The Assessment of Dietary Organic Zinc on Zinc Homeostasis, Antioxidant Capacity, Immune Response, Glycolysis and Intestinal Microbiota in White Shrimp (Litopenaeus vannamei Boone, 1931)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Trial and Sample Collection
2.3. Vibrio Parahaemolyticus Challenge
2.4. Growth Performance and Hemocyte Count
2.5. Zinc Accumulation Analysis
2.6. Biochemical Analysis
2.7. RNA Extraction and qPCR
2.8. Intestinal Microbiota DNA Extraction and Sequencing
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Zinc Accumulation and Zinc Transport
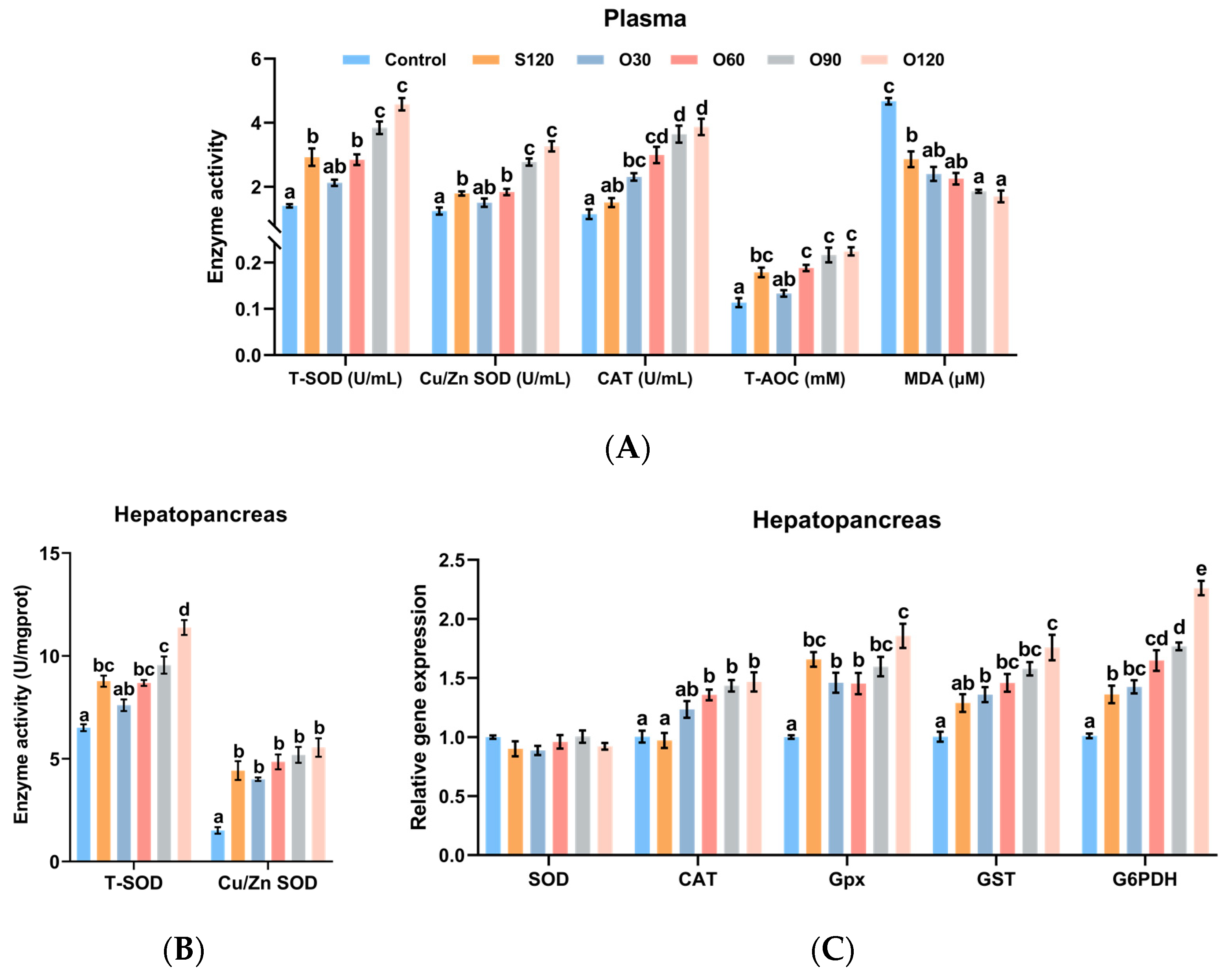
3.3. Antioxidant Capacity
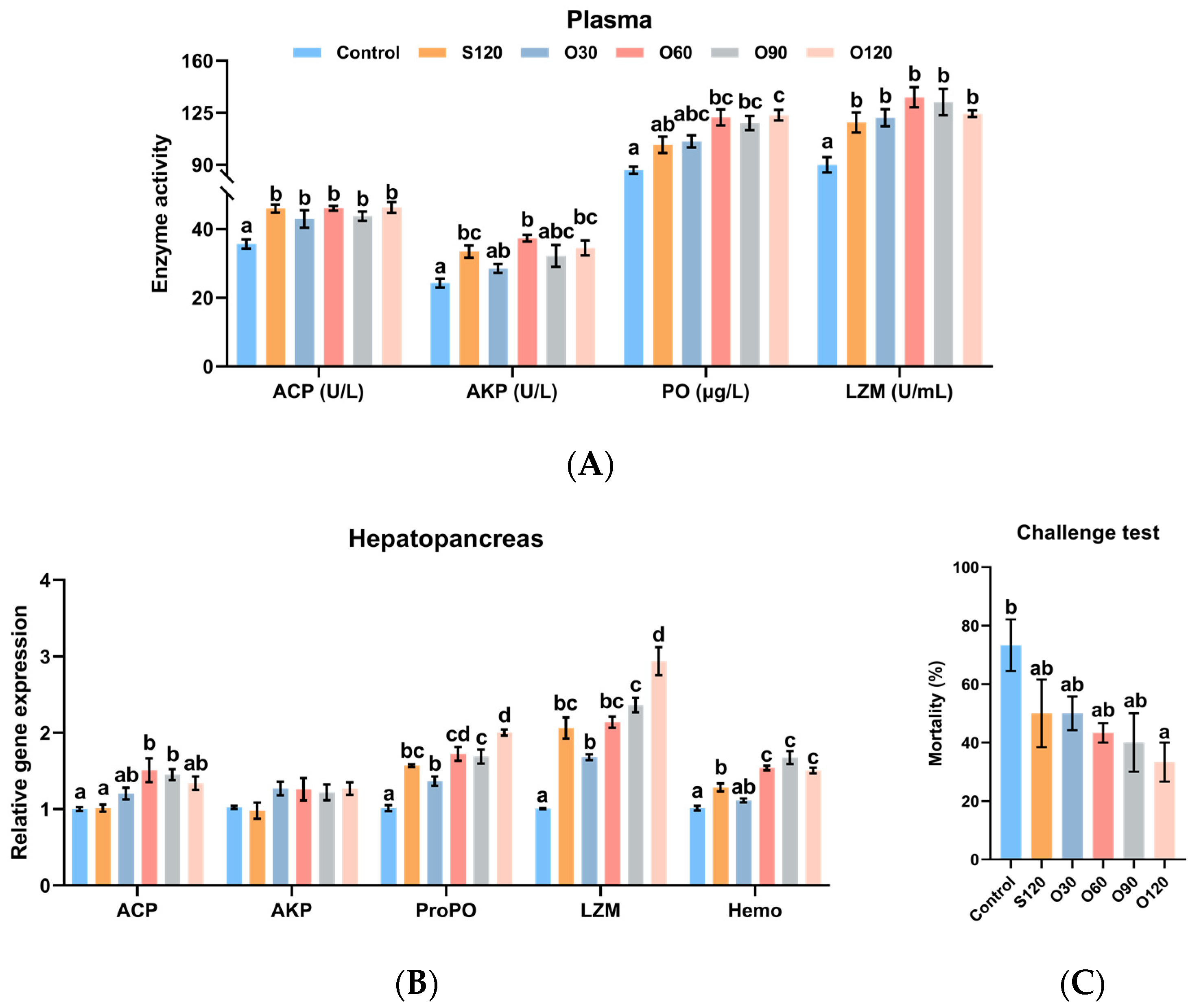
3.4. Immunity
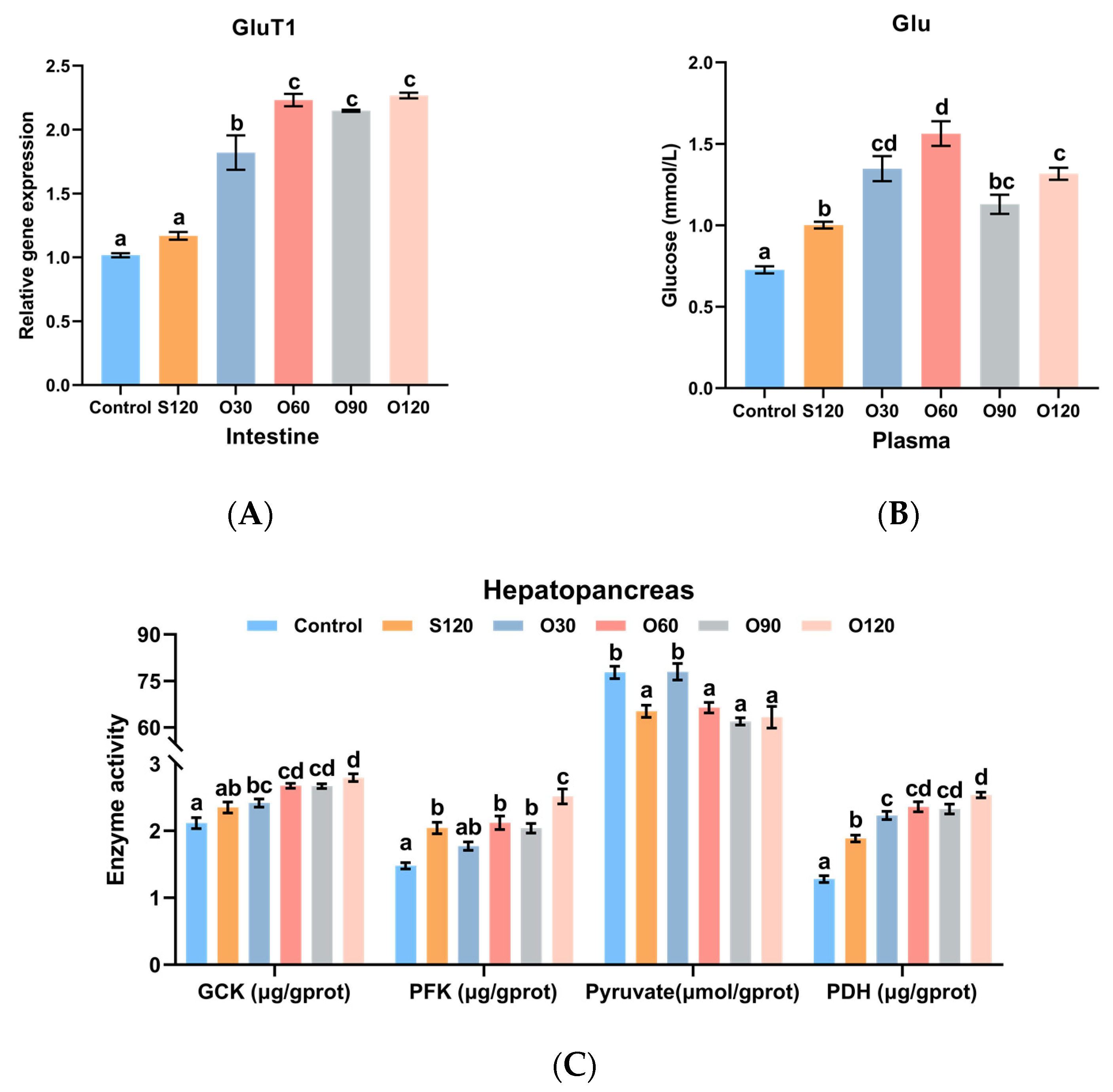
3.5. Glucose Transport and Glycolysis
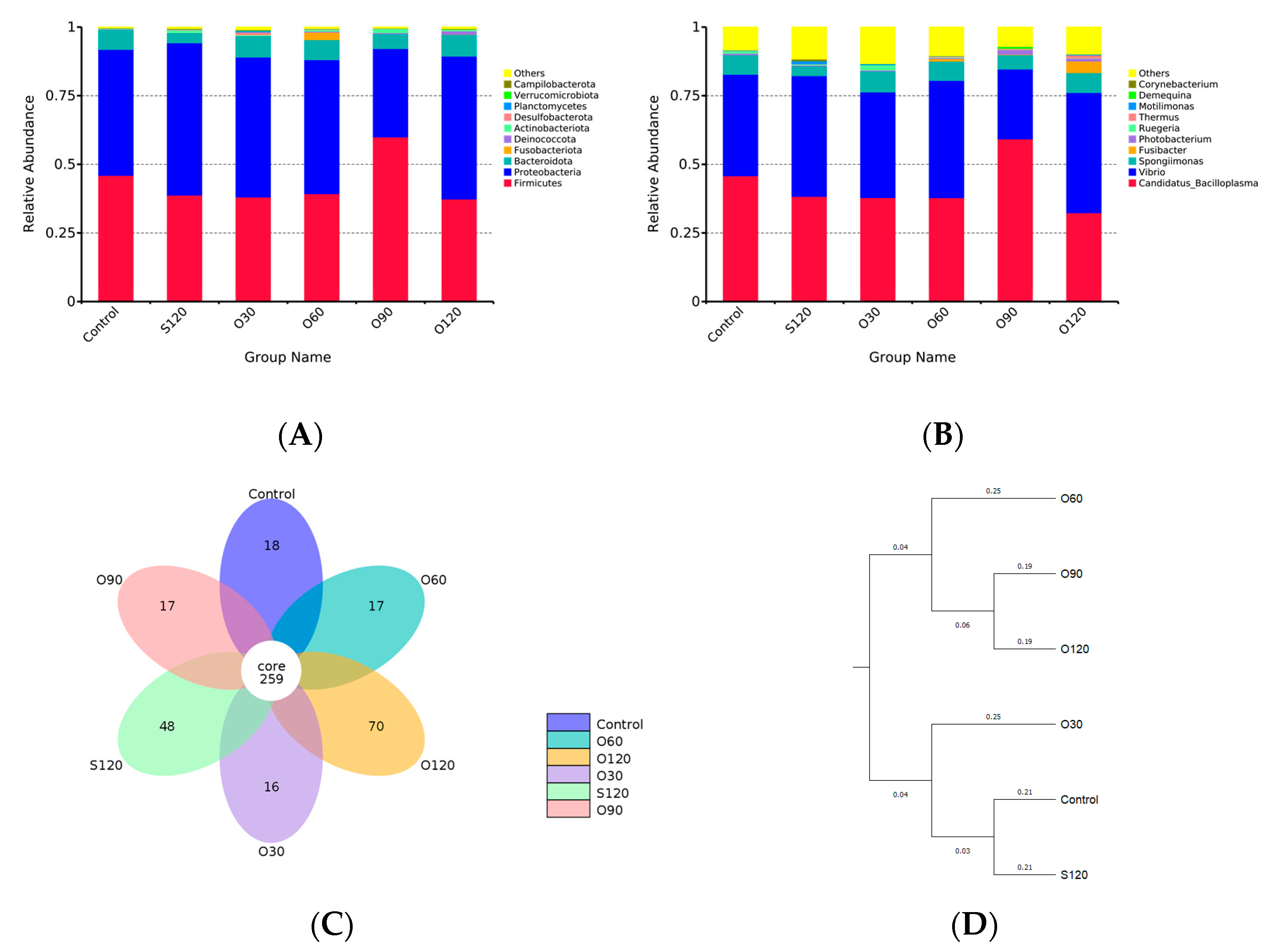
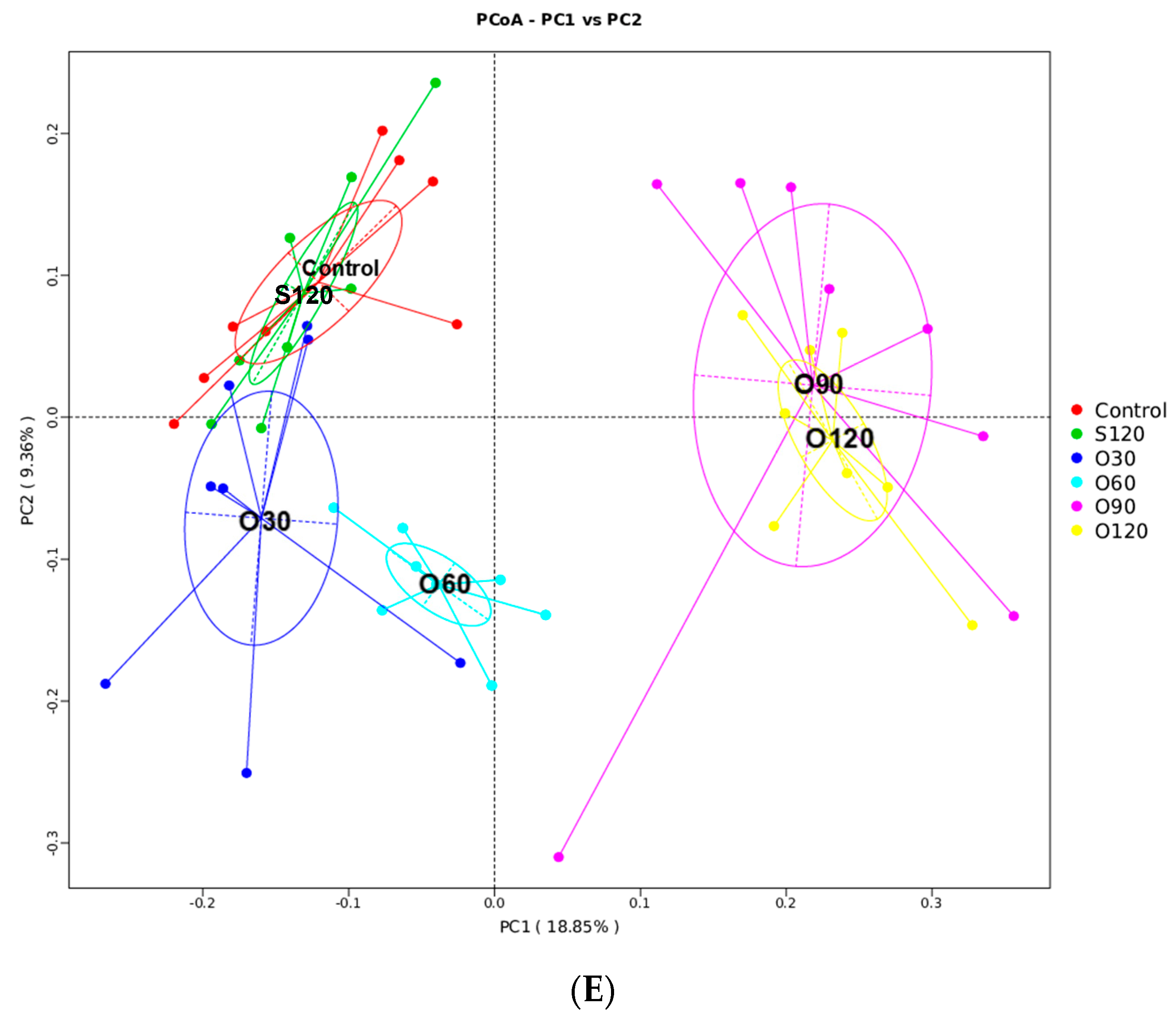
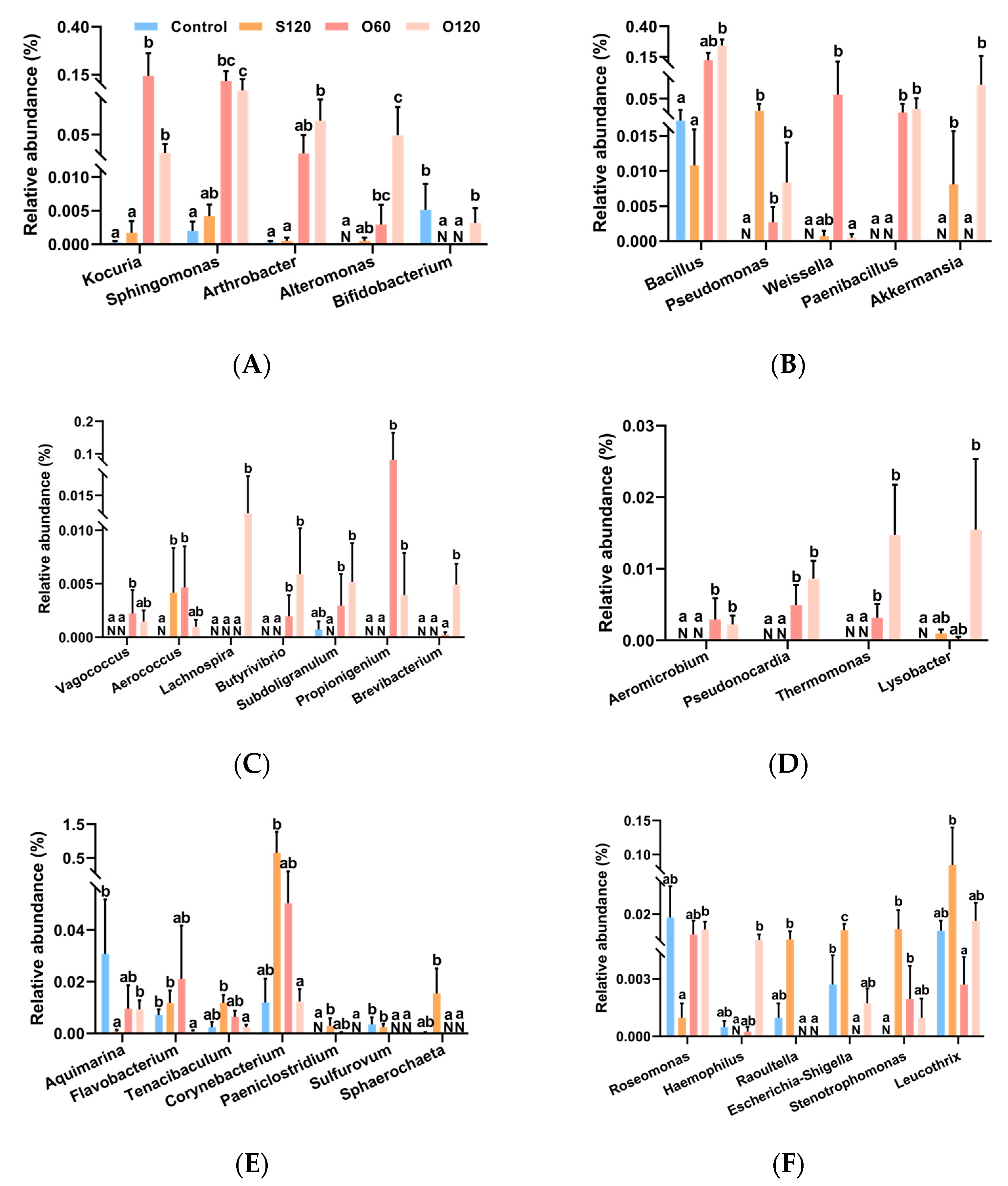
