Ultraviolet Light Protection: Is It Really Enough?
Abstract
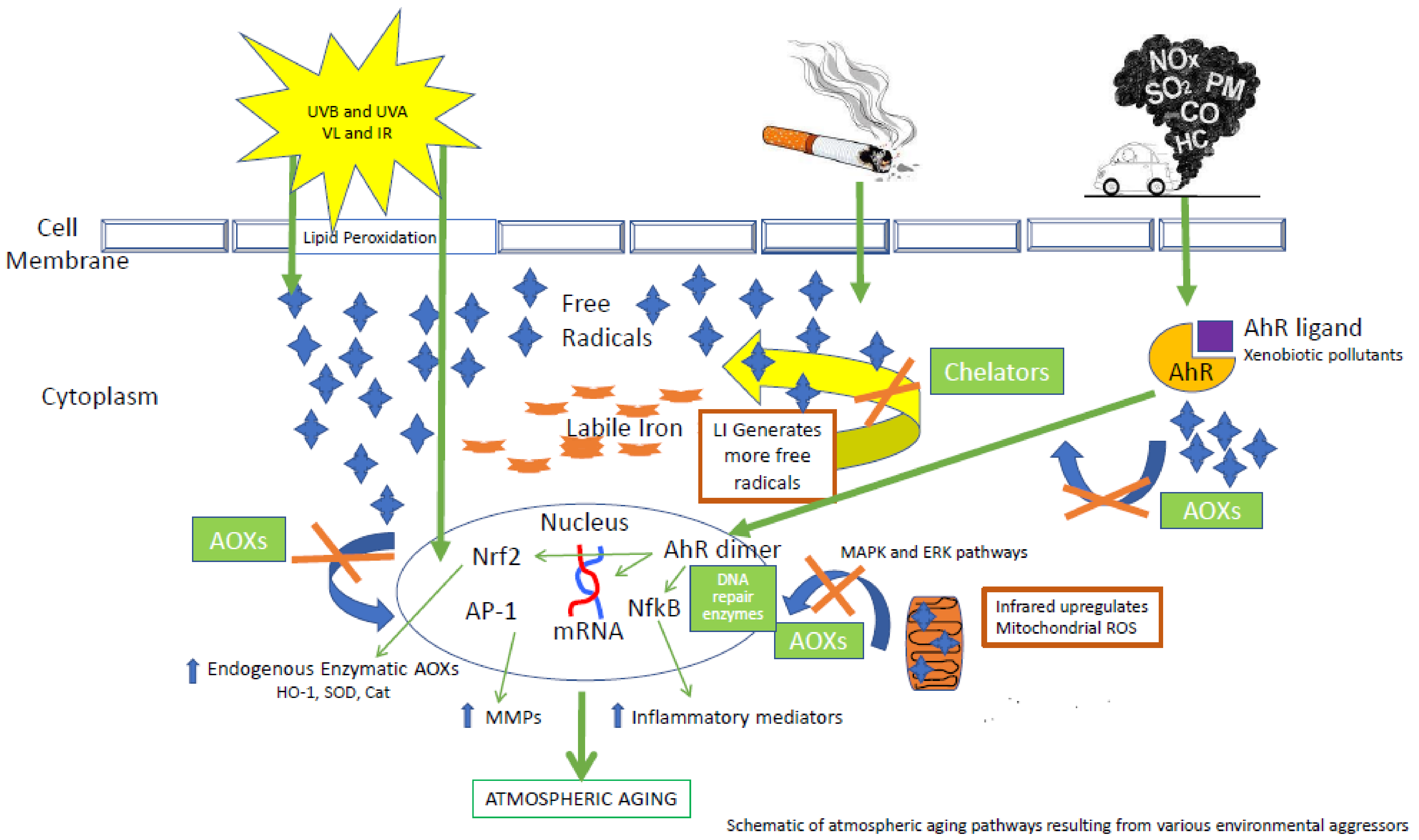
:1. Introduction
2. Ultraviolet Light
3. Beyond UV: Visible Light and Infrared
4. Environmental Pollutants
5. Ozone (O3)
6. Particulate Matter (PM)
7. Cigarette Smoke (CS)
8. Synergy between Solar Radiation and Pollution
9. Environmental Protection Strategies
9.1. Sunscreen
9.2. Antioxidants Compounds as a Therapeutic Approach to Prevent the OxInflammatory Damage within the Skin
9.2.1. Endogenous Defensive Enzymes
9.2.2. Skin Micronutrients and Topical Antioxidant Application
9.3. Chelating Agents to Modulate and Iron and Redox Homeostasis in Skin
| Environmental Aggressor | Solar Radiation | Pollution | ||||
|---|---|---|---|---|---|---|
| UVA/UVB | VL and IR | Tropospheric Ozone (O3) | Particulate Matter | Transition Metals | Cigarette Smoke | |
| Biomarkers of Exposure and Damage | UVB (290–320 nm) [5,8]
| VL (400–700 mm) [23,24,25,26,27,28,29]
|
| Particulate matter (PM)
|
|
|
| Protection | Physical Barriers-
Diet, Micronutrients [131] | Physical Barriers-
Diet and Micronutrients [131] | Topical AOX- [42,146,147]
| Topical AOX- [42]
| Combination- [89]
| Topical AOX- [148]
|
9.4. DNA Repair Enzymes
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kligman, L.H.; Kligman, A.M. The nature of photoaging: Its prevention and repair. Photodermatology 1986, 3, 215–227. [Google Scholar] [PubMed]
- Krutmann, J.; Gilchrest, B.A. Photoaging of skin. In Skin Aging; Gilchrest, B.A., Krutmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–43. [Google Scholar]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging-Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M. Clinical and histological features of intrinsic versus extrinsic skin aging. In Skin Aging; Gilchrest, B.A., Krutmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 9–21. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation, and the skin. Int. J. Mol Sci. 2013, 14, 12222–122248. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin. Exp. Dermatol. 2001, 26, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, C.; Roza, L.; Epe, B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997, 18, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishigori, C.; Yarosh, D.B.; Donawho, C.; Kripke, M.L. The immune system in ultraviolet carcinogenesis. J. Investig. Dermatol. Symp. Proc. 1996, 1, 143–146. [Google Scholar]
- Kitazawa, M.; Iwasaki, K.; Sakamoto, K. Iron chelators may help prevent photoaging. J. Cosmet. Dermatol. 2006, 5, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Pourzand, C.; Watkin, R.D.; Brown, J.E.; Tyrrell, R.M. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: The role of ferritin. Proc. Natl. Acad. Sci. USA 1999, 96, 6751–6756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ryšavá, A.; Vostálová, J.; Svobodová, A.R. Effect of ultraviolet radiation on the Nrf2 signaling pathway in skin cells. Int. J. Radiat. Biol. 2021, 97, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Voorhees, J.J. Molecular Mechanisms of Photoaging and its Prevention by Retinoic Acid: Ultraviolet Irradiation Induces MAP Kinase Signal Transduction Cascades that Induce Ap-1-Regulated Matrix Metalloproteinases that Degrade Human Skin In Vivo. J. Investig. Dermatol. 1998, 3, 61–68. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Senfetleben, U.; Karin, M. The IKK/NF-kappaB pathway. Crit. Care Med. 2002, 30, S18–S26. [Google Scholar]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.; Watson, R.E. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Mahmoud, B.H.; Hexsel, C.L.; Hamzavi, I.H.; Lim, H.W. Effects of Visible Light on the Skin. Photochem. Photobiol. 2008, 84, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on mechanisms of skin photoaging. Photodermatol. Photoimmunol. Photomed. 2021, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Eggers, K.; Rippke, F.; Tesch, M.; Buerger, A.; Darvin, M.E.; Schanzer, S.; Meinke, M.C.; Lademann, J.; Kolbe, L. High-energy visible light at ambient doses and intensities induces oxidative stress of skin—Protective effects of the antioxidant and Nrf2 inducer Licochalcone A in vitro and in vivo. Photodermatol. Photoimmunol. Photomed. 2020, 36, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of Skin with Visible Light Induces Reactive Oxygen Species and Matrix-Degrading Enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [Green Version]
