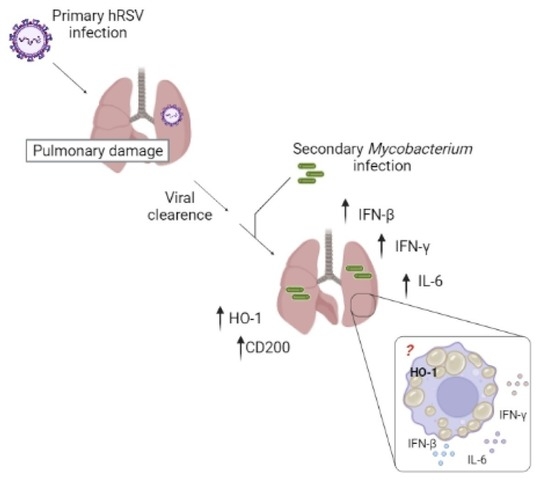
Increased Heme Oxygenase 1 Expression upon a Primary Exposure to the Respiratory Syncytial Virus and a Secondary Mycobacterium bovis Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Viral Propagation and Titration
2.3. Mycobacterium Bovis-BCG-Culture, and Storage
2.4. Mouse Immunization and Viral Infection
2.5. Mouse Viral and Mycobacterial Infections
2.6. Evaluation of hRSV-Associated Disease Parameters
2.7. Lung Histopathology Analyses
2.8. Relative Expression by RT-qPCR
2.9. Statistical Analyses
3. Results
3.1. HRSV Infection Induces de Expression of Immunomodulatory Molecules
3.2. Previous hRSV Infection Causes Further Long-Term Susceptibility to Mycobacterium Bovis-Driven Pneumonia
3.3. Characterization of the Inflammatory Landscape of the Lungs
3.4. rBCG-N-hRSV Immunization Promotes a Higher Induction of HO-1 on Epithelial Cells than in DCs at Late Infection Times
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwane, M.K.; Farnon, E.C.; Gerber, S.I. Importance of Global Surveillance for Respiratory Syncytial Virus. J. Infect. Dis. 2013, 208, S165–S166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proesmans, M.; Rector, A.; Keyaerts, E.; Vandendijck, Y.; Vermeulen, F.; Sauer, K.; Reynders, M.; Verschelde, A.; Laffut, W.; Garmyn, K.; et al. Risk factors for disease severity and increased medical resource utilization in respiratory syncytial virus (+) hospitalized children: A descriptive study conducted in four Belgian hospitals. PLoS ONE 2022, 17, e0268532. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.B.; Rosario, C.S.; Santos, J.S.; Avanzi, V.M.; Nogueira, M.B.; Vidal, L.R.; Raboni, S.M. Molecular characterization and clinical epidemiology of human respiratory syncytial virus (HRSV) A and B in hospitalized children, Southern Brazil. J. Med. Virol. 2017, 89, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Gálvez, N.M.S.; Canedo-Marroquín, G.; Pizarro-Ortega, M.S.; Andrade-Parra, C.; Gómez-Santander, F.; Kalergis, A.M. Contribution of Cytokines to Tissue Damage During Human Respiratory Syncytial Virus Infection. Front. Immunol. 2019, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Fernandez, R.; Tapia, L.I.; Yang, C.-F.; Torres, J.P.; Chavez-Bueno, S.; Garcia, C.; Jaramillo, L.M.; Moore-Clingenpeel, M.; Jafri, H.; Peeples, M.E.; et al. Respiratory Syncytial Virus Genotypes, Host Immune Profiles, and Disease Severity in Young Children Hospitalized with Bronchiolitis. J. Infect. Dis. 2018, 217, 24–34. [Google Scholar] [CrossRef] [Green Version]
- González, A.E.; Lay, M.K.; Jara, E.L.; Espinoza, J.A.; Gómez, R.S.; Soto, J.; Rivera, C.A.; Abarca, K.; Bueno, S.M.; Riedel, C.A.; et al. Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection. Virulence 2017, 8, 685–704. [Google Scholar] [CrossRef]
- Sigurs, N.; Aljassim, F.; Kjellman, B.; Robinson, P.D.; Sigurbergsson, F.; Bjarnason, R.; Gustafsson, P.M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010, 65, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Mavunda, K.; Krilov, L.R. Current State of Respiratory Syncytial Virus Disease and Management. Infect. Dis. Ther. 2021, 10, 5–16. [Google Scholar] [CrossRef]
- Abramson, J.S.; Wheeler, J.G. Virus-induced neutrophil dysfunction: Role in the pathogenesis of bacterial infections. Pediatr. Infect. Dis. J. 1994, 13, 643–652. [Google Scholar] [CrossRef]
