Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Irradiation
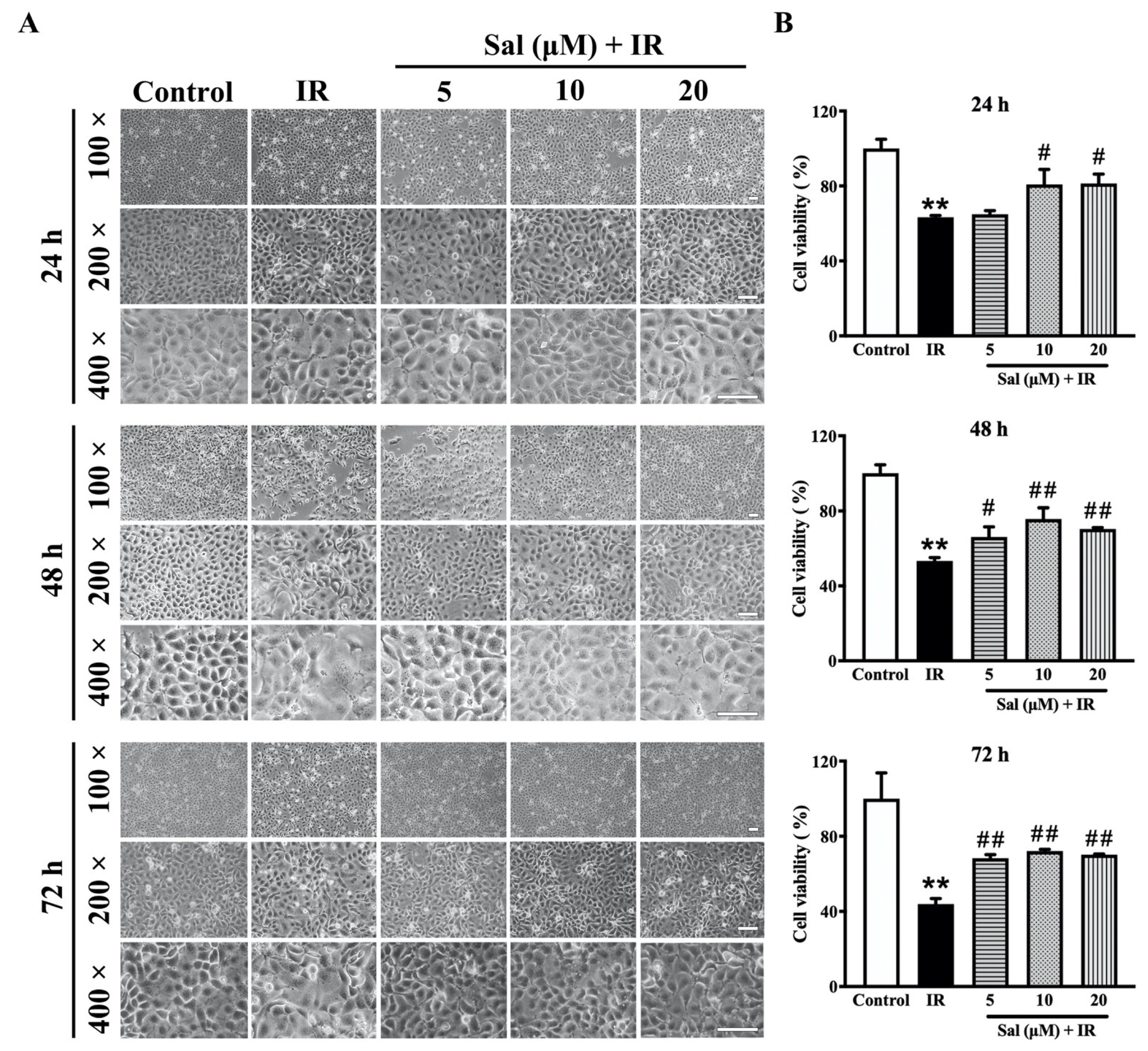
2.2. Cellular Morphologic Observation
2.3. Cell Viability Assay
2.4. Examination of Antioxidant Parameters
2.5. Mitochondrial ROS Production Assay
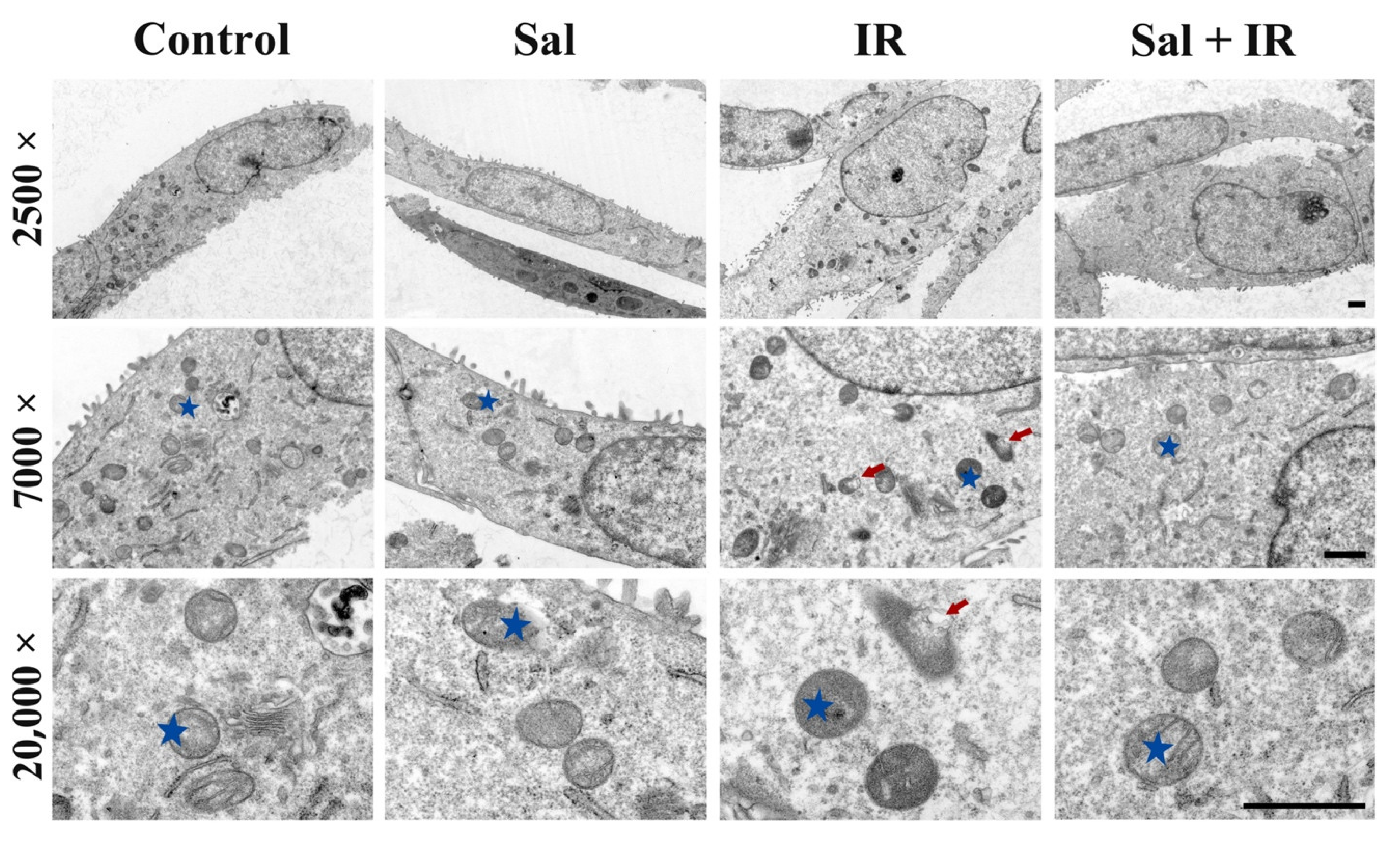
2.6. Transmission Electron Microscopy (TEM)
2.7. Mitochondrial Membrane Potential (MMP) Measurement
2.8. ATP Assay
2.9. Isolation of Mitochondrial and Cytosolic Fraction
2.10. Western Blot
2.11. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Staining
2.12. Animals and Irradiation
2.13. Hematoxylin-Eosin (HE) Staining
2.14. Measurement of Pilocarpine-Stimulated Saliva Flow
2.15. Statistical Analysis
3. Results
3.1. Salidroside Mitigated Radiation Damage in SMG Cells
3.2. Salidroside Enhanced Antioxidant Defense and Reduced Mitochondrial ROS Generation in Irradiated SMG Cells
3.3. Salidroside Alleviated Mitochondrial Ultrastructural Damage in Irradiated SMG Cells
3.4. Salidroside Maintained MMP and Enhanced ATP Production in Irradiated SMG Cells
3.5. Salidroside Inhibited Cellular Apoptosis by Suppressing Cytochrome c and Cleaved-Caspase 3 in Irradiated SMG Cells
3.6. Salidroside Alleviated Glandular Atrophy and Promoted Secretion Function in Irradiated SMG Tissues
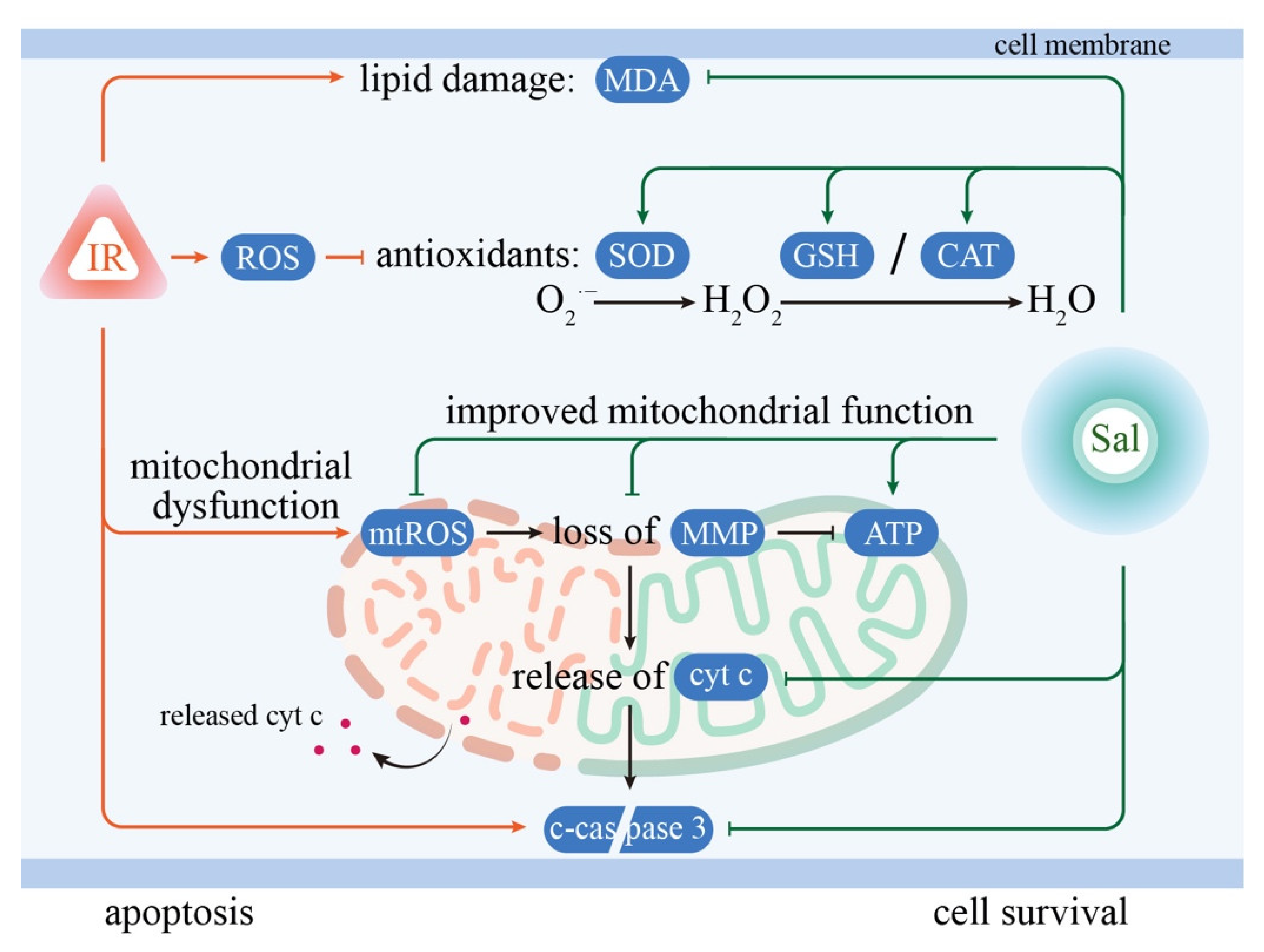
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.W.C.; Leung, K.Y. A Review on the Assessment of Radiation Induced Salivary Gland Damage After Radiotherapy. Front. Oncol. 2019, 9, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudelet, M.; Msc, L.V.D.S.; Tomassen, P.; Bonte, K.; Deron, P.; Huvenne, W.; Rottey, S.; De Neve, W.; Sundahl, N.; Van Nuffelen, G.; et al. Very late xerostomia, dysphagia, and neck fibrosis after head and neck radiotherapy. Head Neck 2019, 41, 3594–3603. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of Amifostine for Cytoprotection during Radiation Therapy: A Review. Oncology 2019, 98, 61–80. [Google Scholar] [CrossRef]