- Zamarrón, A.; Lorrio, S.; González, S.; Juarranz, A. Fernblock Prevents Dermal Cell Damage Induced by Visible and Infrared A Radiation. Int. J. Mol. Sci. 2018, 19, 2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.; De Donatis, G.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocyts sense blue light and regulate pigmentation through opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, B.H.; Ruvolo, E.; Hexsel, C.L.; Liu, Y.; Owen, M.R.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Impact of Long-Wavelength UVA and Visible Light on Melanocompetent Skin. J. Investig. Dermatol. 2010, 130, 2092–2097. [Google Scholar] [CrossRef] [Green Version]
- Dutiel, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.P.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths; a clinical and histological study in comparison to UVB exposure. Pigment Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, D.H.; Cho, S.; Chung, J.H. Minimal heating dose: A novel biological unit to measure infrared irradiation. Photodermatol. Photoimmunol. Photomed. 2006, 22, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.-E.; Lee, Y.M.; Park, C.-H.; Chung, J.H. Effects of Infrared Radiation and Heat on Human Skin Aging in vivo. J. Investig. Dermatol. Symp. Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Seo, J.Y.; Kim, Y.K.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Heat modulation of tropoelastin, fibrillin-1 and matrix metalloproteinase-12 in human skin in vivo. J. Investig. Dermatol. 2005, 124, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Kim, Y.; Cho, K.; Chung, J. Infrared exposure induces an angiogenic switch in human skin that is partially mediated by heat. Br. J. Dermatol. 2006, 155, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous effects of infrared radiation: From clinical observations to molecular response mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, Y.K.; Cho, K.H.; Chung, J.H. Regulation of type I procollagen and MMP-1 expression after single or repeated exposure to infrared radiation in human skin. Mech. Ageing Dev. 2006, 127, 875–882. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, M.J.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech. Ageing Dev. 2005, 126, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schroeder, P. Role of Mitochondria in Photoaging of Human Skin: The Defective Powerhouse Model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shroeder, P.; Calles, C.; Benesova, T.; Macaluso, F.; Krutmann, J. Photoprotection beyond ultraviolet radiation-effective sun protection must include protection against infrared A radiation-induced skin damage. Skin Pharmacol. Physiol. 2010, 23, 15–17. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. BioFactors 2019, 45, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, F.; Woodby, B.; Pecorelli, A.; Schiavone, M.L.; Pambianchi, E.; Messano, N.; Therrien, J.-P.; Choudhary, H.; Valacchi, G. Additive effect of combined pollutants to UV induced skin OxInflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. 2020, 34, 101481. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef]
- Cross, C.E.; Valacchi, G.; Schock, B.; Wilson, M.; Weber, S.; Eiserich, J.; van der Vliet, A. Environmental Oxidant Pollutant Effects on Biologic Systems: A focus on micronutrient antioxidant—oxidant interactions. Am. J. Respir. Crit. Care Med. 2002, 166, S44–S50. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumby, S.; Chung, K.F.; Adcock, I.M. Transcriptional Effects of Ozone and Impact on Airway Inflammation. Front. Immunol. 2019, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-H.; Yi, H.-J.; Kim, Y.-S.; Ko, Y.; Kim, Y.-S. Association between Diurnal Variation of Ozone Concentration and Stroke Occurrence: 24-Hour Time Series Study. PLoS ONE 2016, 11, e0152433. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pagnin, E.; Corbacho, A.M.; Olano, E.; David, P.A.; Packer, L.; Cross, C.E. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic. Biol. Med. 2004, 36, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Pambianchi, E.; Pecorelli, A.; Woodby, B.; Messano, N.; Therrien, J.-P.; Lila, M.A.; Valacchi, G. Redox regulation of cutaneous inflammasome by ozone exposure. Free Radic. Biol. Med. 2019, 152, 561–570. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Niki, E. Lipid oxidation in the skin. Free Radic. Res. 2015, 49, 827–834. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lyga, J. Inflammaging in skin and other tissues—The roles of complement system and macrophage. Inflamm. Allergy-Drug Targets 2014, 13, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valacchi, G.; van der Vliet, A.; Schock, B.C.; Okamoto, T.; Obermuller-Jevic, U.; Cross, C.E.; Packer, L. Ozone exposure activates oxidative stress responses in murine skin. Toxicology 2002, 179, 163–170. [Google Scholar] [CrossRef]
