The Role of Concomitant Nrf2 Targeting and Stem Cell Therapy in Cerebrovascular Disease
Abstract
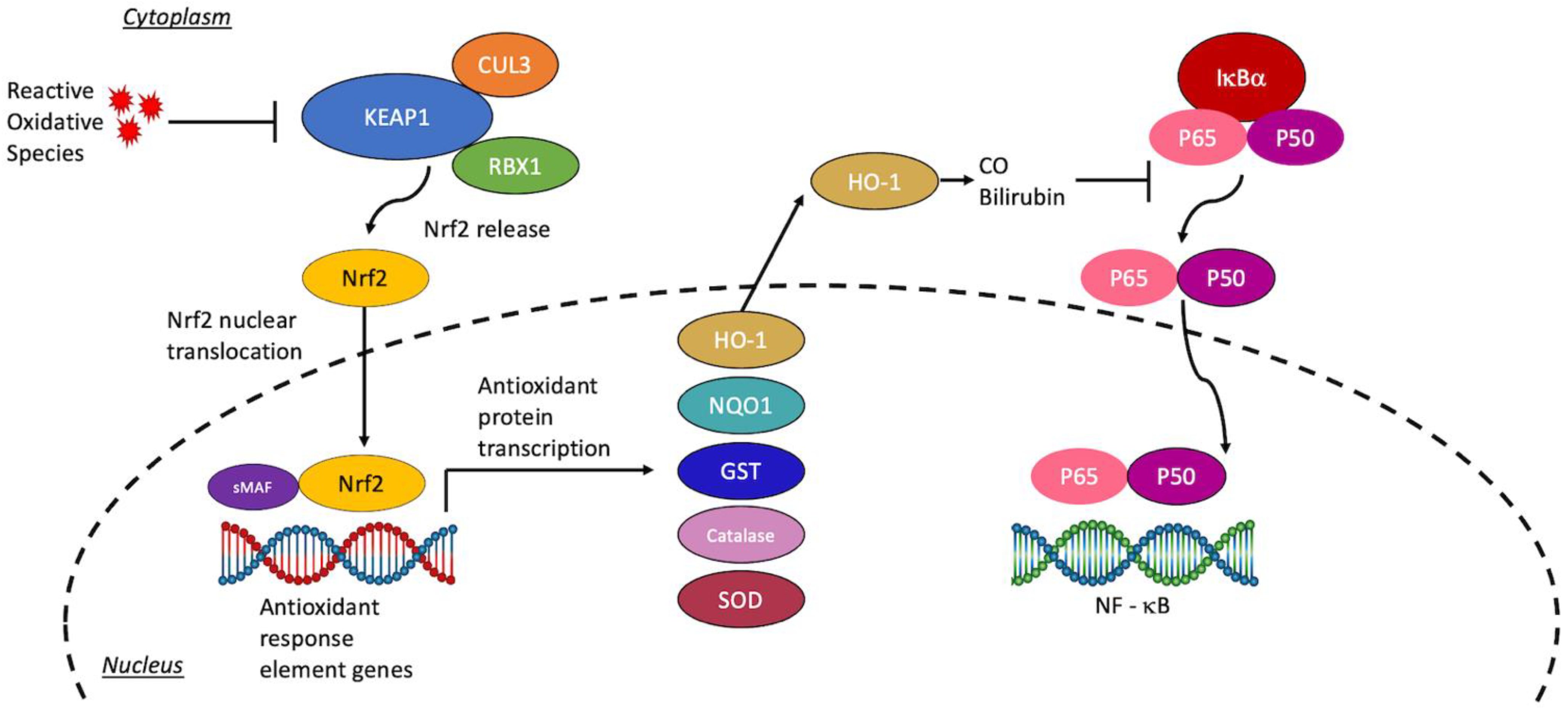
:1. Introduction
2. Neuroinflammation and Oxidative Stress in Stroke
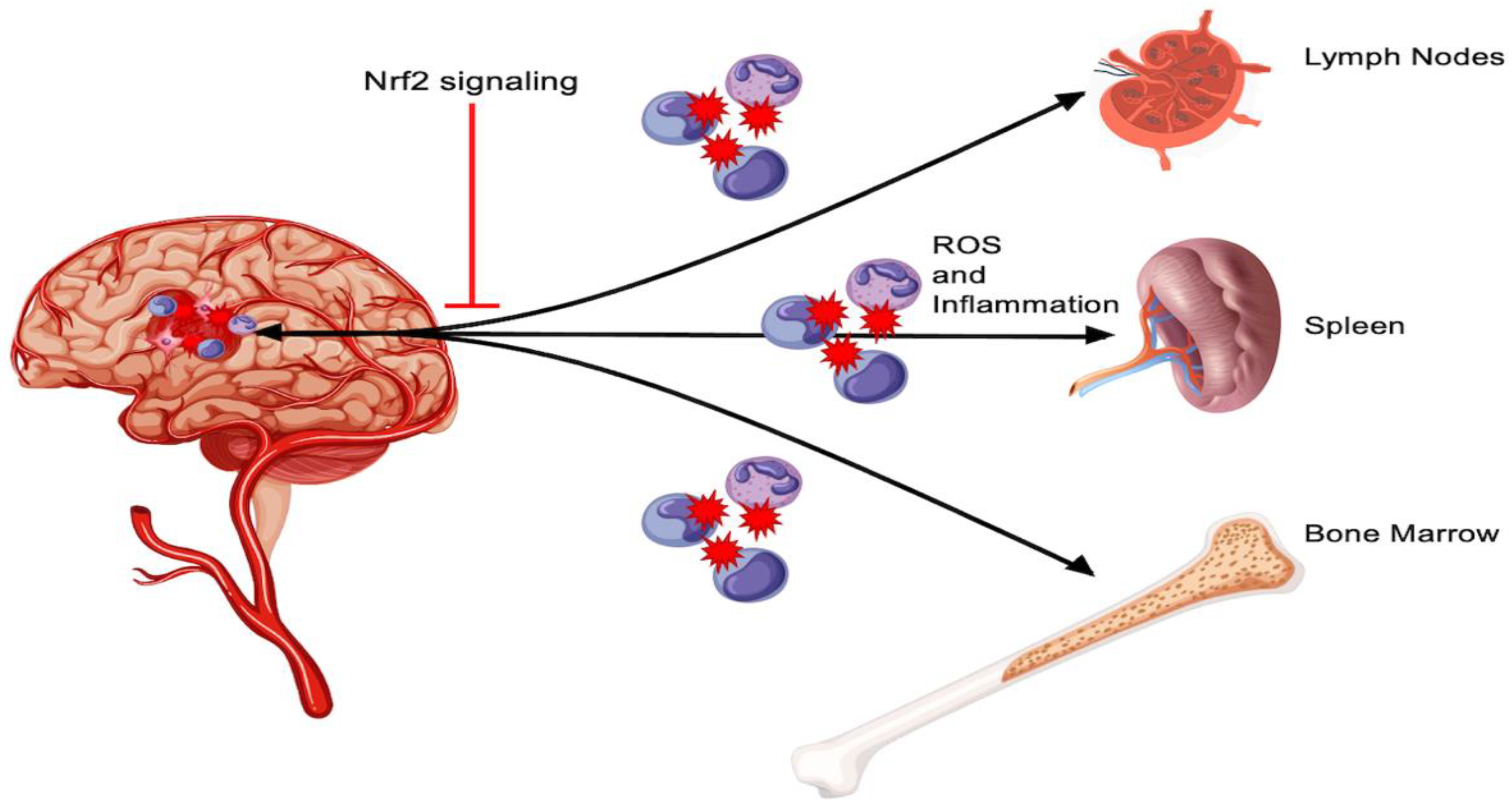
3. Peripheral Inflammation and Nrf2
3.1. Mechanistic Interrogation of Peripheral Inflammation
3.2. Stem Cells and Peripheral Inflammation
4. Current Nrf2-Directed Treatment Modalities
4.1. Stem Cell Therapies and Nrf2
4.2. Targeting Nrf2 to Enhance Stem Cell Therapy
4.3. Downstream Targets of the Nrf2 Pathway
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.K.; Olsen, T.S.; Dehlendorff, C.; Kammersgaard, L.P. Hemorrhagic and ischemic strokes compared: Stroke severity, mortality, and risk factors. Stroke 2009, 40, 2068–2072. [Google Scholar] [CrossRef] [Green Version]
- Marcet, P.; Santos, N.; Borlongan, C.V. When friend turns foe: Central and peripheral neuroinflammation in central nervous system injury. Neuroimmunol. Neuroinflamm. 2017, 4, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, S.A.; Tajiri, N.; de la Pena, I.; Bastawrous, M.; Sanberg, P.R.; Kaneko, Y.; Borlongan, C.V. Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson’s disease. J. Cell. Physiol. 2015, 230, 1024–1032. [Google Scholar] [CrossRef]
- Gumbinger, C.; Reuter, B.; Stock, C.; Sauer, T.; Wietholter, H.; Bruder, I.; Rode, S.; Kern, R.; Ringleb, P.; Hennerici, M.G.; et al. Time to treatment with recombinant tissue plasminogen activator and outcome of stroke in clinical practice: Retrospective analysis of hospital quality assurance data with comparison with results from randomised clinical trials. BMJ 2014, 348, g3429. [Google Scholar] [CrossRef] [Green Version]
- Kaesmacher, J.; Kaesmacher, M.; Maegerlein, C.; Zimmer, C.; Gersing, A.S.; Wunderlich, S.; Friedrich, B.; Boeckh-Behrens, T.; Kleine, J.F. Hemorrhagic Transformations after Thrombectomy: Risk Factors and Clinical Relevance. Cerebrovasc. Dis. 2017, 43, 294–304. [Google Scholar] [CrossRef]
- Nguyen, H.; Lee, J.Y.; Sanberg, P.R.; Napoli, E.; Borlongan, C.V. Eye Opener in Stroke. Stroke 2019, 50, 2197–2206. [Google Scholar] [CrossRef]
- Lees, K.R.; Bluhmki, E.; von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A.; et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef]
- Mao, L.; Li, P.; Zhu, W.; Cai, W.; Liu, Z.; Wang, Y.; Luo, W.; Stetler, R.A.; Leak, R.K.; Yu, W.; et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain 2017, 140, 1914–1931. [Google Scholar] [CrossRef]
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral haemorrhage: Current approaches to acute management. Lancet 2018, 392, 1257–1268. [Google Scholar] [CrossRef]
- Imai, T.; Matsubara, H.; Hara, H. Potential therapeutic effects of Nrf2 activators on intracranial hemorrhage. J. Cereb. Blood Flow Metab. 2021, 41, 1483–1500. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.; Cabantan, D.; Monsour, M.; Borlongan, C.V. Neuroinflammation, Stem Cells, and Stroke. Stroke 2022, 53, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, F.; Leak, R.K.; Chen, J.; Hu, X. Regulation of Neuroinflammation through Programed Death-1/Programed Death Ligand Signaling in Neurological Disorders. Front. Cell. Neurosci. 2014, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007, 182, 1. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Huang, C.S.; Anderson, M.E.; Meister, A. Amino acid sequence and function of the light subunit of rat kidney gamma-glutamylcysteine synthetase. J. Biol. Chem. 1993, 268, 20578–20583. [Google Scholar] [CrossRef]
- Meister, A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974, 15, 177–190. [Google Scholar] [CrossRef]
- Boyland, E.; Chasseaud, L.F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 173–219. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mazur, A.; Fangman, M.; Ashouri, R.; Arcenas, A.; Dore, S. Nrf2 as a therapeutic target in ischemic stroke. Expert Opin. Ther. Targets 2021, 25, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, C.C.; Dore, S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr. Neurosci. 2011, 14, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Cho, S. Microglia and Monocyte-Derived Macrophages in Stroke. Neurotherapeutics 2016, 13, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Christopher, E.; Loan, J.J.M.; Samarasekera, N.; McDade, K.; Rose, J.; Barrington, J.; Hughes, J.; Smith, C.; Al-Shahi Salman, R. Nrf2 activation in the human brain after stroke due to supratentorial intracerebral haemorrhage: A case-control study. BMJ Neurol. Open 2022, 4, e000238. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Kitashoji, A.; Iwawaki, T.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. Temporal activation of Nrf2 in the penumbra and Nrf2 activator-mediated neuroprotection in ischemia-reperfusion injury. Free Radic. Biol. Med. 2014, 72, 124–133. [Google Scholar] [CrossRef]
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [Green Version]
- Morooka, H.; Hirotsune, N.; Wani, T.; Ohmoto, T. Histochemical demonstration of free radicals (H2O2) in ischemic brain edema and protective effects of human recombinant superoxide dismutase on ischemic neuronal damage. Acta Neurochir. Suppl. 1994, 60, 307–309. [Google Scholar] [CrossRef]
- Purdom-Dickinson, S.E.; Sheveleva, E.V.; Sun, H.; Chen, Q.M. Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol. Pharmacol. 2007, 72, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Vollmer, M.K.; Fernandez, V.M.; Dweik, Y.; Kim, H.; Dore, S. Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely through Nrf2. Front. Cell. Neurosci. 2018, 12, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fields, J.; Zhao, C.; Langer, J.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Dore, S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic. Biol. Med. 2007, 43, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Sun, G.; Zhang, J.; Strong, R.; Dash, P.K.; Kan, Y.W.; Grotta, J.C.; Aronowski, J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 2007, 38, 3280–3286. [Google Scholar] [CrossRef]
- Wagner, K.R.; Sharp, F.R.; Ardizzone, T.D.; Lu, A.; Clark, J.F. Heme and iron metabolism: Role in cerebral hemorrhage. J. Cereb. Blood Flow Metab. 2003, 23, 629–652. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Sun, G.; Ting, S.M.; Song, S.; Zhang, J.; Edwards, N.J.; Aronowski, J. Cleaning up after ICH: The role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem. 2015, 133, 144–152. [Google Scholar] [CrossRef]
