Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Design
2.3. Histological Examination and Immunohistochemistry
2.4. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) and Biochemical Analyses
2.6. Real-Time PCR
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Effects of COS on Body Weight
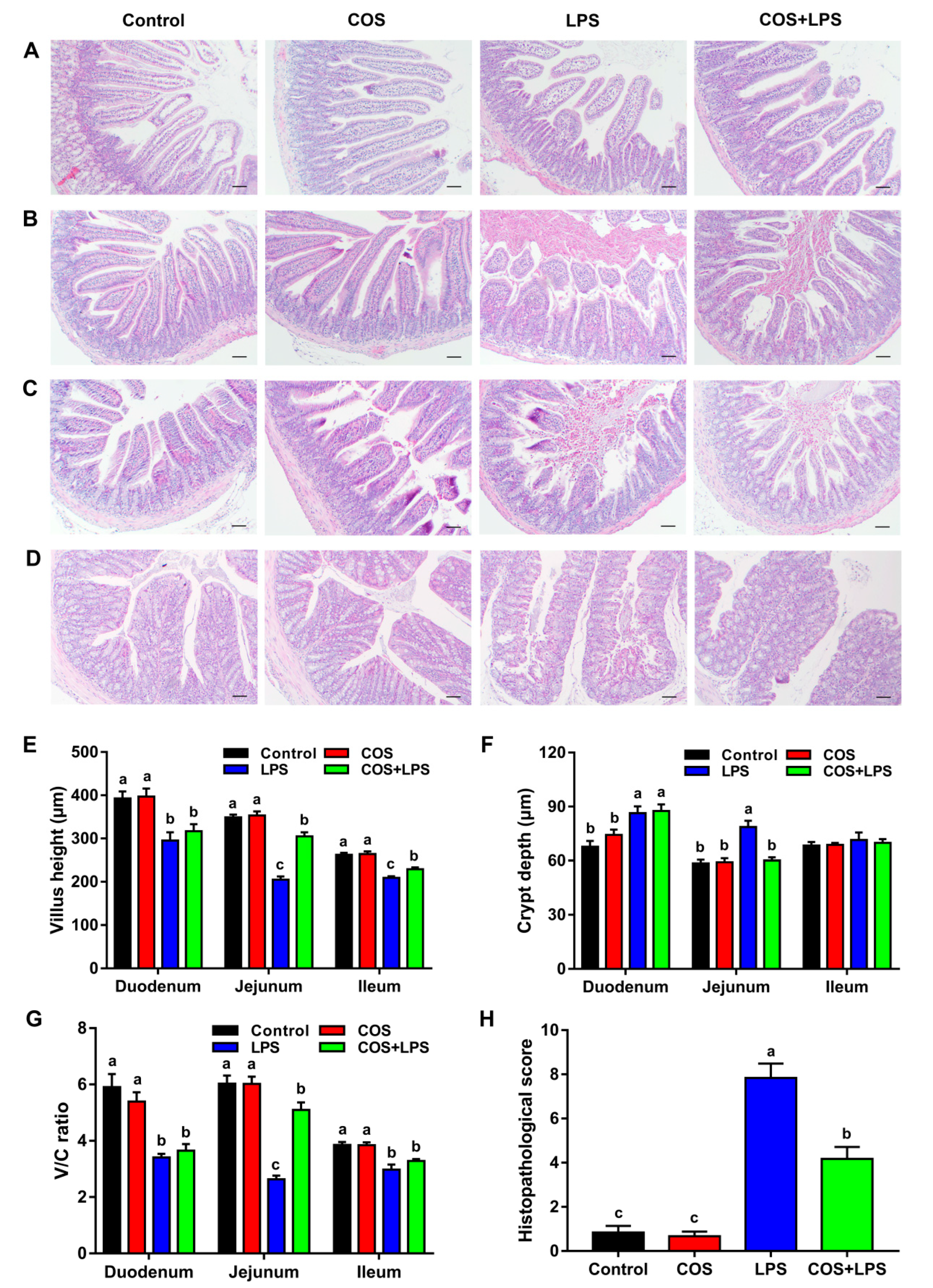
3.2. Effects of COS on Intestinal Morphology and Ultrastructure
3.3. Effects of COS on Intestinal Barrier Functions
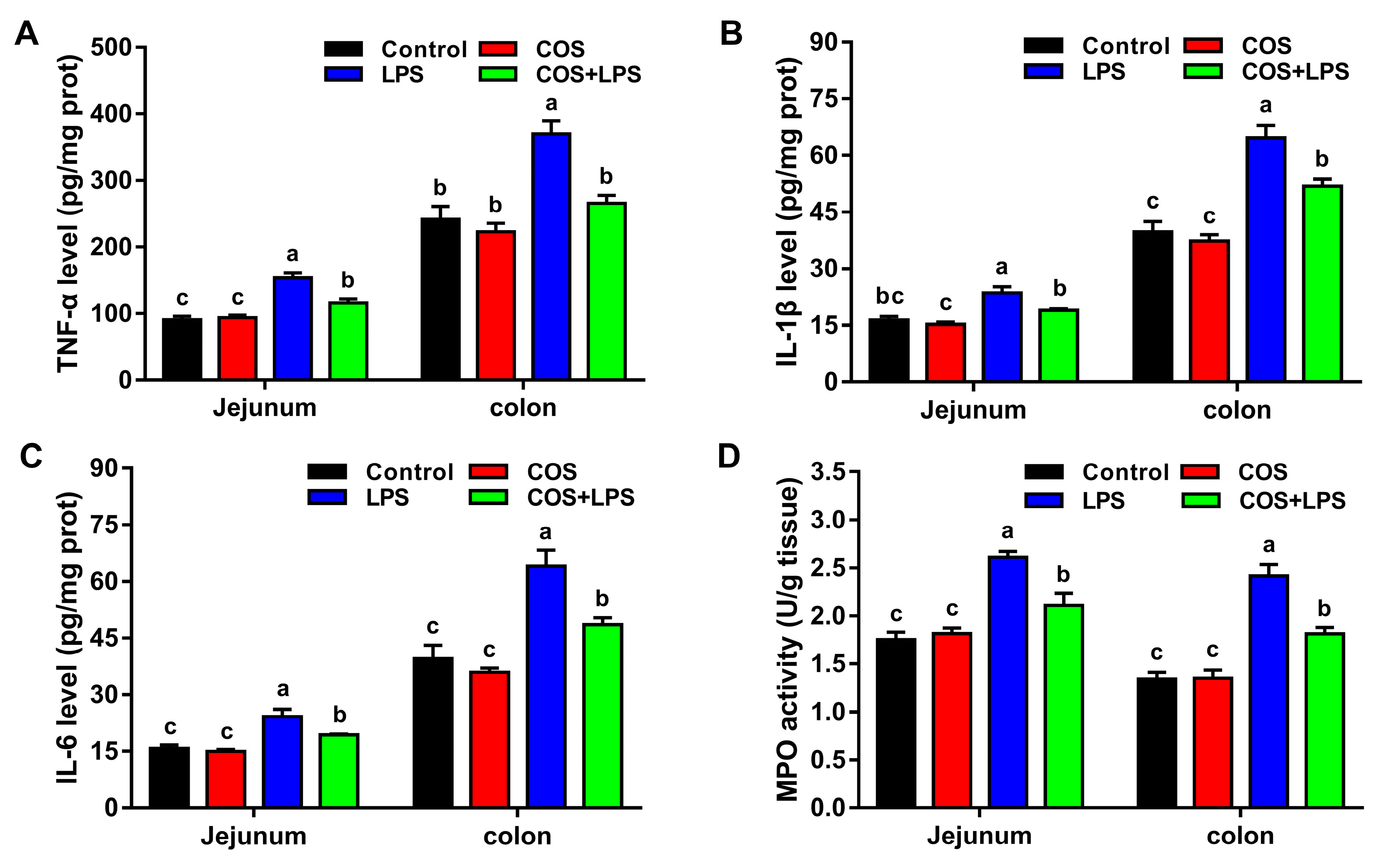
3.4. Effects of COS on Intestinal Inflammation
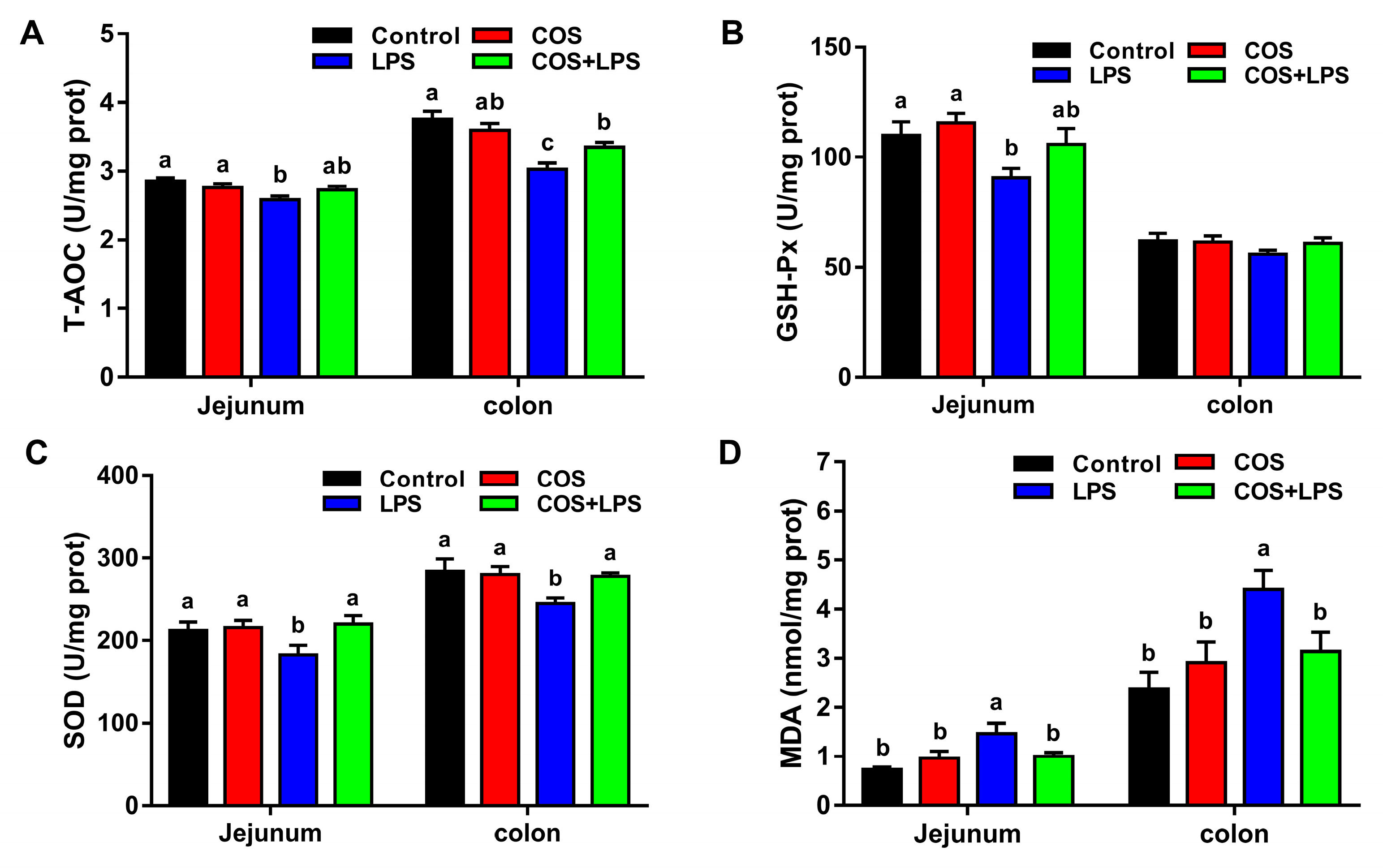
3.5. Effects of COS on Intestinal Oxidative Stress
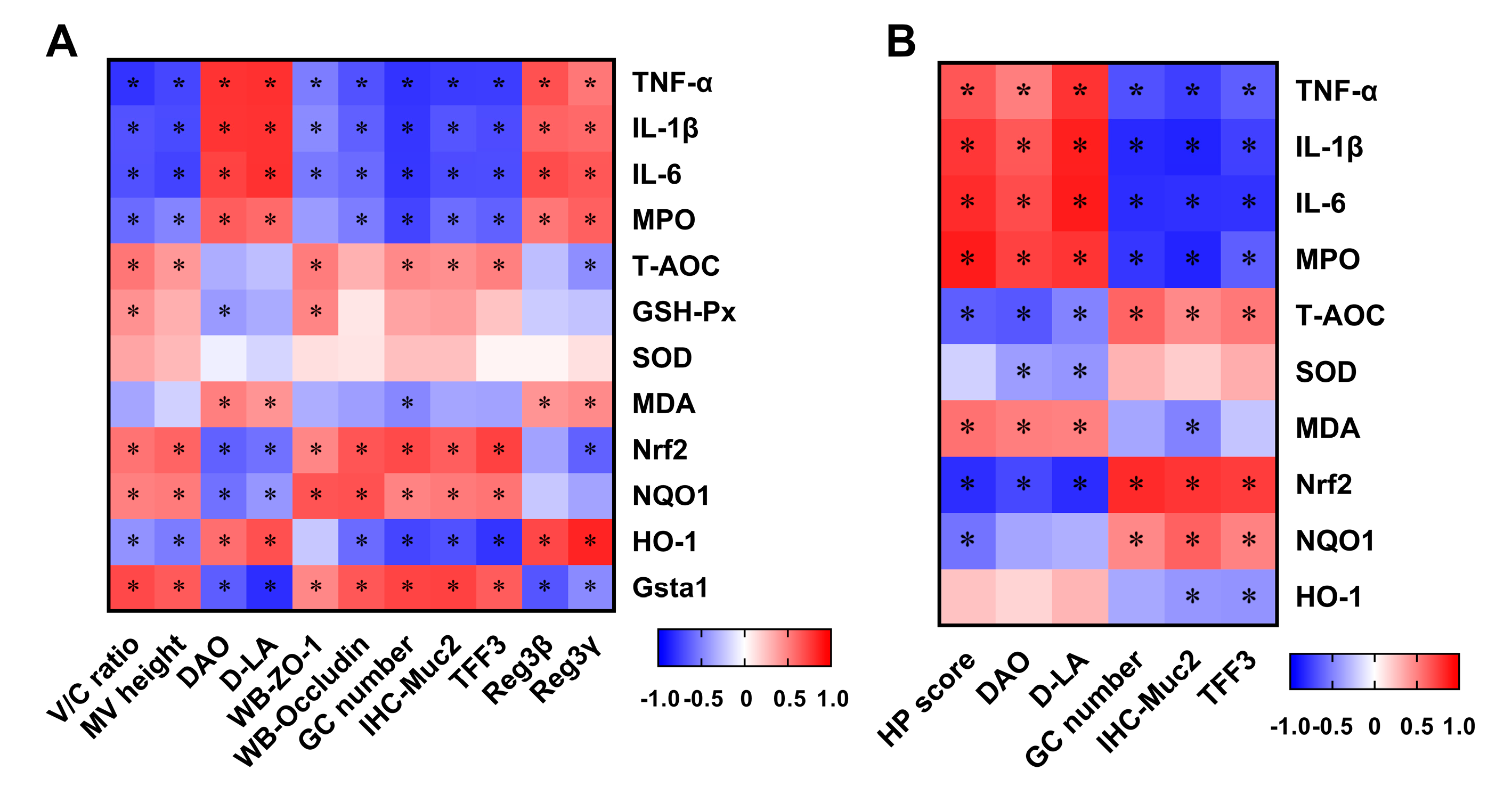
3.6. Correlation between Intestinal Damage Biomarkers and Indices Related to Inflammation and Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal. Immunol. 2015, 8, 712–719. [Google Scholar]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar]
- Naydenov, N.G.; Baranwal, S.; Khan, S.; Feygin, A.; Gupta, P.; Ivanov, A.I. Novel mechanism of cytokine-induced disruption of epithelial barriers: Janus kinase and protein kinase D-dependent downregulation of junction protein expression. Tissue Barriers 2013, 1, e25231. [Google Scholar]