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Skin Health from the Inside Out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valacchi, G.; Fortino, V.; Bocci, V. The dual action of ozone on the skin. Br. J. Dermatol. 2005, 153, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Savelli, V.; Evelson, P.; Valacchi, G. Modulation of cutaneous scavenger receptor B1 levels by exogenous stressors impairs “in vitro” wound closure. Mech. Ageing Dev. 2018, 172, 78–85. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Leikauf, G.D.; Kim, S.-H.; Jang, A.-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hopke, P.K.; Croft, D.; Zhang, W.; Lin, S.; Masiol, M.; Squizzato, S.; Thurston, S.W.; van Wijngaarden, E.; Utell, M.J.; Rich, D.Q. Changes in the acute response of respiratory diseases to PM 2.5 in New York State from 2005 to 2016. Sci. Total Environ. 2019, 677, 328–339. [Google Scholar] [CrossRef]
- Doiron, D.; De Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; De Matteis, S.; Hansell, A.L. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54, 1802140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, L.; Tang, M. Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease. J. Appl. Toxicol. 2017, 37, 644–667. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.A.; Valacchi, G. Skin Damage Mechanisms Related to Airborne Particulate Matter Exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schikowski, T.; Hüls, A. Air Pollution and Skin Aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.-K.; Seo, S.J. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Bruch-Gerharz, D.; Ruzicka, T.; Kolb-Bachofen, V. Nitric oxide in human skin: Current status and future prospects. J. Investig. Dermatol. 1998, 110, 1–7. [Google Scholar] [PubMed] [Green Version]
- Hüls, A.; Vierkötter, A.; Gao, W.; Krämer, U.; Yang, Y.; Ding, A.; Stolz, S.; Matsui, M.; Kan, H.; Wang, S.; et al. Traffic-Related Air Pollution Contributes to Development of Facial Lentigines: Further Epidemiological Evidence from Caucasians and Asians. J. Investig. Dermatol. 2016, 136, 1053–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, I.; Mihele, C.; Lu, G.; Narayan, J.; Brook, J.R. Evaluating Multipollutant Exposure and Urban Air Quality: Pollutant Interrelationships, Neighborhood Variability, and Nitrogen Dioxide as a Proxy Pollutant. Environ. Health Perspect. 2014, 122, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.A.; Tomkins, B.; Guerin, M.R. The Chemistry of Environmental Tobacco Smoke: Composition and Measurement; CRC Press LLC: Boca Raton, FL, USA, 2000; pp. 5–14. [Google Scholar]
- Eiserich, J.; Vossen, V.; O’Neill, C.A.; Halliwell, B.; Cross, C.E.; Van Der Vliet, A. Molecular mechanisms of damage by excess nitrogen oxides: Nitration of tyrosine by gas-phase cigarette smoke. FEBS Lett. 1994, 353, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Schick, S.; Glantz, S. Philip Morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob. Control 2005, 14, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; Liu, X.; Li, J.; Ouyang, R.; Chen, P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin 2019, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, Y.; Shi, J.; Larson, D.F.; Watson, R.R. Side-Stream Cigarette Smoke Induces Dose–Response in Systemic Inflammatory Cytokine Production and Oxidative Stress. Exp. Biol. Med. 2002, 227, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wong, L.S.; Bhattacharya, M.; Ma, C.; Zafarani, M.; Yao, M.; Schneider, M.; Pitas, R.E.; Martins-Green, M. The effects of second-hand smoke on biological processes important in atherogenesis. BMC Cardiovasc. Disord. 2007, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular Basis of Tobacco Smoke-Induced Premature Skin Aging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 53–55. [Google Scholar] [CrossRef]
- Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Arezzini, B.; Gardi, C.; Maioli, E.; Miracco, C.; Toscano, M.; Forman, H.J.; Valacchi, G. Cigarette Smoke Affects Keratinocytes SRB1 Expression and Localization via H2O2 Production and HNE Protein Adducts Formation. PLoS ONE 2012, 7, e33592. [Google Scholar] [CrossRef] [Green Version]
- Just, M.; Ribera, M.; Monso, E.; Lorenzo, J.; Ferrándiz, C. Effect of smoking on skin elastic fibres: Morphometric and immunohistochemical analysis. Br. J. Dermatol. 2007, 156, 85–91. [Google Scholar] [CrossRef]