- Belcher, J.D.; Beckman, J.D.; Balla, G.; Balla, J.; Vercellotti, G. Heme degradation and vascular injury. Antioxid. Redox Signal. 2010, 12, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Regan, R.F. Time course of increased heme oxygenase activity and expression after experimental intracerebral hemorrhage: Correlation with oxidative injury. J. Neurochem. 2007, 103, 2015–2021. [Google Scholar] [CrossRef]
- Shang, H.; Yang, D.; Zhang, W.; Li, T.; Ren, X.; Wang, X.; Zhao, W. Time course of Keap1-Nrf2 pathway expression after experimental intracerebral haemorrhage: Correlation with brain oedema and neurological deficit. Free Radic. Res. 2013, 47, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxid. Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Pourbagher-Shahri, A.M.; Azimi-Nezhad, M.; Forouzanfar, F.; Brockmueller, A.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies. Molecules 2021, 26, 3157. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Hernandez-Ontiveros, D.G.; Tajiri, N.; Acosta, S.; Giunta, B.; Tan, J.; Borlongan, C.V. Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 2013, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ziai, W.C. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke 2013, 44, S74–S78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Iwata, S.; Hirayama, T.; Nagasawa, H.; Nakamura, S.; Shimazawa, M.; Hara, H. Intracellular Fe2+ accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci. Rep. 2019, 9, 6228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meguro, T.; Chen, B.; Lancon, J.; Zhang, J.H. Oxyhemoglobin induces caspase-mediated cell death in cerebral endothelial cells. J. Neurochem. 2001, 77, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Zille, M.; Karuppagounder, S.S.; Chen, Y.; Gough, P.J.; Bertin, J.; Finger, J.; Milner, T.A.; Jonas, E.A.; Ratan, R.R. Neuronal Death After Hemorrhagic Stroke In Vitro and In Vivo Shares Features of Ferroptosis and Necroptosis. Stroke 2017, 48, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Hua, Y.; Keep, R.F.; Nakamura, T.; Hoff, J.T.; Xi, G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke 2003, 34, 2964–2969. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Fang, Y.; Gao, S.; Wang, X.; Cao, Y.; Lu, J.; Chen, S.; Lenahan, C.; Zhang, J.H.; Shao, A.; Zhang, J. Programmed Cell Deaths and Potential Crosstalk With Blood-Brain Barrier Dysfunction After Hemorrhagic Stroke. Front. Cell. Neurosci. 2020, 14, 68. [Google Scholar] [CrossRef]
- Li, Q.; Weiland, A.; Chen, X.; Lan, X.; Han, X.; Durham, F.; Liu, X.; Wan, J.; Ziai, W.C.; Hanley, D.F.; et al. Ultrastructural Characteristics of Neuronal Death and White Matter Injury in Mouse Brain Tissues After Intracerebral Hemorrhage: Coexistence of Ferroptosis, Autophagy, and Necrosis. Front. Neurol. 2018, 9, 581. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhao, H.; Ji, X.; Luo, Y. Peripheral to central: Organ interactions in stroke pathophysiology. Exp. Neurol. 2015, 272, 41–49. [Google Scholar] [CrossRef]
- Allan, S.M.; Rothwell, N.J. Inflammation in central nervous system injury. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2003, 358, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.J.; Emsley, H.C.; Gavin, C.M.; Georgiou, R.F.; Vail, A.; Barberan, E.M.; del Zoppo, G.J.; Hallenbeck, J.M.; Rothwell, N.J.; Hopkins, S.J.; et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Emsley, H.C.; Smith, C.J.; Gavin, C.M.; Georgiou, R.F.; Vail, A.; Barberan, E.M.; Hallenbeck, J.M.; del Zoppo, G.J.; Rothwell, N.J.; Tyrrell, P.J.; et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J. Neuroimmunol. 2003, 139, 93–101. [Google Scholar] [CrossRef]
- Ajmo, C.T., Jr.; Vernon, D.O.; Collier, L.; Hall, A.A.; Garbuzova-Davis, S.; Willing, A.; Pennypacker, K.R. The spleen contributes to stroke-induced neurodegeneration. J. Neurosci. Res. 2008, 86, 2227–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planas, A.M.; Gómez-Choco, M.; Urra, X.; Gorina, R.; Caballero, M.; Chamorro, Á. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J. Immunol. 2012, 188, 2156–2163. [Google Scholar] [CrossRef]
- Courties, G.; Herisson, F.; Sager, H.B.; Heidt, T.; Ye, Y.; Wei, Y.; Sun, Y.; Severe, N.; Dutta, P.; Scharff, J.; et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ. Res. 2015, 116, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Gendron, A.; Teitelbaum, J.; Cossette, C.; Nuara, S.; Dumont, M.; Geadah, D.; du Souich, P.; Kouassi, E. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 2002, 955, 85–97. [Google Scholar] [CrossRef]
- Offner, H.; Subramanian, S.; Parker, S.M.; Wang, C.; Afentoulis, M.E.; Lewis, A.; Vandenbark, A.A.; Hurn, P.D. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 2006, 176, 6523–6531. [Google Scholar] [CrossRef] [Green Version]
- Seifert, H.A.; Hall, A.A.; Chapman, C.B.; Collier, L.A.; Willing, A.E.; Pennypacker, K.R. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J. Neuroimmune Pharmacol. 2012, 7, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Dotson, A.L.; Chen, Y.; Zhu, W.; Libal, N.; Alkayed, N.J.; Offner, H. Partial MHC Constructs Treat Thromboembolic Ischemic Stroke Characterized by Early Immune Expansion. Transl. Stroke Res. 2016, 7, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Ajmo, C.T., Jr.; Collier, L.A.; Leonardo, C.C.; Hall, A.A.; Green, S.M.; Womble, T.A.; Cuevas, J.; Willing, A.E.; Pennypacker, K.R. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp. Neurol. 2009, 218, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, N.L.; Kaiser, B.; Nguyen, Y.V.; Welbourne, S.; Lall, C.; Cramer, S.C. The Volume of the Spleen and Its Correlates after Acute Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2958–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Ahn, B.J.; Shi, J.; Nakamura, Y.; Park, J.H.; Mandeville, E.T.; Yu, Z.; Chan, S.J.; Desai, R.; Hayakawa, A.; et al. Brain-to-cervical lymph node signaling after stroke. Nat. Commun. 2019, 10, 5306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.M.; Behrouz, R. Impact of Infection on Stroke Morbidity and Outcomes. Curr. Neurol. Neurosci. Rep. 2016, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.W.; Yang, H.; Yan, F.L.; Wang, H.; Xing, F.L.; Zuo, L.; Zhang, H.Q. Blocking Sympathetic Nervous System Reverses Partially Stroke-Induced Immunosuppression but does not Aggravate Functional Outcome After Experimental Stroke in Rats. Neurochem. Res. 2016, 41, 1877–1886. [Google Scholar] [CrossRef]