- Aguilera, E.R.; Pfeiffer, J.K. Strength in numbers: Mechanisms of viral co-infection. Virus Res. 2019, 265, 43–46. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and immunological outcomes of coinfections. Clin. Microbiol. Rev. 2018, 31, e00111-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sande, C.J.; Njunge, J.M.; Ngoi, J.M.; Mutunga, M.N.; Chege, T.; Gicheru, E.T.; Gardiner, E.M.; Gwela, A.; Green, C.A.; Drysdale, S.B.; et al. Airway response to respiratory syncytial virus has incidental antibacterial effects. Nat. Commun. 2019, 10, 2218. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Stark, M.A.; Colasurdo, G.N.; LeVine, A.M. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J. Med. Virol. 2006, 78, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Gálvez, N.M.; Andrade, C.A.; Ramírez, M.A.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. BCG vaccination induces cross-protective immunity against pathogenic microorganisms. Trends Immunol. 2022, 43, 322–335. [Google Scholar] [CrossRef]
- Tree, J.A.; Williams, A.; Clark, S.; Hall, G.; Marsh, P.D.; Ivanyi, J. Intranasal bacille Calmette–Guérin (BCG) vaccine dosage needs balancing between protection and lung pathology. Clin. Exp. Immunol. 2004, 138, 405–409. [Google Scholar] [CrossRef]
- Harris, S.A.; White, A.; Stockdale, L.; Tanner, R.; Sibley, L.; Sarfas, C.; Meyer, J.; Peter, J.; O’Shea, M.K.; Thomas, Z.-R.M.; et al. Development of a non-human primate BCG infection model for the evaluation of candidate tuberculosis vaccines. Tuberculosis 2017, 108, 99–105. [Google Scholar] [CrossRef]
- Leversen, N.A.; Sviland, L.; Wiker, H.G.; Mustafa, T. Long-Term Persistence of BCG Pasteur in Lungs of C57BL/6 Mice Following Intranasal Infection. Scand. J. Immunol. 2012, 75, 489–499. [Google Scholar] [CrossRef]
- Dunn, L.L.; Midwinter, R.G.; Ni, J.; Hamid, H.A.; Parish, C.R.; Stocker, R. New Insights into Intracellular Locations and Functions of Heme Oxygenase-1. Antioxid. Redox Signal. 2014, 20, 1723–1742. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, J.A.; León, M.A.; Céspedes-Donoso, P.F.; Gómez, R.S.; Canedo-Marroquín, G.; Riquelme, S.A.; Salazar-Echegarai, F.J.; Blancou, P.; Simon, T.; Anegon, I.; et al. Heme Oxygenase-1 Modulates Human Respiratory Syncytial Virus Replication and Lung Pathogenesis during Infection. J. Immunol. 2017, 199, 212–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill-Batorski, L.; Halfmann, P.; Neumann, G.; Kawaoka, Y. The Cytoprotective Enzyme Heme Oxygenase-1 Suppresses Ebola Virus Replication. J. Virol. 2013, 87, 13795–13802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharn, C.R.; Collins, A.C.; Nair, V.R.; Stamm, C.E.; Marciano, D.K.; Graviss, E.A.; Shiloh, M.U. Heme Oxygenase-1 Regulates Inflammation and Mycobacterial Survival in Human Macrophages during Mycobacterium tuberculosis Infection. J. Immunol. 2016, 196, 4641–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacharia, V.M.; Shiloh, M.U. Effect of carbon monoxide on Mycobacterium tuberculosis pathogenesis. Med. Gas Res. 2012, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- He, G. Hypoxia increases Nrf2-induced HO-1 expression via the PI3K Akt pathway. Front. Biosci. 2016, 21, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A.; Edwards, K.M. (Eds.) Plotkin’s Vaccines; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Bohmwald, K.; Céspedes, P.F.; Gómez, R.S.; Riquelme, S.A.; Cortés, C.M.; Valenzuela, J.A.; Sandoval, R.A.; Pancetti, F.C.; Bueno, S.M.; et al. Impaired learning resulting from Respiratory Syncytial Virus infection. Proc. Natl. Acad. Sci. USA 2013, 110, 9112–9117. [Google Scholar] [CrossRef] [Green Version]