- Yin, L.; Morita, A.; Tsuji, T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch. Dermatol. Res 2000, 292, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Nanjappa, V.; Raja, R.; Jain, A.P.; Mangalaparthi, K.K.; Sathe, G.J.; Babu, N.; Patel, K.; Cavusoglu, N.; Soeur, J.; et al. How Does Chronic Cigarette Smoke Exposure Affect Human Skin? A Global Proteomics Study in Primary Human Keratinocytes. OMICS A J. Integr. Biol. 2016, 20, 615–626. [Google Scholar] [CrossRef]
- Sticozzi, C.; Belmonte, G.; Cervellati, F.; Muresan, X.; Pessina, F.; Lim, Y.; Forman, H.; Valacchi, G. Resveratrol protects SR-B1 levels in keratinocytes exposed to cigarette smoke. Free Radic. Biol. Med. 2014, 69, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Kavli, G.; Forde, O.H.; Arnesen, E.; E Stenvold, S. Psoriasis: Familial predisposition and environmental factors. BMJ 1985, 291, 999–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naldi, L.; Peli, L.; Parazzini, F. Association of early-stage psoriasis with smoking and male alcohol consumption: Evidence from an Italian case-control study. Arch. Dermatol. 1999, 135, 1479–1484. [Google Scholar] [CrossRef] [Green Version]
- Naldi, L. Psoriasis: Targets and Therapy Dovepress Psoriasis and smoking: Links and risks. Psoriasis Targets Ther. 2016, 6, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, K.; Wei, H. Synergistic damage by UVA radiation and pollutants. Toxicol. Ind. Health 2009, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khan, H.; Bahadar, R.; Riaz, A.; Bin Asad, M.H.H. The impact of airborne pollution and exposure to solar ultraviolet radiation on skin: Mechanistic and physiological insight. Environ. Sci. Pollut. Res. 2020, 27, 28730–28736. [Google Scholar] [CrossRef] [PubMed]
- Pambianchi, E.; Ferrara, F.; Pecorelli, A.; Benedusi, M.; Choudhary, H.; Therrien, J.-P.; Valacchi, G. Deferoxamine Treatment Improves Antioxidant Cosmeceutical Formulation Protection against Cutaneous Diesel Engine Exhaust Exposure. Antioxidants 2021, 10, 1928. [Google Scholar] [CrossRef] [PubMed]
- Mokrzyński, K.; Krzysztyńska-Kuleta, O.; Zawrotniak, M.; Sarna, M.; Sarna, T. Fine Particulate Matter-Induced Oxidative Stress Mediated by UVA-Visible Light Leads to Keratinocyte Damage. Int. J. Mol. Sci. 2021, 22, 10645. [Google Scholar] [CrossRef]
- Toyooka, T.; Ibuki, Y. DNA damage induced by coexposure to PAHs and light. Environ. Toxicol. Pharmacol. 2007, 23, 256–263. [Google Scholar] [CrossRef]
- Bao, L.; Xu, A.; Tong, L.; Chen, S.; Zhu, L.; Zhao, Y.; Zhao, G.; Jiang, E.; Wang, J.; Wu, L. Activated Toxicity of Diesel Particulate Extract by Ultraviolet A Radiation in Mammalian Cells: Role of Singlet Oxygen. Environ. Heath Perspect. 2009, 117, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, D.; Atencio, D.P.; Perez, E.; Saladi, R.; Moore, J.; Guevara, D.; Rosenstein, B.S.; Lebwohl, M.; Wei, H. Combined subcarcinogenic benzo[a]pyrene and UVA synergistically caused high tumor incidence and mutations in H-ras gene, but notp53, in SKH-1 hairless mouse skin. Int. J. Cancer 2005, 116, 193–199. [Google Scholar] [CrossRef]
- Teranishi, M.; Toyooka, T.; Ohura, T.; Masuda, S.; Ibuki, Y. Benzo[a]pyrene exposed to solar-simulated light inhibits apoptosis and augments carcinogenicity. Chem. Interactions 2010, 185, 4–11. [Google Scholar] [CrossRef]
- Soeur, J.; Belaïdi, J.-P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.; Arakane, K.; Koide, C.; Arai, H.; Nagano, T. Squalene as a Target Molecule in Skin Hyperpigmentation Caused by Singlet Oxygen. Biol. Pharm. Bull. 2009, 32, 1504–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussouira, B.; Pham, D.M. Squalene and Skin Barrier Function: From Molecular Target to Biomarker of Environmental Exposure. In Skin Stress Response Pathways; Springer: Cham, Switzerland, 2016; pp. 29–48. [Google Scholar] [CrossRef]
- Kostyuk, V.; Potapovich, A.; Stancato, A.; De Luca, C.; Lulli, D.; Pastore, S.; Korkina, L. Photo-Oxidation Products of Skin Surface Squalene Mediate Metabolic and Inflammatory Responses to Solar UV in Human Keratinocytes. PLoS ONE 2012, 7, e44472. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Weber, S.U.; Luu, C.; Cross, C.E.; Packer, L. Ozone potentiates vitamin E depletion by ultraviolet radiation in the murine stratum corneum. FEBS Lett. 2000, 466, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Packer, L.; Valacchi, G. Antioxidants and the Response of Skin to Oxidative Stress: Vitamin E as a Key Indicator. Ski. Pharmacol. Physiol. 2002, 15, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Moravcová, M.; Libra, A.; Dvořáková, J.; Víšková, A.; Muthny, T.; Velebný, V.; Kubala, L. Modulation of keratin 1, 10 and involucrin expression as part of the complex response of the human keratinocyte cell line HaCaT to ultraviolet radiation. Interdiscip. Toxicol. 2013, 6, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-W.; Lin, Z.-C.; Hu, S.C.-S.; Chiang, Y.-C.; Hsu, L.-F.; Lin, Y.-C.; Lee, I.-T.; Tsai, M.-H.; Fang, J.-Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef] [Green Version]