- Zuo, L.; Shi, L.; Yan, F. The reciprocal interaction of sympathetic nervous system and cAMP-PKA-NF-kB pathway in immune suppression after experimental stroke. Neurosci. Lett. 2016, 627, 205–210. [Google Scholar] [CrossRef]
- Liu, D.D.; Chu, S.F.; Chen, C.; Yang, P.F.; Chen, N.H.; He, X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem. Int. 2018, 114, 42–54. [Google Scholar] [CrossRef]
- Martelli, D.; McKinley, M.J.; McAllen, R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci. 2014, 182, 65–69. [Google Scholar] [CrossRef]
- Mracsko, E.; Liesz, A.; Karcher, S.; Zorn, M.; Bari, F.; Veltkamp, R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav. Immun. 2014, 41, 200–209. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, B.H.; Lundberg, L.; Yli-Karjanmaa, M.; Martin, N.A.; Svensson, M.; Alfsen, M.Z.; Flæng, S.B.; Lyngsø, K.; Boza-Serrano, A.; Nielsen, H.H.; et al. Fumarate decreases edema volume and improves functional outcome after experimental stroke. Exp. Neurol. 2017, 295, 144–154. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef] [Green Version]
- Vendrame, M.; Gemma, C.; Pennypacker, K.R.; Bickford, P.C.; Davis Sanberg, C.; Sanberg, P.R.; Willing, A.E. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp. Neurol. 2006, 199, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.A.; Offner, H. The splenic response to stroke: From rodents to stroke subjects. J. Neuroinflamm. 2018, 15, 195. [Google Scholar] [CrossRef] [Green Version]
- Vendrame, M.; Cassady, J.; Newcomb, J.; Butler, T.; Pennypacker, K.R.; Zigova, T.; Sanberg, C.D.; Sanberg, P.R.; Willing, A.E. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 2004, 35, 2390–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Hamilton, J.A.; Valenzuela, K.S.; Bogaerts, A.; Xi, X.; Aronowski, J.; Mays, R.W.; Savitz, S.I. Multipotent Adult Progenitor Cells Enhance Recovery After Stroke by Modulating the Immune Response from the Spleen. Stem Cells 2017, 35, 1290–1302. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Lee, J.Y.; Kaneko, Y.; Tuazon, J.P.; Vale, F.; van Loveren, H.; Borlongan, C.V. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica 2019, 104, 1062–1073. [Google Scholar] [CrossRef]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Suzuki, T.; Sato, Y.; Kushida, Y.; Tsuji, M.; Wakao, S.; Ueda, K.; Imai, K.; Iitani, Y.; Shimizu, S.; Hida, H.; et al. Intravenously delivered multilineage-differentiating stress enduring cells dampen excessive glutamate metabolism and microglial activation in experimental perinatal hypoxic ischemic encephalopathy. J. Cereb. Blood Flow Metab. 2021, 41, 1707–1720. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doeppner, T.R.; El Aanbouri, M.; Dietz, G.P.; Weise, J.; Schwarting, S.; Bähr, M. Transplantation of TAT-Bcl-xL-transduced neural precursor cells: Long-term neuroprotection after stroke. Neurobiol. Dis. 2010, 40, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Drecun, S.; Varga, B.V.; Vonderwalde, I.; Siu, R.; Nagy, A.; Morshead, C.M. Transplantation of Human Cortically-Specified Neuroepithelial Progenitor Cells Leads to Improved Functional Outcomes in a Mouse Model of Stroke. Front. Cell. Neurosci. 2021, 15, 4290. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Hoover, J.; Kaneko, Y.; Borlongan, C.V. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke 2015, 46, 2616–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghuman, H.; Perry, N.; Grice, L.; Gerwig, M.; Moorhead, J., Jr.; Nitzsche, F.; Poplawsky, A.J.; Ambrosio, F.; Modo, M. Physical therapy exerts sub-additive and suppressive effects on intracerebral neural stem cell implantation in a rat model of stroke. J. Cereb. Blood Flow Metab. 2022, 42, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Park, J.; Kim, K.M.; Jin, H.J.; Wu, H.; Rajadas, J.; Kim, D.H.; Steinberg, G.K.; Lee, W. A neurovascular-unit-on-a-chip for the evaluation of the restorative potential of stem cell therapies for ischaemic stroke. Nat. Biomed. Eng. 2021, 5, 847–863. [Google Scholar] [CrossRef]
- Park, Y.J.; Borlongan, C.V. Recent advances in cell therapy for stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2797–2799. [Google Scholar] [CrossRef]
- Borlongan, M.C.; Farooq, J.; Sadanandan, N.; Wang, Z.J.; Cozene, B.; Lee, J.Y.; Steinberg, G.K. Stem Cells for Aging-Related Disorders. Stem Cell Rev. Rep. 2021, 17, 2054–2058. [Google Scholar] [CrossRef]
- Berlet, R.; Anthony, S.; Brooks, B.; Wang, Z.J.; Sadanandan, N.; Shear, A.; Cozene, B.; Gonzales-Portillo, B.; Parsons, B.; Salazar, F.E.; et al. Combination of Stem Cells and Rehabilitation Therapies for Ischemic Stroke. Biomolecules 2021, 11, 1316. [Google Scholar] [CrossRef]
- Prakash, R.; Fauzia, E.; Siddiqui, A.J.; Yadav, S.K.; Kumari, N.; Shams, M.T.; Naeem, A.; Praharaj, P.P.; Khan, M.A.; Bhutia, S.K.; et al. Oxidative Stress-induced Autophagy Compromises Stem Cell Viability. Stem Cells 2022, 40, 468–478. [Google Scholar] [CrossRef]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006, 7, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chuikov, S.; Taitano, S.; Wu, Q.; Rastogi, A.; Tuck, S.J.; Corey, J.M.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Protects Neural Stem/Progenitor Cells and Neurons from Oxidative Damage through Nrf2-ERK1/2 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 13885–13907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, M.; Smith, J.; Power, J.; Mayer-Pröschel, M. Redox state as a central modulator of precursor cell function. Ann. N. Y. Acad. Sci. 2003, 991, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Rafalski, V.A.; Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011, 93, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Corenblum, M.J.; Ray, S.; Remley, Q.W.; Long, M.; Harder, B.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell 2016, 15, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 2017, 13, 393–401. [Google Scholar] [CrossRef]
- Kernie, S.G.; Parent, J.M. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol. Dis. 2010, 37, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Kokaia, Z.; Lindvall, O. Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol. 2003, 13, 127–132. [Google Scholar] [CrossRef]
