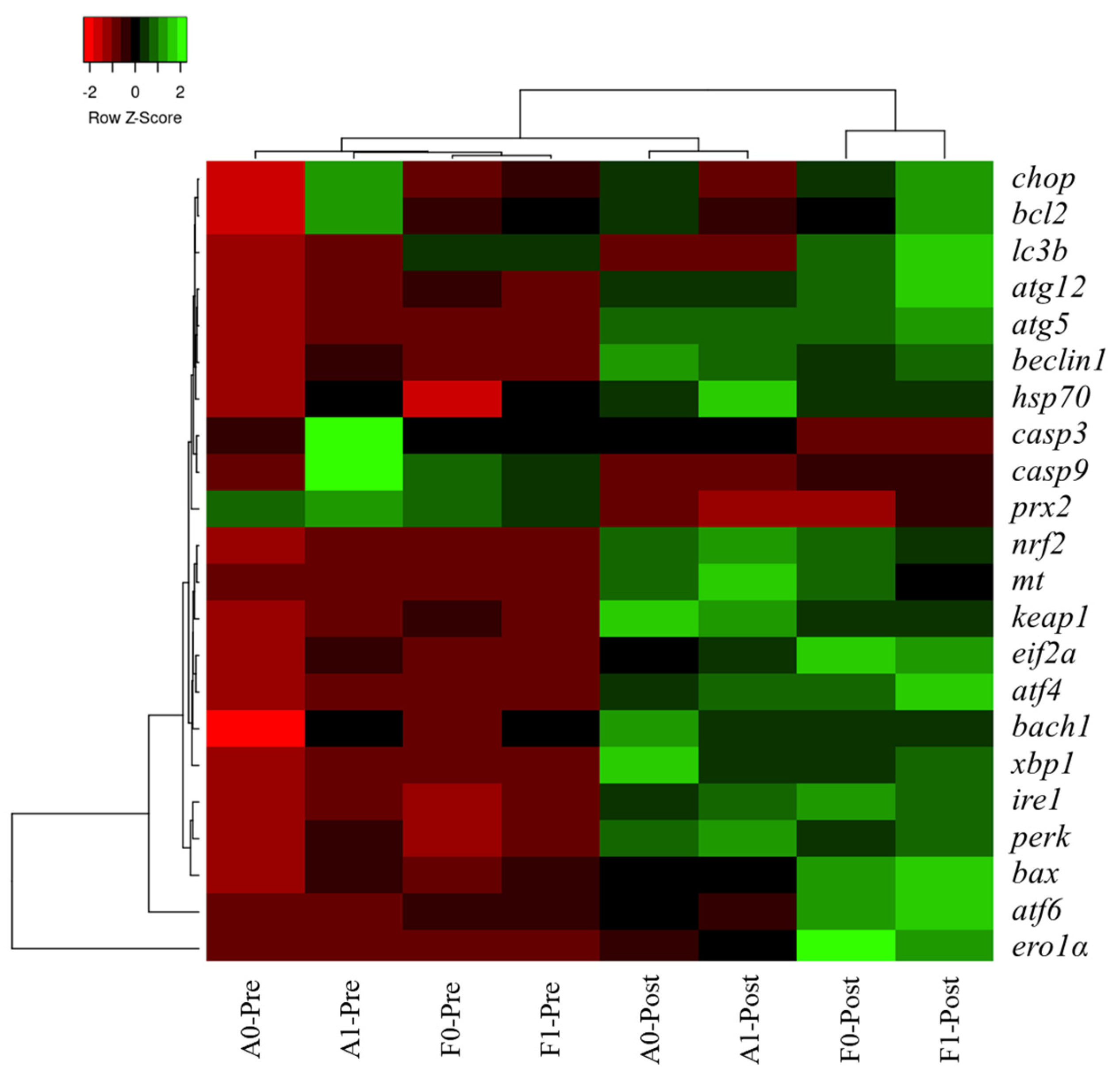
3.7. Heatmap Cluster Analysis
The mean values of molecular (gene expression) signatures of all treatments are presented in the clustering heatmap (Figure 9). Obvious differences were observed in the two strains before or after Cd exposure. Molecular expression of genes involved in antioxidant response, ER stress, and autophagy in the F strain after Cd exposure was not in a cluster with other treatments, especially in the F strain fed the taurine diet.