- McDonald, S.; Meyerowitz, C.; Smudzin, T.; Rubin, P. Preliminary results of a pilot study using WR-2721 before fractionated irradiation of the head and neck to reduce salivary gland dysfunction. Int. J. Radiat. Oncol. 1994, 29, 747–754. [Google Scholar] [CrossRef]
- Rades, D.; Fehlauer, F.; Bajrovic, A.; Mahlmann, B.; Richter, E.; Alberti, W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. 2004, 70, 261–264. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Faulds, D. Oral pilocarpine: A review of its pharmacological properties and clinical potential in xerostomia. Drugs 1995, 49, 143–155. [Google Scholar] [CrossRef]
- Farag, A.M.; Holliday, C.; Cimmino, J.; Roomian, T.; Papas, A. Comparing the effectiveness and adverse effects of pilocarpine and cevimeline in patients with hyposalivation. Oral Dis. 2019, 25, 1937–1944. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Zhu, F.; Deng, S.n.; Li, X.; He, Y. Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury. Cell Death Dis. 2019, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Rong, R.; Xia, X.; Peng, H.; Li, H.; You, M.; Liang, Z.; Yao, F.; Yao, X.; Xiong, K.; Huang, J.; et al. Cdk5-mediated Drp1 phosphorylation drives mitochondrial defects and neuronal apoptosis in radiation-induced optic neuropathy. Cell Death Dis. 2020, 11, 720. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, J.; Zhang, F.Y.; Liu, K.J.; Xiang, B. Phenylephrine Alleviates 131I Radiation Damage in Submandibular Gland Through Maintaining Mitochondrial Homeostasis. Int. J. Radiat. Oncol. 2019, 104, 644–655. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef]
- Li, R.; Guo, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Yan, T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int. J. Mol. Sci. 2019, 20, 1103. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Li, L.; Huang, Y.; Qian, D.; Liu, W.; Zhang, C.; Luo, Y.; Zhou, Z.; Kong, F.; Zhao, X.; et al. Salidroside Ameliorates Mitochondria-Dependent Neuronal Apoptosis after Spinal Cord Ischemia-Reperfusion Injury Partially through Inhibiting Oxidative Stress and Promoting Mitophagy. Oxidative Med. Cell. Longev. 2020, 2020, 3549704. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Pang, X.-W.; Zhang, G.-Q.; Guo, J.-Y. Salidroside’s protection against UVB-mediated oxidative damage and apoptosis is associated with the upregulation of Nrf2 expression. Photomed. Laser Surg. 2017, 35, 49–56. [Google Scholar] [CrossRef]
- Feng, T.; Wang, L.; Zhou, N.; Liu, C.; Cui, J.; Wu, R.; Jing, J.; Zhang, S.; Chen, H.; Wang, S. Salidroside, a scavenger of ROS, enhances the radioprotective effect of Ex-RAD® via a p53-dependent apoptotic pathway. Oncol. Rep. 2017, 38, 3094–3102. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.-X.; Xing, W.-M.; Wen, X.-L.; Jia, B.-B.; Yang, Z.-X.; Wang, Y.-Z.; Jin, X.-Q.; Wang, G.-F.; Yan, J. Salidroside protects against premature senescence induced by ultraviolet B irradiation in human dermal fibroblasts. Int. J. Cosmet. Sci. 2015, 37, 321–328. [Google Scholar] [CrossRef]
- Fan, F.; Yang, L.; Li, R.; Zou, X.; Li, N.; Meng, X.; Zhang, Y.; Wang, X. Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharmacother. 2020, 129, 110458. [Google Scholar] [CrossRef]
- Liu, X.; Cotrim, A.P.; Teos, L.Y.; Zheng, C.; Swaim, W.D.; Mitchell, J.B.; Mori, Y.; Ambudkar, I.S. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat. Commun. 2013, 4, 1515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhong, L.J.; Wang, Y.; Liu, L.M.; Cong, X.; Xiang, R.L.; Wu, L.L.; Yu, G.Y.; Zhang, Y. Proteomic analysis reveals an impaired Ca2+/AQP5 pathway in the submandibular gland in hypertension. Sci. Rep. 2017, 7, 14524. [Google Scholar]
- Liu, Z.; Dong, L.; Zheng, Z.; Liu, S.; Gong, S.; Meng, L.; Xin, Y.; Jiang, X. Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress. Antioxidants 2021, 10, 1666. [Google Scholar] [CrossRef]