- Hieda, D.S.; Carvalho, L.A.D.C.; de Mello, B.V.; de Oliveira, E.A.; de Assis, S.R.; Wu, J.; Du-Thumm, L.; da Silva, C.L.V.; Roubicek, D.A.; Maria-Engler, S.S.; et al. Air Particulate Matter Induces Skin Barrier Dysfunction and Water Transport Alteration on a Reconstructed Human Epidermis Model. J. Investig. Dermatol. 2020, 140, 2343–2352. [Google Scholar] [CrossRef]
- Ferrara, F.; Pambianchi, E.; Woodby, B.; Messano, N.; Therrien, J.-P.; Pecorelli, A.; Canella, R.; Valacchi, G. Evaluating the effect of ozone in UV induced skin damage. Toxicol. Lett. 2021, 338, 40–50. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp. Dermatol. 2018, 27, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balda, M.S.; Matter, K. Epidermal tight junctions in health and disease. Semin. Cell Dev. Biol. 2014, 36, 147–148. [Google Scholar] [CrossRef]
- Yuki, T.; Hachiya, A.; Kusaka, A.; Sriwiriyanont, P.; Visscher, M.O.; Morita, K.; Muto, M.; Miyachi, Y.; Sugiyama, Y.; Inoue, S. Characterization of tight junctions and their disruption by UVB in human and cultured keratinocytes. J. Investig. Dermatol. 2011, 131, 744–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wang, L.; Moreno-Vinasco, L.; Lang, G.D.; Siegler, J.H.; Mathew, B.; Usatyuk, P.V.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part. Fibre Toxicol. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Elias, P.M. Could inflammaging and its sequelae be prevented or mitigated? Clin. Interv. Aging 2019, 14, 2301–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegarska, B.; Pietkun, K.; Zegarski, W.; Bolibok, P.; Wiśniewski, M.; Roszek, K.; Czarnecka, J.; Nowacki, M. Air pollution, UV irradiation and skin carcinogenesis: What we know, where we stand and what is likely to happen in the future? Adv. Dermatol. Allergol. 2017, 34, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Fenini, G.; Grossi, S.; Contassot, E.; Biedermann, T.; Reichmann, E.; French, L.E.; Beer, H.-D. Genome Editing of Human Primary Keratinocytes by CRISPR/Cas9 Reveals an Essential Role of the NLRP1 Inflammasome in UVB Sensing. J. Investig. Dermatol. 2018, 138, 2644–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, F.; Prieux, R.; Woodby, B.; Valacchi, G. Inflammasome Activation in Pollution-Induced Skin Conditions. Plast. Reconstr. Surg. 2021, 147, 15S–24S. [Google Scholar] [CrossRef] [PubMed]
- Prieux, R.; Ferrara, F.; Cervellati, F.; Guiotto, A.; Benedusi, M.; Valacchi, G. Inflammasome involvement in CS-induced damage in HaCaT keratinocytes. Vitr. Cell. Dev. Biol.-Anim. 2022, 58, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Kanneganti, T.D. Autoinflammatory Skin Disorders: The Inflammasomme in Focus. Trends Mol. Med. 2016, 22, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Vogeley, C.; Esser, C.; Tüting, T.; Krutmann, J.; Haarmann-Stemmann, T. Role of the Aryl Hydrocarbon Receptor in Environmentally Induced Skin Aging and Skin Carcinogenesis. Int. J. Mol. Sci. 2019, 20, 6005. [Google Scholar] [CrossRef] [Green Version]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Abu Zaid, M.; Pelle, E.; Khan, N.; Syed, D.N.; Matsui, M.S.; Maes, D.; Mukhtar, H. Aryl Hydrocarbon Receptor Is an Ozone Sensor in Human Skin. J. Investig. Dermatol. 2009, 129, 2396–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Li, Q.; Du, H.-Y.; Wang, Q.-W.; Huang, Y.; Liu, W. Airborne polycyclic aromatic hydrocarbons trigger human skin cells aging through aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2017, 488, 445–452. [Google Scholar] [CrossRef]
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the aryl hydrocarbon receptor in tobacco smoke extract-induced matrix metalloproteinase-1 expression. Exp. Dermatol. 2013, 22, 349–353. [Google Scholar] [CrossRef]
- Liu, H.; Shi, L.; Giesy, J.P.; Yu, H. Polychlorinated diphenyl sulfides can induce ROS and genotoxicity via the AhR-CYP1A1 pathway. Chemosphere 2019, 223, 165–170. [Google Scholar] [CrossRef]