- Boese, A.C.; Le, Q.E.; Pham, D.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Lee, J.P.; McKercher, S.; Muller, F.J.; Snyder, E.Y. Neural stem cell transplantation in mouse brain. Curr. Protoc. Neurosci. 2008, 42, 3.10.1–3.10.23. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Niizuma, K.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Narasimhan, P.; Maier, C.M.; Nishiyama, Y.; Chan, P.H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012, 32, 3462–3473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahroba, H.; Davatgaran-Taghipour, Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie 2020, 171–172, 103–109. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, Y.; Qu, T.; Zhang, J.; Duan, X.; Xu, H.; Mu, Y.; Ma, H.; Wang, F. Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro. Life Sci. 2020, 254, 117772. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, P.K.; Bhasin, A.; Padma, M.V.; Mohanty, S. Application of Stem Cells in Stroke: A Multifactorial Approach. Front. Neurosci. 2020, 14, 473. [Google Scholar] [CrossRef]
- Bomprezzi, R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: An overview. Ther. Adv. Neurol. Disord. 2015, 8, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Miao, W.; Liu, Z.; Han, W.; Shi, K.; Shen, Y.; Li, H.; Liu, Q.; Fu, Y.; Huang, D.; et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res. 2016, 7, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.X.; Lisi, L.; Dello Russo, C.; Polak, P.E.; Sharp, A.; Weinberg, G.; Kalinin, S.; Feinstein, D.L. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro 2011, 3, AN20100033. [Google Scholar] [CrossRef]
- Chen, H.; Assmann, J.C.; Krenz, A.; Rahman, M.; Grimm, M.; Karsten, C.M.; Köhl, J.; Offermanns, S.; Wettschureck, N.; Schwaninger, M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J. Clin. Investig. 2014, 124, 2188–2192. [Google Scholar] [CrossRef]
- Safari, A.; Badeli-Sarkala, H.; Namavar, M.R.; Kargar-Abarghouei, E.; Anssari, N.; Izadi, S.; Borhani-Haghighi, A. Neuroprotective effect of dimethyl fumarate in stroke: The role of nuclear factor erythroid 2-related factor 2. Iran. J. Neurol. 2019, 18, 108–113. [Google Scholar] [CrossRef]
- Shou, J.W.; Shaw, P.C. Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms. Cells 2022, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Berberine Hydrochloride Protects C2C12 Myoblast Cells Against Oxidative Stress-Induced Damage via Induction of Nrf-2-Mediated HO-1 Expression. Drug Dev. Res. 2016, 77, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Fekri, H.S.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell. Biochem. 2020, 121, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.W.; Li, X.X.; Tang, Y.S.; Lim-Ho Kong, B.; Wu, H.Y.; Xiao, M.J.; Cheung, C.K.; Shaw, P.C. Novel mechanistic insight on the neuroprotective effect of berberine: The role of PPARδ for antioxidant action. Free Radic. Biol. Med. 2022, 181, 62–71. [Google Scholar] [CrossRef]
- Stucki, D.; Steinhausen, J.; Westhoff, P.; Krahl, H.; Brilhaus, D.; Massenberg, A.; Weber, A.P.M.; Reichert, A.S.; Brenneisen, P.; Stahl, W. Endogenous Carbon Monoxide Signaling Modulates Mitochondrial Function and Intracellular Glucose Utilization: Impact of the Heme Oxygenase Substrate Hemin. Antioxidants 2020, 9, 652. [Google Scholar] [CrossRef]
- Zhengxing, X.; Aiying, H.; Zongqiang, Z.; Zengli, M. Carbon Monoxide Protects Neural Stem Cells Against Iron Overload by Modulating the Crosstalk Between Nrf2 and NF-κB Signaling. Neurochem. Res. 2022, 47, 1383–1394. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, T.; Mao, L.; Zhang, F. Sulforaphane Protects against Brain Diseases: Roles of Cytoprotective Enzymes. Austin J. Cerebrovasc. Dis. Stroke 2017, 4, 1054. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yang, T.; Leak, R.K.; Chen, J.; Zhang, F. Preventive and Protective Roles of Dietary Nrf2 Activators Against Central Nervous System Diseases. CNS Neurol. Disord. Drug Targets 2017, 16, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Ping, Z.; Liu, W.; Kang, Z.; Cai, J.; Wang, Q.; Cheng, N.; Wang, S.; Wang, S.; Zhang, J.H.; Sun, X. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010, 1343, 178–185. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, A.; Srivastava, S.; Siow, R.C.M.; Cash, D.; Modo, M.; Duchen, M.R.; Fraser, P.A.; Williams, S.C.R.; Mann, G.E. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood–brain barrier disruption and neurological deficits in stroke. Free Radic. Biol. Med. 2013, 65, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xu, Q.; Li, C.; Zhao, H. Effects of sulforaphane on neural stem cell proliferation and differentiation. Genesis 2017, 55, e23022. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Cell Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, D.L.; Duryee, M.J.; Sarmiento, C.; Chiou, A.; McGowan, J.D.; Hunter, C.D.; Schlichte, S.L.; Tian, J.; Klassen, L.W.; O’Dell, J.R.; et al. Novel Antioxidant Properties of Doxycycline. Int. J. Mol. Sci. 2018, 19, 4078. [Google Scholar] [CrossRef] [Green Version]
- Yrjänheikki, J.; Keinänen, R.; Pellikka, M.; Hökfelt, T.; Koistinaho, J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 15769–15774. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xue, Y.; Jiao, H.; Liu, Y.; Wang, P. Doxycycline-mediated protective effect against focal cerebral ischemia-reperfusion injury through the modulation of tight junctions and PKCδ signaling in rats. J. Mol. Neurosci. 2012, 47, 89–100. [Google Scholar] [CrossRef]
- Clark, W.M.; Calcagno, F.A.; Gabler, W.L.; Smith, J.R.; Coull, B.M. Reduction of central nervous system reperfusion injury in rabbits using doxycycline treatment. Stroke 1994, 25, 1411–1415; discussion 1416. [Google Scholar] [CrossRef] [Green Version]
- Clark, W.M.; Lessov, N.; Lauten, J.D.; Hazel, K. Doxycycline treatment reduces ischemic brain damage in transient middle cerebral artery occlusion in the rat. J. Mol. Neurosci. 1997, 9, 103–108. [Google Scholar] [CrossRef]
- Malik, Y.S.; Sheikh, M.A.; Zhu, X. Doxycycline can stimulate cytoprotection in neural stem cells with oxygen-glucose deprivation-reoxygenation injury: A potential approach to enhance effectiveness of cell transplantation therapy. Biochem. Biophys. Res. Commun. 2013, 432, 355–358. [Google Scholar] [CrossRef]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2021, 65, 101211. [Google Scholar] [CrossRef]