- Siemen, D.; Ziemer, M. What is the nature of the mitochondrial permeability transition pore and what is it not? IUBMB Life 2013, 65, 255–262. [Google Scholar] [CrossRef]
- Chomette, G.; Auriol, M.; Vaillant, J.M.; Bertrand, J.C.; Chenal, C. Effects of irradiation on the submandibular gland of the rat. An enzyme histochemical and ultrastructural study. Virchows Arch. 1981, 391, 291–299. [Google Scholar] [CrossRef]
- Espinal, E.G.; De Rey, B.M.; Cabrini, R.L. Radiation effects on submandibular gland of the rat. Stereological and ultrastructural study. Strahlentherapie 1983, 159, 290–295. [Google Scholar]
- Norberg, L.E.; Lundquist, P.-G. An Ultrastructural Study of Salivary Gland Radiosensitivity After Alpha-Adrenergic Stimulation. Auris Nasus Larynx 1988, 15, 1–17. [Google Scholar] [CrossRef]
- Tateishi, Y.; Sasabe, E.; Ueta, E.; Yamamoto, T. Ionizing irradiation induces apoptotic damage of salivary gland acinar cells via NADPH oxidase 1-dependent superoxide generation. Biochem. Biophys. Res. Commun. 2008, 366, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambudkar, I. Calcium signaling defects underlying salivary gland dysfunction. Biochim. Biophys. Acta 2018, 1865, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, A.; Cotrim, A.P.; Katabi, N.; Mitchell, J.B.; Verheij, M.; Haimovitz-Friedman, A. Radiation-Induced Microvascular Injury as a Mechanism of Salivary Gland Hypofunction and Potential Target for Radioprotectors. Radiat. Res. 2016, 186, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Hu, L.; Xu, Y.; Li, X.; Ma, L.; Feng, X.; Wang, J.; Zhang, C.; Wang, S. Inorganic Nitrate Alleviates Total Body Irradiation-Induced Systemic Damage by Decreasing Reactive Oxygen Species Levels. Int. J. Radiat. Oncol. 2019, 103, 945–957. [Google Scholar] [CrossRef]