- Ibrahim, M.; MacFarlane, E.M.; Matteo, G.; Hoyeck, M.P.; Rick, K.R.C.; Farokhi, S.; Copley, C.M.; O’Dwyer, S.; Bruin, J.E. Functional cytochrome P450 1A enzymes are induced in mouse and human islets following pollutant exposure. Diabetologia 2020, 63, 162–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of Aryl Hydrocarbon Receptor-mediated Induction of the CYP1 Enzymes in Environmental Toxicity and Cancer. J. Biol. Chem. 2004, 279, 23847–23850. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Lim, H.; Mohammad, T.F. Sunscreens and photoaging: A review of current literature. Am. J. Clin. Dermatol. 2021, 22, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Haywood, R.; Wardman, P.; Sanders, R.; Linge, C. Sunscreens inadequately protect against ultraviolet-A-induced free radicals in skin: Implications for skin aging and melanoma? J. Investig. Dermatol. 2003, 121, 862–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumbuya, H.; Grimes, P.; Lynch, S.; Ji, K.; Brahmachary, M.; Zheng, Q.; Bouez, C.; Wangari-Talbot, J. Impact of iron-oxide containiing formulations agasint visible light-induced skin pigmentation in skin of color individuals. J. Drug. Dermatol. 2020, 19, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Creusot, T.; Catoire, S.; Egles, C.; Ficheux, H. Mechanisms of pollutant-induced toxicity in skin and detoxification: Anti-pollution strategies and perspectives for cosmetic products. Ann. Pharm. Fr. 2019, 77, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Kohen, R. Skin antioxidants: Their role in aging and in oxidative stress—New approaches for their evaluation. Biomed. Pharmacother. 1999, 53, 181–192. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and Non-Enzymic Antioxidants in Epidermis and Dermis of Human Skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [Green Version]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 2006, 1763, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findlay, G.H. Catalase Activity in Human Epidermis. Br. J. Dermatol. 1963, 75, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Carels, C.E.; Lundvig, D. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Kökçam, I.; Naziroǧlu, M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clin. Chim. Acta 1999, 289, 23–31. [Google Scholar] [CrossRef]
- Drewa, G.; Krzyzyńska-Malinowska, E.; Woźniak, A.; Protas-Drozd, F.; Mila-Kierzenkowska, C.; Rozwodowska, M.; Kowaliszyn, B.; Czajkowski, R. Activity of superoxide dismutase and catalase and the level of lipid peroxidation products reactive with TBA in patients with psoriasis. Med Sci. Monit. 2002, 8, 338–344. [Google Scholar]
- Romani, A.; Cervellati, C.; Muresan, X.M.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Benedusi, M.; Evelson, P.A.; Valacchi, G. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Treiber, N.; Maity, P.; Singh, K.; Ferchiu, F.; Wlaschek, M.; Scharffetter-Kochanek, K. The role of manganese superoxide dismutase in skin aging. Dermato-Endocrinology 2012, 4, 232–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.H.; Rhie, G.-E.; Kim, Y.K.; Park, C.-H.; Cho, K.H.; Kim, K.H.; Eun, H.C.; Chung, J.H. H2O2 Accumulation by Catalase Reduction Changes MAP Kinase Signaling in Aged Human Skin In Vivo. J. Investig. Dermatol. 2005, 125, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef]
- Syed, D.N.; Mukhtar, H. Gender Bias in Skin Cancer: Role of Catalase Revealed. J. Investig. Dermatol. 2012, 132, 512–514. [Google Scholar] [CrossRef] [Green Version]
- Niwa, Y.; Sumi, H.; Kawahira, K.; Terashima, T.; Nakamura, T.; Akamatsu, H. Protein oxidative damage in the stratum corneum: Evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br. J. Dermatol. 2003, 149, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C Compound Mixtures Prevent Ozone-Induced Oxidative Damage in Human Keratinocytes as Initial Assessment of Pollution Protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Demaude, J.; Krol, Y.; Oresajo, C. Ozone-induced damage in 3D-Skin Model is prevented by topical vitamin C and vitamin E compound mixtures application. J. Dermatol. Sci. 2016, 82, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Sticozzi, C.; Cervellati, F.; Muresan, X.M.; Cervellati, C.; Valacchi, G. Resveratrol prevents cigarette smoke-induced keratinocytes damage. Food Funct. 2014, 5, 2348–2356. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Aspects Med. 2007, 28, 646–667. [Google Scholar] [CrossRef] [PubMed]
- Al-Niami, F.; Yi Zhen Chiang, N. Topical Vitamin C and the Skin. J. Clin. Aesthethetic Dermatol. 2017, 10, 14–17. [Google Scholar]
- Burke, K. Interaction of vitamins C and E as better cosmeceuticals. Dermatol. Ther. 2007, 20, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Selim, M.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, J.J.; Traber, M.G.; Packer, L. Depletion of Human Stratum Corneum Vitamin E: An Early and Sensitive In Vivo Marker of UV Induced Photo-Oxidation. J. Investig. Dermatol. 1998, 110, 756–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, J.J.; Traber, M.G.; Tsang, K.; Cross, C.E.; Packer, L. In Vivo Exposure to Ozone Depletes Vitamins C and E and Induces Lipid Peroxidation in Epidermal Layers of Murine Skin. Free Radic. Biol. Med. 1997, 23, 385–391. [Google Scholar] [CrossRef]