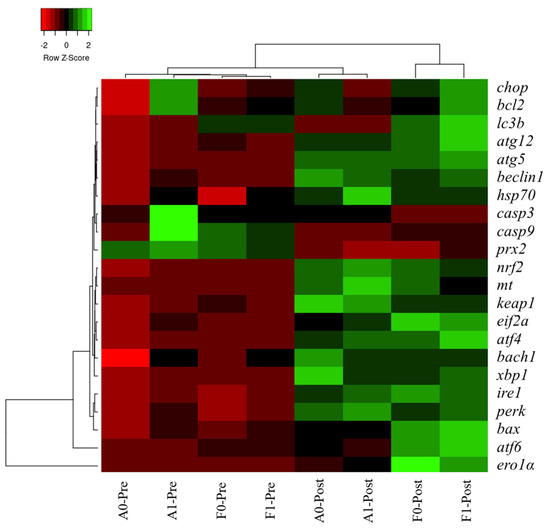
Figure 9.
Gene expression heatmap of genes related to antioxidation, ER stress, autophagy, and apoptosis in the livers of gibel carp.
4. Discussion
Taurine has been reported to have beneficial effects on the growth performance of aquatic animals fed low fish meal diets in species such as black carp and white shrimp [30,31]. In the Phase 1 period of the present study, no significant effects on FBW or SGR were observed in either strain of gibel carp subjected to 8 weeks of taurine supplementation. Consistent with our results, the positive effects of dietary taurine supplementation (0, 0.5, 1.0, 1.5, and 2.0%) on growth improvement in yellowtail disappeared after six weeks of feeding, although higher final body weight was observed in fish fed a taurine diet for three weeks [32]. Dietary taurine supplementation significantly decreased FE and increased FCR in both strains. When the dietary taurine level exceeds the basic nutritional requirement, this can lead to feed intake reduction, as has been reported in Nile tilapia [33]. Therefore, the effects of dietary taurine supplement on growth of aquatic animals may be dose or time dependent.
As a semi-essential amino acid, taurine has physiological functions in the antioxidant and anti-apoptosis responses. Being a non-essential heavy metal, Cd exerts its effects and causes damage to tissues primarily through peroxidation and apoptosis [34]. Reports have shown that dietary taurine supplementation could mitigate Cd toxicity in catfish and red sea bream [16,24]. In the present study, histological observations showed that 96 h Cd exposure caused degenerated cristae, swelling of mitochondria, and irregular parallel stacked endoplasmic reticulum, and plaques in the cytoplasm of hepatocytes of both strains, suggesting that Cd damaged the mitochondria and endoplasmic reticulum in the liver cells of gibel carp. Meanwhile, Cd triggered apoptosis signals, as shown by the TUNEL results. Nevertheless, the apoptosis index was significantly lower in both strains fed diets with taurine supplementation compared to the control groups. Therefore, dietary taurine supplementation could apparently mitigate Cd-induced hepatic damage in gibel carp as in catfish and red sea bream [16,24]. Metallothionein (mt) is considered as a biomarker in the Cd detoxification process, as it can combine with Cd to form a Cd-MT complex [35]. In the present study, hepatic mRNA levels of mt increased significantly after Cd exposure, indicating that Cd triggered the protective proteins to counteract the damage to the liver. To further elucidate the potential protective effects of taurine in gibel carp against Cd toxicity, we investigated the antioxidant response, ER stress, autophagy, and apoptosis.
Induction of oxidative stress is one of the toxicological mechanisms involved in heavy metal stress in fish, where the production of ROS (reactive oxygen species) causes oxidative damage to cells. Previous studies have shown that the hepatic enzyme activity of SOD increased significantly in rainbow trout after 7 days of waterborne Cd exposure [36]. Meanwhile, 21 days of waterborne Cd exposure enhanced the hepatic enzyme activity of SOD in catfish [37]. In the present study, Cd exposure elevated the SOD activity in gibel carp fed the control diet. However, no significant differences were found in hepatic SOD activities in fish fed diets with taurine supplementation, implying the protective role of taurine against Cd exposure in gibel carp. MDA is considered as a biomarker of the lipid peroxidation level under oxidative stress [38]. Dietary taurine supplementation significantly decreased MDA levels in the liver, which is consistent with the results for hepatic SOD activity. Cd exposure suppressed the enzyme activity of CAT in the livers of both strains, but the F strain showed higher levels than the A strain. Moreover, Cd exposure inhibited the activities of T-AOC while elevating the activity of Casp3 and the contents of GSH. However, no significant differences were observed in the activities of T-AOC or Casp3 or in the content of GSH in the F strain fed the taurine diet. Additionally, even though the activity of GSH-Px showed no variation among groups, the lowest level was found in the F strain fed the diet with taurine supplementation. Overall, taurine may exert its protective function against Cd poisoning more efficiently in the F strain than in the A strain.