- Othman, F.A.; Tan, S.C. Preconditioning Strategies to Enhance Neural Stem Cell-Based Therapy for Ischemic Stroke. Brain Sci. 2020, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Yrjänheikki, J.; Tikka, T.; Keinänen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, K.; Chang, J.J.; Khunger, A.; Blacker, D.; Switzer, J.A.; Goyal, N.; Hernandez, A.V.; Pasupuleti, V.; Alexandrov, A.V.; Tsivgoulis, G. Minocycline for acute stroke treatment: A systematic review and meta-analysis of randomized clinical trials. J. Neurol. 2018, 265, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Rueger, M.A.; Muesken, S.; Walberer, M.; Jantzen, S.U.; Schnakenburg, K.; Backes, H.; Graf, R.; Neumaier, B.; Hoehn, M.; Fink, G.R.; et al. Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience 2012, 215, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The promise of minocycline in neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef]
- Vay, S.U.; Blaschke, S.; Klein, R.; Fink, G.R.; Schroeter, M.; Rueger, M.A. Minocycline mitigates the gliogenic effects of proinflammatory cytokines on neural stem cells. J. Neurosci. Res. 2016, 94, 149–160. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, Y.; Won, S.J.; Neumann, M.; Hu, D.; Zhou, L.; Weinstein, P.R.; Liu, J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 2007, 38, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.J.; Liou, D.Y.; Chu, Y.C.; Chen, Y.; Huang, M.C.; Huang, W.C.; Cheng, H.; Tsai, S.K.; Huang, S.S. Minocycline exhibits synergism with conditioned medium of bone marrow mesenchymal stem cells against ischemic stroke. J. Tissue Eng. Regen. Med. 2021, 15, 279–292. [Google Scholar] [CrossRef]
- Franco, E.C.; Cardoso, M.M.; Gouvêia, A.; Pereira, A.; Gomes-Leal, W. Modulation of microglial activation enhances neuroprotection and functional recovery derived from bone marrow mononuclear cell transplantation after cortical ischemia. Neurosci. Res. 2012, 73, 122–132. [Google Scholar] [CrossRef]
- Cho, D.Y.; Jeun, S.S. Combination therapy of human bone marrow-derived mesenchymal stem cells and minocycline improves neuronal function in a rat middle cerebral artery occlusion model. Stem Cell Res. Ther. 2018, 9, 309. [Google Scholar] [CrossRef]
- Li, J.; Johnson, D.; Calkins, M.; Wright, L.; Svendsen, C.; Johnson, J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol. Sci. 2005, 83, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Yang, Y.; Zhang, L.; Cui, L.; Zhang, C.; Chen, R.; Xie, Y.; He, J.; He, W. Tert-butylhydroquinone enhanced angiogenesis and astrocyte activation by activating nuclear factor-E2-related factor 2/heme oxygenase-1 after focal cerebral ischemia in mice. Microvasc. Res. 2019, 126, 103891. [Google Scholar] [CrossRef] [PubMed]
- Sukumari-Ramesh, S.; Alleyne, C.H., Jr. Post-Injury Administration of Tert-butylhydroquinone Attenuates Acute Neurological Injury After Intracerebral Hemorrhage in Mice. J. Mol. Neurosci. 2016, 58, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ji, C.; Wu, L.; Qiu, J.; Li, Q.; Shao, Z.; Chen, G. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: Role of Keap1/Nrf2/ARE pathway. PLoS ONE 2014, 9, e97685. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, R.; Kobayashi, M.; Kobashi, R.; Onishi, M.; Maeda, M.; Kataoka, Y.; Imaoka, S. Brain pericytes acquire stemness via the Nrf2-dependent antioxidant system. Stem Cells 2022, sxac024. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. Biofactors 2010, 36, 401–406. [Google Scholar] [CrossRef]
- Gu, J.; Wang, C.Q.; Fan, H.H.; Ding, H.Y.; Xie, X.L.; Xu, Y.M.; Wang, B.Y.; Huang, D.J. Effects of resveratrol on endothelial progenitor cells and their contributions to reendothelialization in intima-injured rats. J. Cardiovasc. Pharmacol. 2006, 47, 711–721. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, J.; Huang, Y.; Hu, Z. Resveratrol has an Overall Neuroprotective Role in Ischemic Stroke: A Meta-Analysis in Rodents. Front. Pharmacol. 2021, 12, 795409. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Li, F.; Lu, S.; Ren, L.; Bian, S.; Liu, M.; Zhao, D.; Wang, S.; Wang, J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 2022, 283, 114739. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mok, H.; Yeo, W.S.; Ahn, J.H.; Choi, Y.K. Role of ginseng in the neurovascular unit of neuroinflammatory diseases focused on the blood-brain barrier. J. Ginseng Res. 2021, 45, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Q.; Cheng, W.; Wang, Y.; Wang, X.M.; Zhao, S.Z.; Zhou, Y.; Liu, S.J.; Wang, X.T. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J. Ethnopharmacol. 2011, 133, 724–728. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Jin, F.; Wang, Y.; Li, F.; Wang, L.; Wang, Q.; Ren, Z.; Wang, Y. In vitro and in vivo anti-inflammatory effects of theaflavin-3,3′-digallate on lipopolysaccharide-induced inflammation. Eur. J. Pharmacol. 2017, 794, 52–60. [Google Scholar] [CrossRef]
- Takemoto, M.; Takemoto, H. Synthesis of Theaflavins and Their Functions. Molecules 2018, 23, 918. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, J.; Sun, X.; Li, Y.; Duan, Y.; Yao, H. Theaflavic acid from black tea protects PC12 cells against ROS-mediated mitochondrial apoptosis induced by OGD/R via activating Nrf2/ARE signaling pathway. J. Nat. Med. 2020, 74, 238–246. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Wu, H.; Yang, Z.; Fei, L.; Zhu, J. Theaflavin attenuates cerebral ischemia/reperfusion injury by abolishing miRNA-128-3p-mediated Nrf2 inhibition and reducing oxidative stress. Mol. Med. Rep. 2019, 20, 4893–4904. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Qin, H.; Jiao, M.; Hua, S.; Sun, T. Modeling the neuro-protection of theaflavic acid from black tea and its synergy with nimodipine via mitochondria apoptotic pathway. J. Zhejiang Univ. Sci. B 2021, 22, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef]
- Chen, J.; Tang, X.Q.; Zhi, J.L.; Cui, Y.; Yu, H.M.; Tang, E.H.; Sun, S.N.; Feng, J.Q.; Chen, P.X. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis 2006, 11, 943–953. [Google Scholar] [CrossRef]
- Bavarsad, K.; Barreto, G.E.; Hadjzadeh, M.A.; Sahebkar, A. Protective Effects of Curcumin Against Ischemia-Reperfusion Injury in the Nervous System. Mol. Neurobiol. 2019, 56, 1391–1404. [Google Scholar] [CrossRef]
- Ma, W.; Xu, D.; Zhao, L.; Yuan, M.; Cui, Y.L.; Li, Y. Therapeutic role of curcumin in adult neurogenesis for management of psychiatric and neurological disorders: A scientometric study to an in-depth review. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Chumboatong, W.; Thummayot, S.; Govitrapong, P.; Tocharus, C.; Jittiwat, J.; Tocharus, J. Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem. Int. 2017, 102, 114–122. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [CrossRef]
- Balla, J.; Vercellotti, G.M.; Jeney, V.; Yachie, A.; Varga, Z.; Jacob, H.S.; Eaton, J.W.; Balla, G. Heme, heme oxygenase, and ferritin: How the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid. Redox Signal. 2007, 9, 2119–2137. [Google Scholar] [CrossRef] [PubMed]