- Thiele, J.J.; Traber, M.G.; Polefka, T.G.; Cross, C.E.; Packer, L. Ozone-Exposure Depletes Vitamin E and Induces Lipid Peroxidation in Murine Stratum Corneum. J. Investig. Dermatol. 1997, 108, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Fahrenholtz, S.R.; Doleiden, F.H.; Trozzolo, A.M.; Lamola, A.A. On the Quenching of Singlet Oxygen by α-Tocopherol. Photochem. Photobiol. 1974, 20, 505–509. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Krol, E.S.; Kramer-Stickland, K.A.; Liebler, D. Photoprotective Actions Of Topically Applied Vitamin E. Drug Metab. Rev. 2000, 32, 413–420. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C. Partners in defense, vitamin E and vitamin C. Can. J. Physiol. Pharmacol. 1993, 71, 725–731. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-Ascorbic Acid: Percutaneous Absorption Studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Choi, Y.W. Skin Permeation Enhancement of Ascorbyl Palmitate by Liposomal Hydrogel (Lipogel) Formulation and Electrical Assistance. Biol. Pharm. Bull. 2007, 30, 393–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austria, R.; Semenzato, A.; Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef]
- Lin, F.-H.; Lin, J.-Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles its Photoprotection of Skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef] [PubMed]
- Anstey, A.V. Systemic photoprotection with α-tocopherol (vitamin E) and β-carotene. Clin. Exp. Dermatol. 2002, 27, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Protonmotive redox mechanism of the cytochrome b-c 1 complex in the respiratory chain: Protonmotive ubiquinone cycle. FEBS Lett. 1975, 56, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-xB transcription factor and HIV-1. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar]
- Žmitek, K.; Pogačnik, T.; Mervic, L.; Žmitek, J.; Pravst, I. The effect of dietary intake of coenzyme Q10 on skin parameters and condition: Results of a randomised, placebo-controlled, double-blind study: The Effect of Dietary Intake of Coenzyme Q10 on Skin Parameters and Condition. BioFactors 2017, 43, 132–140. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects. BioFactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Effective photoprotection of human skin against infrared A radiation: Results from a vehicle controlled, randomized, double-blind study. Photochem. Photobiol. 2015, 91, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Photoprotection of human skin beyond ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2014, 30, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kohli, I.; Rubolo, E.; Kolbe, L.; Hamzavi, I. Impact of visible light on skin health: The role of antioxidants and free radical quenchers in skin protection. J. Am. Acad. Dermatol. 2022, 86, S27–S37. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Yen, H.-H.; Yang, T.-H.; Su, C.-C. Tetrathiomolybdate, a copper chelator inhibited imiquimod-induced skin inflammation in mice. J. Dermatol. Sci. 2018, 92, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A. Air pollution particles and iron homeostasis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A.; Madden, M.C. Air pollutants disrupt iron homeostasis to impact oxidant generation, biological effects, and tissue injury. Free Radic. Biol. Med. 2020, 151, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; Richards, T.; Srai, S.K.S. The role of iron in the skin and cutaneous wound healing. Front. Pharmacol. 2014, 5, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reelfs, O.; MEggleston, I.; Pourzand, C. Skin Protection Against UVA-Induced Iron Damage by Multiantioxidants and Iron Chelating Drugs/Prodrugs. Curr. Drug Metab. 2010, 11, 242–249. [Google Scholar] [CrossRef]
- Welch, K.D.; Davis, T.; E Van Eden, M.; Aust, S.D. Deleterious iron-mediated oxidation of biomolecules. Free Radic. Biol. Med. 2002, 32, 577–583. [Google Scholar] [CrossRef]
- Hatcher, H.C.; Singh, R.N.; Torti, F.M.; Torti, S.V. Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med. Chem. 2009, 1, 1643–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2018, 21, S6–S20. [Google Scholar] [CrossRef]
- Applegate, L.A.; Noël, A.; Vile, G.; Frenk, E.; Tyrrell, R.M. Two Genes Contribute to Different Extents to the Heme Oxygenase Enzyme Activity Measured in Cultured Human Skin Fibroblasts and Keratinocytes: Implications for Protection Against Oxidant Stress. Photochem. Photobiol. 1995, 61, 285–291. [Google Scholar] [CrossRef]