Nuclear factor erythroid 2-related factor 2 (nrf2) is a key transcriptional factor involved in the regulation of the cellular antioxidant response [39]. Nrf2 regulates downstream antioxidant-related genes such as keap1, prx2, bach1, and hsp70 to alleviate oxidative stress in organisms [40]. In the present study, the nrf2 signaling pathway was activated, as indicated by upregulation of nrf2 mRNA levels in both strains fed the taurine supplemented diet. In zebrafish, the nrf2 pathway demonstrated protective effects by mitigating Cd-induced cellular oxidative damage [41]. Before Cd exposure, the expression levels of nrf2 and bach1 were significantly higher in both strains fed diets with taurine supplementation than in the control groups, indicating that dietary taurine could enhance the antioxidant potential of gibel carp, while such beneficial effects were not observed after the Cd exposure. Moreover, Cd exposure downregulated the expression levels of prx2, except in the F strain fed the diet with taurine supplementation. Taken together, the results suggest that taurine had a protective role against Cd-induced damage in both strains, especially in the F strain.
The endoplasmic reticulum is a dynamic organelle that is responsible for folding and assembly of proteins [42]. ER stress and its downstream signaling pathways play a crucial regulatory role in response to heavy-metal-induced toxic effects [43]. Previous studies had indicated that Cd waterborne could induce ER stress in both strains of gibel carp [25]. In the present study, the expression levels of the ER-stress-related genes ire1, perk, atf6, xbp1, eif2α, and atf4 were increased after Cd exposure. In other words, all branches of the regulatory pathways of PERK-eIF2a-ATF4, IRE1-XBP1, and ER stress transducers ATF6 were induced after Cd exposure, suggesting the occurrence of ER stress in gibel carp exposed to Cd. The phosphorylation dependence of PERK induces dissociation of Nrf2/Keap1 complexes, thereby triggering the transcription of downstream genes involved in antioxidant pathways [44]. The expression level of perk had a variation trend similar to that of nrf2. Meanwhile, hepatic histological alterations such as swelling of mitochondria and irregular parallel stacks of ER were triggered by Cd exposure, observations that confirmed the ER stress in gibel carp.
Autophagy refers to a catabolic process in which cytoplasmic constituents and organelles in the lysosome are degraded to maintain homeostasis as an adaptative response to stressful conditions [45]. Autophagic pathways can be triggered through induction of ER sensors under long-lasting ER stress [46]. It has been reported that Cd exposure could cause such a stress response, eliciting ER-stress-mediated autophagic and apoptosis processes in both strains of gibel carp [9,25]. Moreover, the formation of autophagosomes requires two ubiquitin-like conjugation pathways: one involves the formation of the multimeric complex of ATG5-ATG12-ATG16 conjugation; the other results in the conjugation of phosphatidylethanolamine (PE) to LC3b for the expansion of autophagic membranes [45,47]. In the present study, Cd exposure upregulated the hepatic mRNA levels of atg5 and atg12 in both strains regardless of the diet effect, suggesting that autophagic processes may be triggered by the increasing level of the ATG5-ATG12 complex. The mRNA levels of lc3b were only elevated in the F strain fed with the taurine diet, implying that more conjugation pathways were stimulated in the F strain. Thus, stronger autophagy may have been triggered in the F strain fed the taurine diet. Furthermore, Beclin-1 is a critical regulator of autophagy, because it participates in the formation of autophagosomes [48]. The transcriptional levels of beclin1 were increased in the A strain fed the control diet and the F strain fed the taurine diet. Taken together, the results suggest that Cd exposure induced the autophagic process, and stronger autophagy responses were observed in the F strain fed the taurine diet.