- John, L.J.; Fromm, M.; Schulzke, J.D. Epithelial barriers in intestinal inflammation. Antioxid. Redox Signal. 2011, 15, 1255–1270. [Google Scholar]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, Y. Metformin protects against LPS-induced intestinal barrier dysfunction by activating AMPK pathway. Mol. Pharm. 2018, 15, 3272–3284. [Google Scholar]
- Zhu, H.; Wang, H.; Wang, S.; Tu, Z.; Zhang, L.; Wang, X.; Hou, Y.; Wang, C.; Chen, J.; Liu, Y. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol. Nutr. Food Res. 2018, 62, e1700814. [Google Scholar]
- Zhuang, S.; Zhong, J.; Bian, Y.; Fan, Y.; Chen, Q.; Liu, P.; Liu, Z. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life Sci. 2019, 216, 168–175. [Google Scholar]
- Jiang, J.; Qi, L.; Lv, Z.; Jin, S.; Wei, X.; Shi, F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants 2019, 8, 575. [Google Scholar]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar]
- Jung, W.J.; Park, R.D. Bioproduction of chitooligosaccharides: Present and perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar]
- Lee, S.H.; Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar]
- Liu, H.T.; Huang, P.; Ma, P.; Liu, Q.S.; Yu, C.; Du, Y.G. Chitosan oligosaccharides suppress LPS-induced IL-8 expression in human umbilical vein endothelial cells through blockade of p38 and Akt protein kinases. Acta Pharmacol. Sin. 2011, 32, 478–486. [Google Scholar]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar]