- Bueno, S.M.; González, P.A.; Cautivo, K.M.; Mora, J.E.; Leiva, E.D.; Tobar, H.E.; Fennelly, G.J.; Eugenin, E.A.; Jacobs, W.R.; Riedel, C.A.; et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci. USA 2008, 105, 20822–20827. [Google Scholar] [CrossRef] [Green Version]
- Soto, J.A.; Gálvez, N.M.S.; Rivera, C.A.; Palavecino, C.E.; Céspedes, P.F.; Rey-Jurado, E.; Bueno, S.M.; Kalergis, A.M. Recombinant BCG Vaccines Reduce Pneumovirus-Caused Airway Pathology by Inducing Protective Humoral Immunity. Front. Immunol. 2018, 9, 2875. [Google Scholar] [CrossRef] [Green Version]
- Soto, J.A.; Gálvez, N.M.; Pacheco, G.A.; Canedo-Marroquín, G.; Bueno, S.M.; Kalergis, A.M. Induction of Protective Immunity by a Single Low Dose of a Master Cell Bank cGMP-rBCG-P Vaccine Against the Human Metapneumovirus in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 1. [Google Scholar] [CrossRef]
- Klopfleisch, R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology—A systematic review. BMC Veter- Res. 2013, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scudamore, C.L. (Ed.) A Practical Guide to the Histology of the Mouse; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Durek, C.; Rüsch-Gerdes, S.; Jocham, D.; Böhle, A. Sensitivity of BCG to modern antibiotics. Eur. Urol. 2000, 37, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Willemse, S.; Oomens, M.; De Lange, J.; Karssemakers, L. Diagnosing nontuberculous mycobacterial cervicofacial lymphadenitis in children: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- González, P.A.; Prado, C.E.; Leiva, E.D.; Carreño, L.J.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells Surface expression of RSV F protein was measured 48 h postinfection at a multiplicity of infection equal to 1, with three. Proc. Natl. Acad. Sci. USA 2008, 30, 14999–15004. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, L.; Wang, J.; Shi, W.; Pawar, R.; Liu, Y.; Xu, C.; Cong, W.; Hu, Q.; Lu, T.; et al. β-Actin is a useful internal control for tissue-specific gene expression studies using quantitative real-time PCR in the half-smooth tongue sole Cynoglossus semilaevis challenged with LPS or Vibrio anguillarum. Fish Shellfish Immunol. 2010, 29, 89–93. [Google Scholar] [CrossRef]
- Domachowske, J.; Bonville, C.A.; Gao, J.-L.; Murphy, P.M.; Easton, A.J.; Rosenberg, H.F. MIP-1α Is Produced but It Does Not Control Pulmonary Inflammation in Response to Respiratory Syncytial Virus Infection in Mice. Cell. Immunol. 2000, 206, 1–6. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Bueno, S.M.; Kalergis, A.M. Carbon monoxide down-modulates Toll-like receptor 4/MD2 expression on innate immune cells and reduces endotoxic shock susceptibility. Immunology 2015, 144, 321–332. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.S.; Perlman, H. Flow Cytometric Analysis of Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Daniel, J.; Maamar, H.; Deb, C.; Sirakova, T.D.; Kolattukudy, P.E. Mycobacterium tuberculosis Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages. PLoS Pathog. 2011, 7, e1002093. [Google Scholar] [CrossRef] [Green Version]