3.6. Intestinal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawood, M.A.O.; Alagawany, M.; Sewilam, H. The role of zinc microelement in aquaculture: A review. Biol. Trace Elem. Res. 2021, 200, 3841–3853. [Google Scholar] [CrossRef] [PubMed]
- Sloup, V.; Jankovská, I.; Nechybová, S.; Peřinková, P.; Langrová, I. Zinc in the animal organism: A review. Sci. Agric. Bohem. 2017, 48, 13–21. [Google Scholar] [CrossRef]
- Wu, G.Y. Principles of Animal Nutrition; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Reilly, C. Zinc. In The Nutritional Trace Metals; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 82–117. [Google Scholar]
- Gibson, R.S. Zinc nutrition in developing countries. Nutr. Res. Rev. 1994, 7, 151–173. [Google Scholar] [CrossRef]
- Daniel, D. A review on replacing fish meal in aqua feeds using plant protein sources. Int. J. Fish. Aquat. Stud. 2018, 6, 164–179. [Google Scholar]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In Feed and Feeding Practices in Aquaculture; Davis., D.A., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 203–233. [Google Scholar]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Feeds for the Aquaculture Sector; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Davis, D.A.; Lawrence, A.L.; Gatlin, D.M. Evaluation of the dietary zinc requirement of Penaeus vannamei and effects of phytic acid on zinc and phosphorus bioavailability. J. World Aquac. Soc. 1993, 24, 40–47. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Patnaik, S.; Browdy, C.L.; Lawrence, A.L. Availability of dietary zinc sources and effects on performance of pacific white shrimp Litopenaeus vannamei (Boone). Int. J. Recirc. Aquac. 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Zak, D.; Hupfer, M.; Cabezas, A.; Jurasinski, G.; Audet, J.; Kleeberg, A.; McInnes, R.; Kristiansen, S.M.; Petersen, R.J.; Liu, H.; et