- Fredenburgh, L.E.; Merz, A.A.; Cheng, S. Haeme oxygenase signalling pathway: Implications for cardiovascular disease. Eur. Heart J. 2015, 36, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, M.; Wang, M.; Li, Y.; Wen, A. Posttreatment with 11-Keto-β-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol. Neurobiol. 2015, 52, 1430–1439. [Google Scholar] [CrossRef]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, Z.A.; Nada, S.E.; Doré, S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience 2011, 180, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, S.; Zhang, M.; Weng, Z.; Li, P.; Gan, Y.; Zhang, L.; Cao, G.; Gao, Y.; Leak, R.K.; et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke 2012, 43, 1390–1397. [Google Scholar] [CrossRef]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef]
- Parada, E.; Buendia, I.; León, R.; Negredo, P.; Romero, A.; Cuadrado, A.; López, M.G.; Egea, J. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J. Pineal Res. 2014, 56, 204–212. [Google Scholar] [CrossRef]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012, 52, 1634–1647. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Jiang, X.; Wang, Y.; Deng, Q.; Lam Yung, K.K.; Zhang, S. Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway. Pharmaceuticals 2021, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, B.; Lu, J.; Wang, G.; Yu, Y. Hemopexin reduces blood-brain barrier injury and protects synaptic plasticity in cerebral ischemic rats by promoting EPCs through the HO-1 pathway. Brain Res. 2018, 1699, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Cang, J.; Wang, H.; Xue, Z. Propofol attenuates cerebral ischemia/reperfusion injury partially using heme oxygenase-1. J. Neurosurg. Anesthesiol. 2013, 25, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.A.; Bidwell, G.L.B., III. Targeting the NF-κB pathway for therapy of ischemic stroke. Ther. Deliv. 2020, 11, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Harari, O.A.; Liao, J.K. NF-κB and innate immunity in ischemic stroke. Ann. N. Y. Acad. Sci. 2010, 1207, 32–40. [Google Scholar] [CrossRef]
- Jover-Mengual, T.; Hwang, J.-Y.; Byun, H.-R.; Court-Vazquez, B.L.; Centeno, J.M.; Burguete, M.C.; Zukin, R.S. The Role of NF-κB Triggered Inflammation in Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 3610. [Google Scholar] [CrossRef]
- Sironi, L.; Banfi, C.; Brioschi, M.; Gelosa, P.; Guerrini, U.; Nobili, E.; Gianella, A.; Paoletti, R.; Tremoli, E.; Cimino, M. Activation of NF-kB and ERK1/2 after permanent focal ischemia is abolished by simvastatin treatment. Neurobiol. Dis. 2006, 22, 445–451. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hou, Y.; Sun, J.; Xu, Q.L.; Zhang, D.Q.; Han, Y.C. Effect of atorvastatin on expression of TLR4 and NF-κB in stroke rats and its protective effect on brain. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10799–10805. [Google Scholar] [CrossRef]
- Hölschermann, H.; Schuster, D.; Parviz, B.; Haberbosch, W.; Tillmanns, H.; Muth, H. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 2006, 185, 240–245. [Google Scholar] [CrossRef]
- Jahromi, G.P.; Shabanzadeh, A.P.; Hashtjini, M.M.; Sadr, S.S.; Vani, J.R.; Sarshoori, J.R.; Charish, J. Bone marrow-derived mesenchymal stem cell and simvastatin treatment leads to improved functional recovery and modified c-Fos expression levels in the brain following ischemic stroke. Iran. J. Basic Med. Sci. 2018, 21, 1004–1012. [Google Scholar] [CrossRef]
- Choi, N.Y.; Kim, J.Y.; Hwang, M.; Lee, E.H.; Choi, H.; Lee, K.Y.; Lee, Y.J.; Koh, S.H. Atorvastatin Rejuvenates Neural Stem Cells Injured by Oxygen-Glucose Deprivation and Induces Neuronal Differentiation through Activating the PI3K/Akt and ERK Pathways. Mol. Neurobiol. 2019, 56, 2964–2977. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.J.; Wu, J.L.; Quan, W.Q.; Xiao, W.D.; Chew, H.; Jiang, C.M.; Li, D. Naloxone attenuates ischemic brain injury in rats through suppressing the NIK/IKKα/NF-κB and neuronal apoptotic pathways. Acta Pharmacol. Sin. 2019, 40, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dang, W.; Zhang, S.; Wang, L.; Zhang, X. Artesunate attenuates inflammatory injury and inhibits the NF-κB pathway in a mouse model of cerebral ischemia. J. Int. Med. Res. 2021, 49, 03000605211053549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, Y.; Ge, H.; Wang, J.; Chen, X.; Lei, X.; Zhong, J.; Zhang, C.; Xian, J.; Lu, Y.; et al. Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates Ischemia-reperfusion Injury through PI3K/Akt/FOXO-3a/p27(kip1) signaling pathway. Aging 2020, 12, 8029–8048. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Y.; Ge, H.; Wang, J.; Lei, X.; Chen, X.; Wan, F.; Feng, H.; Tan, L. Neurogenesis and Proliferation of Neural Stem/Progenitor Cells Conferred by Artesunate via FOXO3a/p27Kip1 Axis in Mouse Stroke Model. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Wang, X.; Shen, B.; Sun, D.; Cui, X. Aspirin ameliorates cerebral infarction through regulation of TLR4/NF-κB-mediated endoplasmic reticulum stress in mouse model. Mol. Med. Rep. 2018, 17, 479–487. [Google Scholar] [CrossRef]
- Sheibani, V.; Esmaeilpour, K.; Eslaminejad, T.; Nematollahi-Mahani, S.N. Coadministration of the Human Umbilical Cord Matrix-Derived Mesenchymal Cells and Aspirin Alters Postischemic Brain Injury in Rats. J. Stroke Cerebrovasc. Dis. 2015, 24, 2005–2016. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Xie, Y.; Zan, J.; Tan, W. Isosteviol Sodium Protects Against Permanent Cerebral Ischemia Injury in Mice via Inhibition of NF-κB-Mediated Inflammatory and Apoptotic Responses. J. Stroke Cerebrovasc. Dis. 2017, 26, 2603–2614. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Xie, Y.; Tian, F.; Hu, H.; Tan, W. Isosteviol Sodium Inhibits Astrogliosis after Cerebral Ischemia/Reperfusion Injury in Rats. Biol. Pharm. Bull. 2018, 41, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lu, M.; Zhang, X.; Kuai, Y.; Mei, Y.; Tan, Q.; Zhong, K.; Sun, X.; Tan, W. Isosteviol Sodium Protects against Ischemic Stroke by Modulating Microglia/Macrophage Polarization via Disruption of GAS5/miR-146a-5p sponge. Sci. Rep. 2019, 9, 12221. [Google Scholar] [CrossRef] [PubMed]