- Snelgrove, R.J.; Goulding, J.; Didierlaurent, A.M.; Lyonga, D.; Vekaria, S.; Edwards, L.; Gwyer, E.; Sedgwick, J.D.; Barclay, A.N.; Hussell, T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008, 9, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Créhan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Veter Res. 2020, 51, 80. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Espinoza, J.A.; Rey-Jurado, E.; Gómez, R.S.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Human Respiratory Syncytial Virus: Infection and Pathology. Semin. Respir. Crit. Care Med. 2016, 37, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Redford, P.S.; Mayer-Barber, K.D.; McNab, F.W.; Stavropoulos, E.; Wack, A.; Sher, A.; O’Garra, A. Influenza A Virus Impairs Control of Mycobacterium tuberculosis Coinfection Through a Type I Interferon Receptor–Dependent Pathway. J. Infect. Dis. 2013, 209, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; Bohmwald, K.; Céspedes, P.F.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M. Modulation of host adaptive immunity by hRSV proteins. Virulence 2014, 5, 740–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagranderie, M.; Nahori, M.-A.; Balazuc, A.-M.; Kiefer-Biasizzo, H.; Silva, J.-R.L.E.; Milon, G.; Marchal, G.; Vargaftig, B.B. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology 2003, 108, 352–364. [Google Scholar] [CrossRef]
- Derrick, S.C.; Kolibab, K.; Yang, A.; Morris, S.L. Intranasal Administration of Mycobacterium bovis BCG Induces Superior Protection against Aerosol Infection with Mycobacterium tuberculosis in Mice. Clin. Vaccine Immunol. 2014, 21, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Estripeaut, D.; Torres, J.P.; Somers, C.S.; Tagliabue, C.; Khokhar, S.; Bhoj, V.G.; Grube, S.M.; Wozniakowski, A.; Gomez, A.M.; Ramilo, O.; et al. Respiratory Syncytial Virus Persistence in the Lungs Correlates with Airway Hyperreactivity in the Mouse Model. J. Infect. Dis. 2008, 198, 1435–1443. [Google Scholar] [CrossRef]
- Tekkanat, K.K.; Maassab, H.; Berlin, A.A.; Lincoln, P.M.; Evanoff, H.L.; Kaplan, M.H.; Lukacs, N.W. Role of Interleukin-12 and Stat-4 in the Regulation of Airway Inflammation and Hyperreactivity in Respiratory Syncytial Virus Infection. Am. J. Pathol. 2001, 159, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Galán, L.; Vesin, D.; Martinvalet, D.; Garcia, I. Low Dose BCG Infection as a Model for Macrophage Activation Maintaining Cell Viability. J. Immunol. Res. 2016, 2016, 4048235. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Miyatake, Y.; Hayashi, D.; Takami, A.; Itoh, S.; Yamamoto, S.; Hida, S.; Onozaki, K.; Takii, T. Early-shared Mycobacterium bovis bacillus Calmette-Guérin sub-strains induce Th1 cytokine productionin vivo. Microbiol. Immunol. 2015, 59, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, S.; Boylan, J.; McLoughlin, P. Hypoxia-Induced Inflammation in the Lung. Am. J. Respir. Cell Mol. Biol. 2013, 48, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Vitali, S.H.; Fernandez-Gonzalez, A.; Nadkarni, J.; Kwong, A.; Rose, C.; Mitsialis, S.A.; Kourembanas, S. Heme oxygenase-1 dampens the macrophage sterile inflammasome response and regulates its components in the hypoxic lung. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L125–L134. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.; Illei, P.B.; Jain, S.; Karakousis, P.C. Characterization of a Novel Necrotic Granuloma Model of Latent Tuberculosis Infection and Reactivation in Mice. Am. J. Pathol. 2014, 184, 2045–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovey, A.; Verma, S.; Kaipilyawar, V.; Ribeiro-Rodrigues, R.; Husain, S.; Palaci, M.; Dietze, R.; Ma, S.; Morrison, R.D.; Sherman, D.R.; et al. Early alveolar macrophage response and IL-1R-dependent T cell priming determine transmissibility of Mycobacterium tuberculosis strains. Nat. Commun. 2022, 13, 884. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Kaufmann, S.H. Mycobacterium tuberculosis: Success through dormancy. FEMS Microbiol. Rev. 2012, 36, 514–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirunavukkarasu, S.; de Silva, K.; Plain, K.M.; Whittington, R.J. Role of host- and pathogen-associated lipids in directing the immune response in mycobacterial infections, with emphasis on Mycobacterium avium subsp. paratuberculosis. Crit. Rev. Microbiol. 2014, 42, 262–275. [Google Scholar] [CrossRef]
- Stehr, M.; Elamin, A.A.; Singh, M. Lipid Inclusions in Mycobacterial Infections. Tuberculosis: Current Issues in Diagnosis and Management; BoD Books on Demand: Norderstedt, Germany, 2013. [Google Scholar] [CrossRef] [Green Version]
- Barisch, C.; Soldati, T. Breaking fat! How mycobacteria and other intracellular pathogens manipulate host lipid droplets. Biochimie 2017, 141, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Janssen, W.J.; Barthel, L.; Muldrow, A.; Oberley-Deegan, R.E.; Kearns, M.T.; Jakubzick, C.; Henson, P.M. Fas Determines Differential Fates of Resident and Recruited Macrophages during Resolution of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2011, 184, 547–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguíluz-Gracia, I.; Schultz, H.H.L.; Sikkeland, L.I.B.; Danilova, E.; Holm, A.M.; Pronk, C.J.H.; Agace, W.W.; Iversen, M.; Andersen, C.; Jahnsen, F.L.; et al. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax 2016, 71, 1006–1011. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Christman, J.W. Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Front. Immunol. 2019, 10, 2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.D.; Antunes, K.H.; Muraro, S.P.; de Souza, G.F.; da Silva, A.G.; de Souza Felipe, J.; Zanetti, L.C.; Czepielewski, R.S.; Magnus, K.; Scotta, M.; et al. TNF-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection. Eur. Respir. J. 2020, 57, 2003764. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Kulikauskaite, J.; Crotta, S.; Patel, H.; Kelly, G.; Hessel, E.M.; Mack, M.; Beinke, S.; Wack, A. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 2020, 21, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kuchtey, J.; Fulton, S.A.; Reba, S.M.; Harding, C.V.; Boom, W.H. Interferon-alphabeta mediates partial control of early pulmonary Mycobacterium bovis bacillus Calmette-Guerin infection. Immunology 2006, 118, 39–49. [Google Scholar] [CrossRef]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. Interleukin-6 Induces Early Gamma Interferon Production in the Infected Lung but Is Not Required for Generation of Specific Immunity to Mycobacterium tuberculosis Infection. Infect. Immun. 