Autophagy may play a protective role in cell survival, and extensive autophagy may trigger apoptosis as an independent pathway of cell death [49]. Apoptosis is also known as a cellular biomarker of metal-induced physiological alterations in aquatic animals [50]. Cd exposure was reported to induce apoptosis in topsmelt, purse red common carp, and gibel carp [25,51,52]. Apoptosis can be triggered by three main pathways, one of which is upstream caspase activation and includes the enzymes Caspase 9 and Caspase 3 [53]. In the present study, the transcriptional levels of casp9 and casp3 were inhibited in the A strain fed the diet supplemented with taurine. The mRNA levels of casp3 were not consistent with the Casp3 activities, possibly due to a feedback response; a similar result has been reported in Litopenaeus vannamei [54]. Meanwhile, the apoptotic index in the A strain after Cd exposure was higher than in other groups as shown by the TUNEL results, suggesting that Cd exposure caused higher levels of apoptosis, and dietary taurine supplementation manifested its antioxidant effects through the regulation of the caspase gene in the A strain. In the apoptotic process, bcl2 is a member of the anti-apoptosis protein family, while bax and ero1α have opposite functions [47,55]. In the present study, no significant variation in bcl2 expression was found after exposure to Cd, while the expression of ero1α was significantly elevated, indicating that apoptosis was induced by Cd exposure. The mRNA levels of bax increased after Cd exposure in all groups, but unaltered mRNA levels of bax were found in the A strain fed taurine, implying that dietary taurine supplementation alleviated the Cd toxicity by attenuating apoptosis in the A strain compared to the F strain.
Hierarchy cluster heatmap analysis showed that significant differences were observed in the two strains before or after Cd exposure, which verified the effects induced by Cd exposure as mentioned above. Expression levels of genes involved in antioxidant response, ER stress, and autophagy in the F strain post Cd exposure was not in cluster with other treatments, especially in the F strain fed the taurine diet, which was in line with previous results. Differential responses between the A and F strains of gibel carp were investigated in our previous studies owing to their genetic differences produced by selection [9,25]. The A strain was produced from eggs of gibel carp D strain and the sperm of gibel carp A strain, while the F strain was produced by the eggs of gibel carp E strain via stimulation with blunt snout bream sperm [56,57]. Therefore, a partial genome from the blunt snout bream may have been introduced into the genome of the F strain; this may have caused genetic differences between the A and F strains that led to differential genomic expression between the two strains upon Cd exposure. In the present study, even the growth performance was not significantly improved by dietary taurine supplement, but the detoxication of taurine might help to increase the survival rate of fish and raise fish quality, thereby improving the economic benefits.
5. Conclusions
Our study found that Cd exposure induced damage and oxidative stress in the livers of both strains of gibel carp, thereby triggering the occurrence of ER stress and the downstream responses of autophagy and apoptosis. Dietary taurine supplementation had no significant effect on the growth performance of gibel carp but did alleviate the Cd toxicity in both strains via specific genetic pathways. Dietary taurine played a protective role in mitigating Cd toxicity in the F strain through the antioxidant response, ER stress response, and autophagy, while in the A strain taurine alleviated cadmium toxicity by attenuation of apoptosis. In conclusion, the present study has provided evidence for the use of taurine in intervention or therapy for Cd poisoning in fish; thus, providing useful information for selective breeding in aquaculture.
Author Contributions
Conceptualization, investigation, methodology, validation, visualization, writing—review and editing, funding acquisition, W.X.; Conceptualization, data curation, methodology, formal analysis, writing—original draft, H.L. (Hongyan Li); Data curation, L.W.; Conceptualization, funding acquisition, project administration, supervision, writing—review and editing, J.J.; Supervision, D.H., X.Z., Y.Y. and H.L. (Haokun Liu); Funding acquisition, investigation, supervision, S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the China Agriculture Research System of MOF and MARA (CARS-45-09), the National Natural Science Foundation of China (32122089; U19A2041; 32102811; 31972805), the National Key R&D Program of China (2018YFD0900605; 2019YFD0900200) and the Strategic Priority Research Program of Chinese Academy of Sciences (XDA24010206).