- Shi, L.; Fang, B.; Yong, Y.; Li, X.; Gong, D.; Li, J.; Yu, T.; Gooneratne, R.; Gao, Z.; Li, S.; et al. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway. Carbohydr. Polym. 2019, 219, 269–279. [Google Scholar]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar]
- Liu, P.; Piao, X.S.; Thacker, P.A.; Zeng, Z.K.; Li, P.F.; Wang, D.; Kim, S.W. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar]
- Ji, Y.; Dai, Z.; Sun, S.; Ma, X.; Yang, Y.; Tso, P.; Wu, G.; Wu, Z. Hydroxyproline attenuates dextran sulfate sodium-induced colitis in mice: Involvment of the NF-κB signaling and oxidative stress. Mol. Nutr. Food Res. 2018, 62, e1800494. [Google Scholar]
- Xie, S.Z.; Liu, B.; Ye, H.Y.; Li, Q.M.; Pan, L.H.; Zha, X.Q.; Liu, J.; Duan, J.; Luo, J.P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar]
- Yi, H.; Zhang, L.; Gan, Z.; Xiong, H.; Yu, C.; Du, H.; Wang, Y. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 2016, 6, 25679. [Google Scholar]
- Catalioto, R.M.; Maggi, C.A.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar]
- Wang, Y.; Liu, S.; Tang, D.; Dong, R.; Feng, Q. Chitosan oligosaccharide ameliorates metabolic syndrome induced by overnutrition via altering intestinal microbiota. Front. Nutr. 2021, 8, 743492. [Google Scholar]
- Wang, Y.; Wen, R.; Liu, D.; Zhang, C. Exploring effects of chitosan oligosaccharides on the DSS-induced intestinal barrier impairment in vitro and in vivo. Molecules 2021, 26, 2199. [Google Scholar]