2000, 68, 3322–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwich, L.; Coma, G.; Peña, R.; Bellido, R.; Blanco, E.J.J.; Este, J.A.; Borras, F.E.; Clotet, B.; Ruiz, L.; Rosell, A.; et al. Secretion of interferon-γ by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 2009, 126, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.K.; Salomonis, N.; Henderlight, M.; Woods, C.; Thakkar, K.; Grom, A.A.; Thornton, S.; Jordan, M.B.; Wikenheiser-Brokamp, K.A.; Schulert, G.S. IFN-γ is essential for alveolar macrophage–driven pulmonary inflammation in macrophage activation syndrome. JCI Insight 2021, 6, e147593. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Wagener, F.A.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Fonseca, W.; Lukacs, N.W.; Ptaschinski, C. Factors Affecting the Immunity to Respiratory Syncytial Virus: From Epigenetics to Microbiome. Front. Immunol. 2018, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yu, S.; Zhang, C.; Kong, A.-N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 337–349. [Google Scholar] [CrossRef] [Green Version]





| Day of the First Inoculation (Mock or Human Respiratory Syncytial Virus) | Day of the Second Inoculation (Vehicle or Bacillus Calmette-Guerin) | Day of the Euthanasia (After the Second Inoculation) | |
|---|---|---|---|
| Short scheme | 0 | 10 | 11 |
| Long scheme | 0 | 21 | 21 |
| Gene | Forward Primer | Reverse Primer | Gene Accession Code |
|---|---|---|---|
| n-hRSV | 5′-GCTAGTGTGCAAGCAGAAATC-3′ | 5′-TGGAGAAGTGAGGAAATTGAGTC-3′ | Gene ID: 1489820 |
| Mouse ho-1 | 5′-CCTCTGACGAAGTGACGCC-3′ | 5′-CAGCCCCACCAAGTTCAAA-3′ | Gene ID: 15368 |
| Mouse nrf2 | 5′-TTCTTTCAGCAGCATCCTCTCCAG-3′ | 5′-ACAGCCTTCAATAGTCCCGTCCAG-3′ | Gene ID: 18024 |
| Mouse ifn-β | 5′-AGCTCCAAGAAAGGACGAACA-3′ | 5′-GCCCTGTAGGTGAGGTTGAT-3′ | Gene ID: 15977 |
| Mouse il-6 | 5′-TAGTCCTTCCTACCC CAATTTCC-3′ | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | Gene ID: 16193 |
| Mouse cd200 | 5′-CTCTCCACCTACAGCCTGATT-3′ | 5′-AGAACATCGTAAGGATGCAGTTG-3′ | Gene ID: 17470 |
| Mouse β-actin | 5′-ACCTTCTACAATGAGCTGCG-3′ | 5′-CTGGATGGCTACGTACATGG-3′ | Gene ID: 11461 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canedo-Marroquín, G.; Soto, J.A.; Andrade, C.A.; Bueno, S.M.; Kalergis, A.M. Increased Heme Oxygenase 1 Expression upon a Primary Exposure to the Respiratory Syncytial Virus and a Secondary Mycobacterium bovis Infection. Antioxidants 2022, 11, 1453. https://doi.org/10.3390/antiox11081453
Canedo-Marroquín G, Soto JA, Andrade CA, Bueno SM, Kalergis AM. Increased Heme Oxygenase 1 Expression upon a Primary Exposure to the Respiratory Syncytial Virus and a Secondary Mycobacterium bovis Infection. Antioxidants. 2022; 11(8):1453. https://doi.org/10.3390/antiox11081453
Chicago/Turabian StyleCanedo-Marroquín, Gisela, Jorge A. Soto, Catalina A. Andrade, Susan M. Bueno, and Alexis M. Kalergis. 2022. "Increased Heme Oxygenase 1 Expression upon a Primary Exposure to the Respiratory Syncytial Virus and a Secondary Mycobacterium bovis Infection" Antioxidants 11, no. 8: 1453. https://doi.org/10.3390/antiox11081453
APA StyleCanedo-Marroquín, G., Soto, J. A., Andrade, C. A., Bueno, S. M., & Kalergis, A. M. (2022). Increased Heme Oxygenase 1 Expression upon a Primary Exposure to the Respiratory Syncytial Virus and a Secondary Mycobacterium bovis Infection. Antioxidants, 11(8), 1453. https://doi.org/10.3390/antiox11081453