Institutional Review Board Statement
All animal care and experimental procedures were approved by the Experimental Animal Ethics Committee of Institute of Hydrobiology, Chinese Academy of Sciences (approval ID: IHB20140724).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank Guanghan Nie for his technical support with the research system.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninkov, M.; Popov Aleksandrov, A.; Demenesku, J.; Mirkov, I.; Mileusnic, D.; Petrovic, A.; Grigorov, A.; Zolotarevski, L.; Tolinacki, M.; Kataranovski, D.; et al. Toxicity of oral cadmium intake: Impact on gut im-munity. Toxicol. Lett. 2015, 237, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Welbourn, P.M. Cadmium in the aquatic environment: A review of ecological, physiological, and toxicological effects on biota. Environ. Rev. 1994, 2, 187–214. [Google Scholar] [CrossRef]
- Deforest, D.K.; Meyer, J.S. Critical review: Toxicity of dietborne metals to aquatic organisms. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1176–1241. [Google Scholar] [CrossRef]
- Driessnack, M.K.; Jamwal, A.; Niyogi, S. Effects of chronic waterborne cadmium and zinc interactions on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Ecotoxicol. Environ. Saf. 2017, 140, 65–75. [Google Scholar] [CrossRef]
- Basha, P.S.; Rani, A.U. Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicol. Environ. Saf. 2003, 56, 218–221. [Google Scholar] [CrossRef]
- Defo, M.A.; Bernatchez, L.; Campbell, P.G.C.; Couture, P. Waterborne cadmium and nickel impact oxidative stress responses and retinoid metabolism in yellow perch. Aquat. Toxicol. 2014, 154, 207–220. [Google Scholar] [CrossRef]
- Isani, G.; Andreani, G.; Cocchioni, F.; Fedeli, D.; Carpené, E.; Falcioni, G. Cadmium accumulation and biochemical responses in Sparus aurata following sub-lethal Cd exposure. Ecotoxicol. Environ. Saf. 2009, 72, 224–230. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Wu, L.; Dong, B.; Jin, J.; Han, D.; Zhu, X.; Yang, Y.; Liu, H.; Xie, S. Distinct dietary cadmium toxic effects and defense strategies in two strains of gibel carp (Carassius gibelio) revealed by a comprehensive perspective. Chemosphere 2020, 261, 127597. [Google Scholar] [CrossRef]
- Méndez-Armenta, M.; Ríos, C. Cadmium neurotoxicity. Environ. Toxicol. Pharmacol. 2007, 23, 350–358. [Google Scholar] [CrossRef]
- Lawal, A.O.; Ellis, E.M. The chemopreventive effects of aged garlic extract against cadmium-induced toxicity. Environ. Toxicol. Pharmacol. 2011, 32, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Obioha, U.E.; Suru, S.M.; Ola-Mudathir, K.F.; Faremi, T.Y. Hepatoprotective Potentials of Onion and Garlic Extracts on Cadmium-Induced Oxidative Damage in Rats. Biol. Trace Elem. Res. 2008, 129, 143. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Lucia, M.; André, J.-M.; Gonzalez, P.; Baudrimont, M.; Bernadet, M.-D.; Gontier, K.; Maury-Brachet, R.; Guy, G.; Davail, S. Effect of dietary cadmium on lipid metabolism and storage of aquatic bird Cairina moschata. Ecotoxicology 2009, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.S.; El-Gazzar, A.M.; El-Nahas, A.F.; Ashry, K.M. Vitamin C modulates cadmium-induced hepatic antioxidants’ gene transcripts and toxicopathic changes in Nile tilapia, Oreochromis niloticus. Environ. Sci. Pollut. Res. 2016, 23, 1664–1670. [Google Scholar] [CrossRef]
- Hano, T.; Ito, K.; Kono, K.; Ito, M.; Ohkubo, N.; Mochida, K. Effect of taurine supplementation on hepatic metabolism and alleviation of cadmium toxicity and bioaccumulation in a marine teleost, red sea bream, Pagrus major. Fish Physiol. Biochem. 2017, 43, 137–152. [Google Scholar] [CrossRef]
- Sampath, W.W.H.A.; Rathnayake, R.M.D.S.; Yang, M.; Zhang, W.; Mai, K. Roles of dietary taurine in fish nutrition. Mar. Life Sci. Technol. 2020, 2, 360–375. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef]