al. Sulphate in freshwater ecosystems: A review of sources, biogeochemical cycles, ecotoxicological effects and bioremediation. Earth-Sci. Rev. 2021, 212, 103446. [Google Scholar] [CrossRef]
- Wang, F.; Chapman, P.M. Biological implications of sulfide in sediment-a review focusing on sediment toxicity. Environ. Toxicol. Chem. 1999, 18, 2526–2532. [Google Scholar]
- Lamers, L.P.M.; Govers, L.L.; Janssen, I.C.J.M.; Geurts, J.J.M.; Van der Welle, M.E.W.; Van Katwijk, M.M.; Van der Heide, T.; Roelofs, J.G.M.; Smolders, A.J.P. Sulfide as a soil phytotoxin–A review. Front. Plant. Sci. 2013, 4, 268. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Q. 2020, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.N.; Hogstrand, C. Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. J. Exp. Biol. 2002, 205, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Antony Jesu Prabhu, P.; Stewart, T.; Silva, M.; Amlund, H.; Ørnsrud, R.; Lock, E.-J.; Waagbo, R.; Hogstrand, C. Zinc uptake in fish intestinal epithelial model RTgutGC: Impact of media ion composition and methionine chelation. J. Trace Elem. Med. Biol. 2018, 50, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.B.; Kuang, Y.G.; Ma, Z.X.; Liu, Y.G. The effect of feeding broiler with inorganic, organic, and coated trace minerals on performance, economics, and retention of copper and zinc. J. Appl. Poult. Res. 2020, 29, 1084–1090. [Google Scholar] [CrossRef]
- Paripatananont, T.; Lovell, R.T. Chelated zinc reduces the dietary zinc requirement of channel catfish, Ictalurus punctatus. Aquaculture 1995, 133, 73–82. [Google Scholar] [CrossRef]
- Paripatananont, T.; Lovell, R.T. Responses of channel catfish fed organic and inorganic sources of zinc to Edwardsiella ictaluri challenge. J. Aquat. Anim. Health 1995, 7, 147–154. [Google Scholar] [CrossRef]