- Rösing, N.; Salvador, E.; Güntzel, P.; Kempe, C.; Burek, M.; Holzgrabe, U.; Soukhoroukov, V.; Wunder, C.; Förster, C. Neuroprotective Effects of Isosteviol Sodium in Murine Brain Capillary Cerebellar Endothelial Cells (cerebEND) After Hypoxia. Front. Cell. Neurosci. 2020, 14, 3950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Wu, F.; Chi, X.; Pang, Y.; Jin, H.; Sun, Y.; Zhang, S. Neuroprotective Effects of Hesperetin in Regulating Microglia Polarization after Ischemic Stroke by Inhibiting TLR4/NF-κB Pathway. J. Healthc. Eng. 2021, 2021, 9938874. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Qie, S.; Gao, F.; Ding, Z.; Yang, S.; Tian, G.; Liu, Z.; Xi, J. Baicalein ameliorates ischemic brain damage through suppressing proinflammatory microglia polarization via inhibiting the TLR4/NF-κB and STAT1 pathway. Brain Res. 2021, 1770, 147626. [Google Scholar] [CrossRef]
- Xue, X.; Qu, X.J.; Yang, Y.; Sheng, X.H.; Cheng, F.; Jiang, E.N.; Wang, J.H.; Bu, W.; Liu, Z.P. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochem. Biophys. Res. Commun. 2010, 403, 398–404. [Google Scholar] [CrossRef]
- Dai, M.; Chen, B.; Wang, X.; Gao, C.; Yu, H. Icariin enhance mild hypothermia-induced neuroprotection via inhibiting the activation of NF-κB in experimental ischemic stroke. Metab. Brain Dis. 2021, 36, 1779–1790. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, D.; Yin, C.; Liu, B.; Shi, J.; Gong, Q. Icariside II protects against cerebral ischemia-reperfusion injury in rats via nuclear factor-κB inhibition and peroxisome proliferator-activated receptor up-regulation. Neurochem. Int. 2016, 96, 56–61. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Xiong, L.; Zhang, L.; Li, X.; Cao, X.; Xue, J.; Li, L.; Huang, C.; Huang, Z. Genistein-3′-sodium sulfonate Attenuates Neuroinflammation in Stroke Rats by Down-Regulating Microglial M1 Polarization through α7nAChR-NF-κB Signaling Pathway. Int. J. Biol. Sci. 2021, 17, 1088–1100. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Tundis, R.; Loizzo, M.R.; Sobarzo-Sanchez, E.; Orhan, I.E.; Nabavi, S.M. Genistein: A Boon for Mitigating Ischemic Stroke. Curr. Top. Med. Chem. 2015, 15, 1714–1721. [Google Scholar] [CrossRef]
- Le, K.; Song, Z.; Deng, J.; Peng, X.; Zhang, J.; Wang, L.; Zhou, L.; Bi, H.; Liao, Z.; Feng, Z. Quercetin alleviates neonatal hypoxic-ischemic brain injury by inhibiting microglia-derived oxidative stress and TLR4-mediated inflammation. Inflamm. Res. 2020, 69, 1201–1213. [Google Scholar] [CrossRef]
- Paramita, P.; Sethu, S.N.; Subhapradha, N.; Ragavan, V.; Ilangovan, R.; Balakrishnan, A.; Srinivasan, N.; Murugesan, R.; Moorthi, A. Neuro-protective effects of nano-formulated hesperetin in a traumatic brain injury model of Danio rerio. Drug Chem. Toxicol. 2022, 45, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ye, Y.; Xu, L.; Yuan, W.; Zhang, Q. Icariin and mesenchymal stem cells synergistically promote angiogenesis and neurogenesis after cerebral ischemia via PI3K and ERK1/2 pathways. Biomed. Pharmacother. 2018, 108, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhang, H.T.; Cai, Y.Q.; Han, Y.J.; Yao, F.; Yuan, Z.H.; Wu, B.Y. Anti-inflammatory Effect of Mesenchymal Stromal Cell Transplantation and Quercetin Treatment in a Rat Model of Experimental Cerebral Ischemia. Cell. Mol. Neurobiol. 2016, 36, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Wilde, G.J.C.; Spiller, D.G.; Kennedy, S.M.; Ray, D.W.; Sullivan, E.; Unitt, J.F.; White, M.R.H. NF-κB signalling is inhibited by glucocorticoid receptor and STAT6 via distinct mechanisms. J. Cell Sci. 2003, 116, 2495–2503. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, P.A.; Soane, T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 2011, CD000064. [Google Scholar] [CrossRef]
- Azadi, R.; Mousavi, S.E.; Kazemi, N.M.; Yousefi-Manesh, H.; Rezayat, S.M.; Jaafari, M.R. Anti-inflammatory efficacy of Berberine Nanomicelle for improvement of cerebral ischemia: Formulation, characterization and evaluation in bilateral common carotid artery occlusion rat model. BMC Pharmacol. Toxicol. 2021, 22, 54. [Google Scholar] [CrossRef]
- Zeynalov, E.; Doré, S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox. Res. 2009, 15, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Kong, X.; Yang, Q.; Zhang, Y.; Han, J.; Li, P.; Xiong, L.; Zhao, G. Ginsenoside rd in experimental stroke: Superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics 2011, 8, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Nada, S.E.; Tulsulkar, J.; Shah, Z.A. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761®) after permanent ischemic stroke in mice. Mol. Neurobiol. 2014, 49, 945–956. [Google Scholar] [CrossRef]
- Li, R.; Si, M.; Jia, H.Y.; Ma, Z.; Li, X.W.; Li, X.Y.; Dai, X.R.; Gong, P.; Luo, S.Y. Anfibatide alleviates inflammation and apoptosis via inhibiting NF-kappaB/NLRP3 axis in ischemic stroke. Eur. J. Pharmacol. 2022, 926, 175032. [Google Scholar] [CrossRef]
- Cai, J.; Liang, J.; Zhang, Y.; Shen, L.; Lin, H.; Hu, T.; Zhan, S.; Xie, M.; Liang, S.; Xian, M.; et al. Cyclo-(Phe-Tyr) as a novel cyclic dipeptide compound alleviates ischemic/reperfusion brain injury via JUNB/JNK/NF-κB and SOX5/PI3K/AKT pathways. Pharmacol. Res. 2022, 180, 106230. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, P.; Guo, X.; Yin, M.; Li, X.; Jiang, L.; Shao, J.; Chen, X.; Jiang, C.; Tao, L.; et al. Maraviroc, an inhibitor of chemokine receptor type 5, alleviates neuroinflammatory response after cerebral Ischemia/reperfusion injury via regulating MAPK/NF-κB signaling. Int. Immunopharmacol. 2022, 108, 108755. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, B. Donepezil ameliorates oxygen-glucose deprivation/reoxygenation-induced brain microvascular endothelial cell dysfunction via the SIRT1/FOXO3a/NF-κB pathways. Bioengineered 2022, 13, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, X.; Zhang, D.; Yu, L.; Li, N.; Lu, Z.; Gao, Y.; Wang, M.; Liu, X.; Zhou, C.; et al. ChAT-positive neurons participate in subventricular zone neurogenesis after middle cerebral artery occlusion in mice. Behav. Brain Res. 2017, 316, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Man, J.; Cui, K.; Fu, X.; Zhang, D.; Lu, Z.; Gao, Y.; Yu, L.; Li, N.; Wang, J. Donepezil promotes neurogenesis via Src signaling pathway in a rat model of chronic cerebral hypoperfusion. Brain Res. 2020, 1736, 146782. [Google Scholar] [CrossRef]
- Liu, W.; Shao, C.; Zang, C.; Sun, J.; Xu, M.; Wang, Y. Protective effects of dexmedetomidine on cerebral ischemia/reperfusion injury via the microRNA-214/ROCK1/NF-κB axis. BMC Anesthesiol. 2021, 21, 203. [Google Scholar] [CrossRef]