- Takeuchi, T. Progress on larval and juvenile nutrition to improve the quality and health of seawater fish: A review. Fish. Sci. 2014, 80, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Chen, K.; Wei, N.; Zhang, Q.; Liu, J.; Mi, M. Dietary taurine reduces retinal damage produced by photochemical stress via antioxidant and anti-apoptotic mechanisms in Sprague-Dawley rats. Br. J. Nutr. 2007, 98, 711–719. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, W.M.; Al-Kahtani, M.A.; Abdel-Moneim, A.M. Prophylactic and therapeutic effects of taurine against aluminum-induced acute hepatotoxicity in mice. J. Hazard. Mater. 2011, 192, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Wang, L.C.; Cheng, H.M. Effect of taurine on toxicity of copper in rats. Food Chem. Toxicol. 1998, 36, 239–244. [Google Scholar] [CrossRef]
- Hwang, D.F.; Wang, L.C. Effect of taurine on toxicity of cadmium in rats. Toxicology 2001, 167, 173–180. [Google Scholar] [CrossRef]
- Kumar, P.; Prasad, Y.; Patra, A.K.; Ranjan, R.; Swarup, D.; Patra, R.C.; Pal, S. Ascorbic acid, garlic extract and taurine alleviate cadmium-induced oxidative stress in freshwater catfish (Clarias batrachus). Sci. Total Environ. 2009, 407, 5024–5030. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Wu, L.; Dong, B.; Jin, J.; Han, D.; Zhu, X.; Yang, Y.; Liu, H.; Xie, S. Differential regulation of endoplasmic reticulum stress-induced autophagy and apoptosis in two strains of gibel carp (Carassius gibelio) exposed to acute waterborne cadmium. Aquat. Toxicol. 2021, 231, 105721. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Ribeyre, F.; Tourencq, J.-N.; Boudou, A. Interspecific comparison of cadmium and zinc contamination in the organs of four fish species along a polymetallic pollution gradient (Lot River, France). Sci. Total Environ. 2000, 248, 11–25. [Google Scholar] [CrossRef]
- Potter, B. Liver-Intermediary Metabolism. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–6. [Google Scholar]
- Li, H.; Xu, W.; Jin, J.; Zhu, X.; Yang, Y.; Han, D.; Liu, H.; Xie, S. Effects of dietary carbohydrate and lipid concentrations on growth performance, feed utilization, glucose, and lipid metabolism in two strains of gibel carp. Front. Vet. Sci. 2019, 6, 165. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, Y.; Tian, L.; Gan, L.; Yang, H.; Liang, G.; He, J. The effect of dietary taurine supplementation on growth performance, feed utilization and taurine contents in tissues of juvenile white shrimp (Litopenaeus vannamei, Boone, 1931) fed with low-fishmeal diets. Aquac. Res. 2013, 44, 1317–1325. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Ai, Q.; Mao, P.; Tian, Q.; Zhong, L.; Xiao, T.; Chu, W. Effect of dietary taurine supplementation on growth performance, digestive enzyme activities and antioxidant status of juvenile black carp (Mylopharyngodon piceus) fed with low fish meal diet. Aquac. Res. 2018, 49, 3187–3195. [Google Scholar] [CrossRef]
- Matsunari, H.; Takeuchi, T.; Takahashi, M.; Mushiake, K. Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata. Fish. Sci. 2005, 71, 1131–1135. [Google Scholar] [CrossRef]
- Al-Feky, S.S.A.; El-Sayed, A.-F.M.; Ezzat, A.A. Dietary taurine enhances growth and feed utilization in larval Nile tilapia (Oreochromis niloticus) fed soybean meal-based diets. Aquac. Nutr. 2016, 22, 457–464. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Dang, F.; Wang, W.X. Assessment of tissue-specific accumulation and effects of cadmium in a marine fish fed contaminated commercially produced diet. Aquat. Toxicol. 2009, 95, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hisar, O.; Yildirim, S.; Sönmez, A.Y.; Aras, H.N.; Gultepe, N. Changes in liver and kidney antioxidant enzyme activities in the rainbow trout (Oncorhynchus mykiss) exposed cadmium. Asian J. Chem. 2009, 21, 3133–3139. [Google Scholar]