- Liu, Y.H.; Li, X.Y.; Chen, C.Y.; Zhang, H.M.; Kang, J.X. Omega-3 fatty acid intervention suppresses lipopolysaccharide-induced inflammation and weight loss in mice. Mar. Drugs 2015, 13, 1026–1036. [Google Scholar]
- He, M.C.; Shi, Z.; Qin, M.; Sha, N.N.; Li, Y.; Liao, D.F.; Lin, F.H.; Shu, B.; Sun, Y.L.; Yuan, T.F.; et al. Muscone ameliorates LPS-induced depressive-like behaviors and inhibits neuroinflammation in prefrontal cortex of mice. Am. J. Chin. Med. 2020, 48, 559–577. [Google Scholar]
- Song, W.B.; Lv, Y.H.; Zhang, Z.S.; Li, Y.N.; Xiao, L.P.; Yu, X.P.; Wang, Y.Y.; Ji, H.L.; Ma, L. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 3916–3919. [Google Scholar]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar]
- Kjellev, S. The trefoil factor family—Small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar]
- Wang, L.; Fouts, D.E.; Stärkel, P.; Hartmann, P.; Chen, P.; Llorente, C.; DePew, J.; Moncera, K.; Ho, S.B.; Brenner, D.A.; et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016, 19, 227–239. [Google Scholar]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 151, 616–632. [Google Scholar]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 2009, 1788, 864–871. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gas-troenterol. Hepatol. 2018, 15, 671–682. [Google Scholar]