- Xian, M.; Cai, J.; Zheng, K.; Liu, Q.; Liu, Y.; Lin, H.; Liang, S.; Wang, S. Aloe-emodin prevents nerve injury and neuroinflammation caused by ischemic stroke via the PI3K/AKT/mTOR and NF-κB pathway. Food Funct. 2021, 12, 8056–8067. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, Y.; Pan, H.; Xuan, Z.; Yan, S.; Mao, Y.; Xiao, X.; Huang, X.; Zhang, H.; Zhou, F.; et al. 9-Methylfascaplysin exerts anti-ischemic stroke neuroprotective effects via the inhibition of neuroinflammation and oxidative stress in rats. Int. Immunopharmacol. 2021, 97, 107656. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Gao, Y.; Lu, J.; Xie, D.; Yu, W.; He, F.; Liu, W.; Hisatome, I.; Yamamoto, T.; et al. Uric acid inhibits HMGB1-TLR4-NF-κB signaling to alleviate oxygen-glucose deprivation/reoxygenation injury of microglia. Biochem. Biophys. Res. Commun. 2021, 540, 22–28. [Google Scholar] [CrossRef]
- Kao, M.H.; Wu, J.S.; Cheung, W.M.; Chen, J.J.; Sun, G.Y.; Ong, W.Y.; Herr, D.R.; Lin, T.N. Clinacanthus nutans Mitigates Neuronal Death and Reduces Ischemic Brain Injury: Role of NF-κB-driven IL-1β Transcription. Neuromol. Med. 2021, 23, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Mi, Y.; Yang, Y.; Li, Q.; Zhou, D.; Wei, K.; Chen, G.; Li, N.; Hou, Y. Pterostilbene alleviates cerebral ischemia and reperfusion injury in rats by modulating microglial activation. Food Funct. 2020, 11, 5432–5445. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Liang, J.; Zhang, C.; Li, R.; Mou, Q.; Qin, J.; Li, X.; Wang, J. Synergistic Effects of Salvianolic Acid B and Puerarin on Cerebral Ischemia Reperfusion Injury. Molecules 2018, 23, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, P.; Zhang, Y.; Cui, G.; Bian, Y.; Zhang, M.; Zhang, J.; Liu, Y.; Yang, X.; Isaiah, A.O.; Lin, Y.; et al. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS ONE 2012, 7, e35636. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.C.; Quang, T.H.; Oh, H.; Kim, Y.C. Steppogenin Isolated from Cudrania tricuspidata Shows Antineuroinflammatory Effects via NF-κB and MAPK Pathways in LPS-Stimulated BV2 and Primary Rat Microglial Cells. Molecules 2017, 22, 2130. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Hu, Z.; Yang, Y.; Yin, Y.; Li, W.; Wu, L.; Fang, M. Anti-Inflammatory and Neuroprotective Effects of Triptolide via the NF-κB Signaling Pathway in a Rat MCAO Model. Anat. Rec. 2016, 299, 256–266. [Google Scholar] [CrossRef] [Green Version]
- El-Sahar, A.E.; Safar, M.M.; Zaki, H.F.; Attia, A.S.; Ain-Shoka, A.A. Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: Implication of the oxidative-inflammatory-apoptotic pathway. Life Sci. 2015, 126, 81–86. [Google Scholar] [CrossRef]
- Tian, M.; Yang, M.; Li, Z.; Wang, Y.; Chen, W.; Yang, L.; Li, Y.; Yuan, H. Fluoxetine suppresses inflammatory reaction in microglia under OGD/R challenge via modulation of NF-κB signaling. Biosci. Rep. 2019, 39, BSR20181584. [Google Scholar] [CrossRef]
- Wei, N.; Li, C.; Zhu, Y.; Zheng, P.; Hu, R.; Chen, J. Fluoxetine regulates the neuronal differentiation of neural stem cells transplanted into rat brains after stroke by increasing the 5HT level. Neurosci. Lett. 2022, 772, 136447. [Google Scholar] [CrossRef]

| Compound | Target(s) | Effect on Stroke Treatment | Citation | Effect on Stem Cells in Setting of Stroke | Citation |
|---|---|---|---|---|---|
| Dimethyl Fumarate | Nrf2 Pathway via KEAP1 |
| [120] |
| [103] |
| Berberine | Nrf2 Pathway via PPARδ |
| [227] |
| [121] |
| Carbon Monoxide | Nrf2 and NF-κB Pathways |
| [228] |
| [126] |
| Sulforaphane | Nrf2 Pathway Upstream |
| [131] |
| [132] |
| Doxycycline | Nrf2 Pathway Upstream |
| [136,137,138] |
| [134,135] |
| Minocycline | Nrf2 Pathway Upstream |
| [143] |
| [112,147] |
| Tert-butylhydroquinone | Nrf2 Pathway Upstream |
| [33] |
| [153,155] |
| Resveratrol | Nrf2 Pathway Upstream |
| [159] |
| [157,158,161] |
| Ginseng | Nrf2 and NF-κB Pathways |
| [229] |
| [164] |
| Theaflavin | Nrf2 Pathway Upstream (via nuclear translocation) |
| [170] |
| [171] |
| Curcumin | Nrf2 Pathway Upstream (via gene transcription) |
| [172,175,176] |
| [178,179] |
| Gingko biloba | HO-1 Downstream |
| [187] |
| [230] |
| 2-cyano-3,12 dioxooleana-1,9 dien-28-oyl imidazoline | HO-1 Downstream |
| [188] | - | - |
| Melatonin | HO-1 Downstream |
| [190] |
| [191] |
| Oleanolic acid | HO-1 Downstream |
| [192] | - | - |
| Hemopexin | HO-1 Downstream |
| [193] | - | - |
| Propofol | HO-1 Downstream |
| [194] | - | - |
| Simvastatin | NF-κB Pathway |
| [198] |
| [201] |
| Atorvastatin | NF-κB Pathway |
| [199] |
| [202] |
| Naloxone | NF-κB Pathway |
| [203] | - | - |
| Artesunate | NF-κB Pathway |
| [204] |
| [205,206] |
| Aspirin | NF-κB Pathway |
| [207] |
| [208] |
| Isosteviol sodium | NF-κB Pathway |
| [209] | - | - |
| Hesperetin | NF-κB Pathway |
| [213] |
| [221] |
| Baicalein | NF-κB Pathway |
| [214] | - | - |
| Icariin | NF-κB Pathway |
| [216] |
| [222] |
| Genistein-3′-sodium sulfonate | NF-κB Pathway |
| [218] | - | - |
| Quercetin | NF-κB Pathway |
| [220] |
| [223] |
| Anfibatide | NF-κB Pathway |
| [231] | - | - |
| Cyclo-(Phe-Tyr) | NF-κB Pathway |
| [232] | - | - |
| Maraviroc | NF-κB Pathway |
| [233] | - | - |
| Donepezil | NF-κB Pathway |
| [234] |
| [235,236] |
| Dexmedetomidine | NF-κB Pathway |
| [237] | - | - |
| Aloe-emodin | NF-κB Pathway |
| [238] | - | - |
| 9-Methylfascaplysin | NF-κB Pathway |
| [239] | - | - |
| Uric acid | NF-κB Pathway |
| [240] | - | - |
| Clinacanthus nutans | NF-κB Pathway |
| [241] | - | - |
| Pterostilbene | NF-κB Pathway |
| [242] | - | - |
| Salvianolic Acid B and Puerarin | NF-κB Pathway |
| [243] | Salvianolic Acid B:
| [244] |
| Steppogenin | NF-κB Pathway |
| [245] | - | - |
| Triptolide | NF-κB Pathway |
| [246] | - | - |
| Sitagliptin | NF-κB Pathway |
| [247] | - | - |
| Fluoxetine | NF-κB Pathway |
| [248] |
| [249] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, J.; Lockard, G.; Monsour, M.; Alayli, A.; Borlongan, C.V. The Role of Concomitant Nrf2 Targeting and Stem Cell Therapy in Cerebrovascular Disease. Antioxidants 2022, 11, 1447. https://doi.org/10.3390/antiox11081447
Gordon J, Lockard G, Monsour M, Alayli A, Borlongan CV. The Role of Concomitant Nrf2 Targeting and Stem Cell Therapy in Cerebrovascular Disease. Antioxidants. 2022; 11(8):1447. https://doi.org/10.3390/antiox11081447
Chicago/Turabian StyleGordon, Jonah, Gavin Lockard, Molly Monsour, Adam Alayli, and Cesario V. Borlongan. 2022. "The Role of Concomitant Nrf2 Targeting and Stem Cell Therapy in Cerebrovascular Disease" Antioxidants 11, no. 8: 1447. https://doi.org/10.3390/antiox11081447