- Liu, X.; Subedi, K.P.; Zheng, C.; Ambudkar, I. Mitochondria-targeted antioxidant protects against irradiation-induced salivary gland hypofunction. Sci. Rep. 2021, 11, 7690. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Luangwattananun, P.; Eiamphungporn, W.; Songtawee, N.; Bülow, L.; Na Ayudhya, C.I.; Prachayasittikul, V.; Yainoy, S. Improving enzymatic activities and thermostability of a tri-functional enzyme with SOD, catalase and cell-permeable activities. J. Biotechnol. 2017, 247, 50–59. [Google Scholar] [CrossRef]
- Yang, N.; Guan, Q.-W.; Chen, F.-H.; Xia, Q.-X.; Yin, X.-X.; Zhou, H.-H.; Mao, X.-Y. Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy. Oxidative Med. Cell. Longev. 2020, 2020, 6687185. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.-H.P.J. Enzymatic regeneration and conservation of ATP: Challenges and opportunities. Crit. Rev. Biotechnol. 2020, 41, 16–33. [Google Scholar] [CrossRef]
- Doczi, J.; Torocsik, B.; Echaniz-Laguna, A.; de Camaret, B.M.; Starkov, A.; Starkova, N.; Gál, A.; Molnár, M.J.; Kawamata, H.; Manfredi, G.; et al. Alterations in voltage-sensing of the mitochondrial permeability transition pore in ANT1-deficient cells. Sci. Rep. 2016, 6, 26700. [Google Scholar] [CrossRef] [Green Version]
- Coppes, R.P.; Zeilstra, L.J.W.; Kampinga, H.; Konings, A.W.T. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br. J. Cancer 2001, 85, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Estaquier, J.; Vallette, F.; Vayssiere, J.-L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar]
- Vissink, A.; Kalicharan, D.; ‘S-Gravenmade, E.; Jongebloed, W.L.; Ligeon, E.E.; Nieuwenhuis, P.; Konings, A.W.T. Acute irradiation effects on morphology and function of rat submandibular glands. J. Oral Pathol. Med. 1991, 20, 449–456. [Google Scholar] [CrossRef]
- Coppes, R.P.; Vissink, A.; Zeilstra, L.J.; Konings, A.W. Muscarinic receptor stimulation increases tolerance of rat salivary gland function to radiation damage. Int. J. Radiat. Biol. 1997, 72, 615–625. [Google Scholar]