- Asagba, S.O.; Eriyamremu, G.E.; Igberaese, M.E. Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus). Fish Physiol. Biochem. 2008, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Luo, Z.; Li, C.-H.; Xiong, B.-X.; Zhao, Y.-H.; Li, X.-D. Antioxidant responses, hepatic intermediary metabolism, histology and ultrastructure in Synechogobius hasta exposed to waterborne cadmium. Ecotoxicol. Environ. Saf. 2011, 74, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Liu, J.J.; Klaassen, C.D. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol. Appl. Pharmacol. 2012, 263, 14–20. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose protects against oxidative stress by regulating the Keap1–Nrf2 and autophagy pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef]
- Wang, L.; Gallagher, E.P. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol. Appl. Pharmacol. 2013, 266, 177–186. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Luo, Z.; Hogstrand, C.; Chen, G.; Wei, C.; Li, D. Zn Stimulates the phospholipids biosynthesis via the pathways of oxidative and endoplasmic reticulum stress in the intestine of freshwater teleost yellow catfish. Environ. Sci. Technol. 2018, 52, 9206–9214. [Google Scholar] [CrossRef] [Green Version]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 Is a Direct PERK Substrate and Effector of PERK-Dependent Cell Survival. Mol. Cell. Biol. 2003, 23, 7198–7209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Ogata, M.; Hino, S.-I.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy Is Activated for Cell Survival after Endoplasmic ReticulumStress. Am. Soc. Microbiol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xia, Q.; Zhou, Y.; Li, J. Endoplasmic reticulum stress and autophagy contribute to cadmium-induced cytotoxicity in retinal pigment epithelial cells. Toxicol. Lett. 2019, 311, 105–113. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Li, Y.; Chen, N.; Fan, Y.; Huang, W.; Hu, S.; Rao, M.; Zhang, Y.; Su, P. Cross-talk between autophagy and apoptosis regulates testicular injury/recovery induced by cadmium via PI3K with mTOR-independent pathway. Cell Death Dis. 2020, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Agnello, M.; Roccheri, M.C. Apoptosis: Focus on sea urchin development. Apoptosis 2010, 15, 322–330. [Google Scholar] [CrossRef]
- Capaldo, A.; Gay, F.; Scudiero, R.; Trinchella, F.; Caputo, I.; Lepretti, M.; Marabotti, A.; Esposito, C.; Laforgia, V. Histological changes, apoptosis and metallothionein levels in Triturus carnifex (Amphibia, Urodela) exposed to environmental cadmium concentrations. Aquat. Toxicol. 2016, 173, 63–73. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Z.E.; Qiao, P.; Liu, S.; Zhang, L.; He, P.; Zhang, X.; Wang, Y.; Min, W. Cadmium induces liver cell apoptosis through caspase-3a activation in purse red common carp (Cyprinus carpio). PLoS ONE 2013, 8, e83423. [Google Scholar]
- Rose, W.L.; Nisbet, R.M.; Green, P.G.; Norris, S.; Fan, T.; Smith, E.H.; Cherr, G.N.; Anderson, S.L. Using an integrated approach to link biomarker responses and physiological stress to growth impairment of cadmium-exposed larval topsmelt. Aquat. Toxicol. 2006, 80, 298–308. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Chang, C.; Yeh, M.; Lin, H.; Cheng, W. The effect of Vibrio alginolyticus infection on caspase-3 expression and activity in white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2008, 25, 672–678. [Google Scholar] [CrossRef]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell. Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wang, Y.; Zhang, Q.-Y.; Mou, C.-Y.; Li, Z.; Deng, Y.-S.; Zhou, L.; Gui, J.-F. Distinct herpesvirus resistances and immune responses of three gynogenetic clones of gibel carp revealed by comprehensive transcriptomes. BMC Genom. 2017, 18, 561. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.-Y.; Zhou, L.; Yu, P.; Wang, Z.-W.; Li, Z.; Zhang, X.-J.; Wang, Y.; Gui, J.-F. Stable Genome Incorporation of Sperm-derived DNA Fragments in Gynogenetic Clone of Gibel Carp. Mar. Biotechnol. 2020, 22, 54–66. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).