- Schreinemachers, D.M.; Ghio, A.J. Article Commentary: Effects of Environmental Pollutants on Cellular Iron Homeostasis and Ultimate Links to Human Disease. Environ. Health Insights 2016, 10, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissett, D.L.; McBride, J.F. Synergistic topical photoprotection by a combination of the iron chelator 2-furildioxime and sunscreen. J. Am. Acad. Dermatol. 1996, 35, 546–549. [Google Scholar] [CrossRef]
- Mitani, H.; Koshiishi, I.; Sumita, T.; Imanari, T. Prevention of the photodamage in the hairless mouse dorsal skin by kojic acid as an iron chelator. Eur. J. Pharmacol. 2001, 411, 169–174. [Google Scholar] [CrossRef]
- Shalev, O.; Hebbel, R. Extremely high avidity association of Fe(III) with the sickle red cell membrane. Blood 1996, 88, 349–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchini, M.; Gandini, G.; Veneri, D.; Aprili, G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload: An update. Blood 2004, 103, 747–748. [Google Scholar] [CrossRef]
- Hou, Z.; Nie, C.; Si, Z.; Ma, Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res. Clin. Pract. 2013, 101, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Neofytou, E.; Wong, V.W.; Maan, Z.N.; Rennert, R.C.; Inayathullah, M.; Januszyk, M.; Rodrigues, M.; Malkovskiy, A.V.; Whitmore, A.J.; et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 2015, 112, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duscher, D.; Trotsyuk, A.A.; Maan, Z.N.; Kwon, S.H.; Rodrigues, M.; Engel, K.; Stern-Buchbinder, Z.A.; Bonham, C.A.; Barrera, J.; Whittam, A.J.; et al. Optimization of transdermal deferoxamine leads to enhanced efficacy in healing skin wounds. J. Control. Release 2019, 308, 232–239. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Davies, M.J.; Donkor, R.; Dunster, C.A.; Gee, C.A.; Jonas, S.; Willson, R.L. Desferrioxamine (Desferal) and superoxide free radicals. Formation of an enzyme-damaging nitroxide. Biochem. J. 1987, 246, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Parvar, M.; Mehrzad, J.; Chaichi, M.J.; Hosseinkhani, S.; Golchoubian, H. Quenching effect of deferoxamine on free radical-mediated photon production in luminol and ortho-phenanthroline-dependent chemiluminescence. Chin. Chem. Lett. 2014, 25, 630–634. [Google Scholar] [CrossRef]
- Hartley, A.; Davies, M.; Rice-Evans, C. Desferrioxamine as a lipid chain-breaking antioxidant in sickle erythrocyte membranes. FEBS Lett. 1990, 264, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N. Shedding a new light on skin aging, iron and redox-homeostasis and emerging natural antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Mulleger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact on photoageing- A systemic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef]
- Kern, J.; Wood, E.; Almukhtar, R.; Angra, K.; Lipp, M.; Goldman, M. Evaluation of an SPF50 sunscreen containing photolyase and antioxidants for its anti-photoaging and photoprotection. J. Drugs Dermatol. JDD 2022, 21, 517–520. [Google Scholar] [PubMed]
- Yarosh, D.B.; Rosenthal, A.; Moy, R. Six critical questions for DNA repair enzymes in skincare products: A review in dialog. Clin. Cosmet. Investig. Dermatol. 2019, 12, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, N.; Berman, B.; Ceilley, R.I.; Kircik, L.H. Understanding the Role of Photolyases: Photoprotection and Beyond. J. Drugs Dermatol. 2017, 16, 61–66. [Google Scholar] [PubMed]
- Kabir, Y.; Seidel, R.; McKnight, B.; Moy, R. DNA repair enzymes: An important role in skin cancer prevention and reversal of photodamage—A review of the literature. J. Drugs Dermatol. 2015, 14, 297–303. [Google Scholar]
- Puviani, M.; Barcella, A.; Milani, M. Efficacy of a photolyase-based device in the treatment of cancerization field in patients with acticinic keratosis and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2013, 148, 693–698. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farris, P.K.; Valacchi, G. Ultraviolet Light Protection: Is It Really Enough? Antioxidants 2022, 11, 1484. https://doi.org/10.3390/antiox11081484
Farris PK, Valacchi G. Ultraviolet Light Protection: Is It Really Enough? Antioxidants. 2022; 11(8):1484. https://doi.org/10.3390/antiox11081484
Chicago/Turabian StyleFarris, Patricia K., and Giuseppe Valacchi. 2022. "Ultraviolet Light Protection: Is It Really Enough?" Antioxidants 11, no. 8: 1484. https://doi.org/10.3390/antiox11081484
APA StyleFarris, P. K., & Valacchi, G. (2022). Ultraviolet Light Protection: Is It Really Enough? Antioxidants, 11(8), 1484. https://doi.org/10.3390/antiox11081484