- Coppes, R.P.; Zeilstra, L.J.W.; Vissink, A.; Konings, A.W.T. Sialogogue-related radioprotection of salivary gland function: The degranulation concept revisited. Radiat. Res. 1997, 148, 240. [Google Scholar] [CrossRef]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.; de Haan, G.; Coppes, R.P. Rescue of Salivary Gland Function after Stem Cell Transplantation in Irradiated Glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An Herb with Anti-Stress, Anti-Aging, and Immunostimulating Properties for Cancer Chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Li, Z. Salidroside suppresses the activation of nasopharyngeal carcinoma cells via targeting miR-4262/GRP78 axis. Cell Cycle 2022, 21, 720–729. [Google Scholar] [CrossRef]
- Shang, H.; Wang, S.; Yao, J.; Guo, C.; Dong, J.; Liao, L. Salidroside inhibits migration and invasion of poorly differentiated thyroid cancer cells. Thorac. Cancer 2019, 10, 1469–1478. [Google Scholar] [CrossRef]
- Tu, Y.; Roberts, L.; Shetty, K.; Schneider, S.S. Rhodiola crenulataInduces Death and Inhibits Growth of Breast Cancer Cell Lines. J. Med. Food 2008, 11, 413–423. [Google Scholar] [CrossRef]
- Udintsev, S.N.; Shakhov, V.P. Decrease in the growth rate of Ehrlich’s tumor and Pliss’ lymphosarcoma with partial hepatectomy. Vopr. Onkol. 1989, 35, 1072–1075. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-M.; Wang, X.-Y.; Zhou, X.-R.; Zhang, C.; Liu, K.-J.; Zhang, F.-Y.; Xiang, B. Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland. Antioxidants 2022, 11, 1414. https://doi.org/10.3390/antiox11071414
Sun Y-M, Wang X-Y, Zhou X-R, Zhang C, Liu K-J, Zhang F-Y, Xiang B. Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland. Antioxidants. 2022; 11(7):1414. https://doi.org/10.3390/antiox11071414
Chicago/Turabian StyleSun, Yue-Mei, Xin-Yue Wang, Xin-Ru Zhou, Chong Zhang, Ke-Jian Liu, Fu-Yin Zhang, and Bin Xiang. 2022. "Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland" Antioxidants 11, no. 7: 1414. https://doi.org/10.3390/antiox11071414