- Sikalidis, A.K.; Maykish, A. The gut microbiome and type 2 diabetes mellitus: Discussing a complex relationship. Biomedicines 2020, 8, 8. [Google Scholar]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar]
- Wang, Q.; Jiang, Y.; Luo, X. Chitooligosaccharides modulate glucose-lipid metabolism by suppressing SMYD3 pathways and regulating gut microflora. Mar. Drugs 2020, 18, 69. [Google Scholar]
- Xiong, W.; Ma, H.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. The protective effect of icariin and phosphorylated icariin against LPS-induced intestinal epithelial cells injury. Biomed. Pharmacother. 2019, 118, 109246. [Google Scholar]
- Chen, F.; Chen, J. Lactobacillus delbrueckii protected intestinal integrity, alleviated intestinal oxidative damage, and activated toll-Like receptor-bruton’s tyrosine kinase-nuclear factor erythroid 2-related factor 2 pathway in weaned piglets challenged with lipopolysaccharide. Antioxidants 2021, 10, 468. [Google Scholar]
- Chorawala, M.R.; Chauhan, S.; Patel, R.; Shah, G. Cell wall contents of probiotics (Lactobacillus species) protect against lipopolysaccharide (LPS)-induced murine colitis by limiting immuno-inflammation and oxidative stress. Probiotics Antimicrob. Proteins 2021, 13, 1005–1017. [Google Scholar]
- Tkachev, V.O.; Menshchikova, E.B.; Zenkov, N.K. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry 2011, 76, 407–422. [Google Scholar]





| Target Genes | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| ZO-1 | D14340.1 | AGGACACCAAAGCATGTGAG | GGCATTCCTGCTGGTTACA |
| Occludin | NM_008756.2 | GGAGATTCCTCTGACCTTGAGTGT | TTCCTGCTTTCCCCTTCGT |
| TFF3 | NM_011575.2 | CCTGGTTGCTGGGTCCTCTG | GCCACGGTTGTTACACTGCTC |
| Reg3β | NM_011036.1 | TGGGAATGGAGTAACAATG | GGCAACTTCACCTCACAT |
| Reg3γ | NM_011260.2 | CCCGACACTGGGCTATGAAC | GGTACCACAGTGATTGCCTGA |
| Nrf2 | NM_010902.5 | CTACTCCCAGGTTGCCCACA | CGACTCATGGTCATCTACAAATGG |
| NQO1 | NM_008706.5 | AGGCTGCTGTAGAGGCTCTGAAG | GCTCAGGCGTCCTTCCTTATATGC |
| HO-1 | NM_010442.2 | GAGCAGAACCAGCCTGAACTA | GGTACAAGGAAGCCATCACCA |
| Gsta1 | NM_008181.3 | TGCCCAATCATTTCAGTCAG | CCAGAGCCATTCTCAACTA |
| β-actin | NM_007393.5 | CGTTGACATCCGTAAAGACC | AACAGTCCGCCTAGAAGCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Wang, G.; Pei, X.; Sun, W.; Wang, M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants 2022, 11, 1384. https://doi.org/10.3390/antiox11071384
Tao W, Wang G, Pei X, Sun W, Wang M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants. 2022; 11(7):1384. https://doi.org/10.3390/antiox11071384
Chicago/Turabian StyleTao, Wenjing, Geng Wang, Xun Pei, Wanjing Sun, and Minqi Wang. 2022. "Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice" Antioxidants 11, no. 7: 1384. https://doi.org/10.3390/antiox11071384