- Mohseni, M.; Hamidoghli, A.; Bai, S.C. Organic and inorganic dietary zinc in beluga sturgeon (Huso huso): Effects on growth, hematology, tissue concertation and oxidative capacity. Aquaculture 2021, 539, 736672. [Google Scholar] [CrossRef]
- Jintasataporn, O.; Ward, T.; Kattakdad, S. The efficacy of organic zinc amino acid complex (Availazn®) on growth performance and immunity of pangasius catfish (Pangasianodon hypophthalmus). Aquac. Indones. 2015, 15, 94–97. [Google Scholar] [CrossRef]
- Tan, B.; Mai, K. Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 2001, 192, 67–84. [Google Scholar] [CrossRef]
- Meiler, K.A.; Cleveland, B.; Radler, L.; Kumar, V. Oxidative stress-related gene expression in diploid and triploid rainbow trout (Oncorhynchus mykiss) fed diets with organic and inorganic zinc. Aquaculture 2021, 533, 736149. [Google Scholar] [CrossRef]
- Meiler, K.A.; Kumar, V. Organic and inorganic zinc in the diet of a commercial strain of diploid and triploid rainbow trout (Oncorhynchus mykiss): Effects on performance and mineral retention. Aquaculture 2021, 545, 737126. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Yang, Y.; Li, F.; Luo, L. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp (Litopenaeus vannamei). Aquaculture 2013, 406–407, 79–84. [Google Scholar] [CrossRef]
- Katya, K.; Lee, S.; Yun, H.; Dagoberto, S.; Browdy, C.L.; Vazquez-Anon, M.; Bai, S.C. Efficacy of inorganic and chelated trace minerals (Cu, Zn and Mn) premix sources in Pacific white shrimp, Litopenaeus vannamei (Boone) fed plant protein based diets. Aquaculture 2016, 459, 117–123. [Google Scholar] [CrossRef]
- Yuan, Y.; Luo, J.; Zhu, T.; Jin, M.; Jiao, L.; Sun, P.; Ward, T.L.; Ji, F.; Xu, G.; Zhou, Q. Alteration of growth performance, meat quality, antioxidant and immune capacity of juvenile Litopenaeus vannamei in response to different dietary dosage forms of zinc: Comparative advantages of zinc amino acid complex. Aquaculture 2020, 522, 735120. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, J.; Xie, J.; Liu, D.; Fan, Y.; Ma, H.; Wang, C.; Hong, Z. Improvement roles of zinc supplementation in low dose lead induced testicular damage and glycolytic inhibition in mice. Toxicology 2021, 462, 152933. [Google Scholar] [CrossRef]
- Tamaki, N.; Ikeda, T.; Funatsuka, A. Zinc as activating cation for muscle glycolysis. J. Nutr. Sci. Vitaminol. 1983, 29, 655–662. [Google Scholar] [CrossRef]
- Brand, I.A.; Kleineke, J. Intracellular zinc movement and its effect on the carbohydrate metabolism of isolated rat hepatocytes. J. Biol. Chem. 1996, 271, 1941–1949. [Google Scholar] [CrossRef]
- Villagómez-Estrada, S.; Pérez, J.F.; Darwich, L.; Vidal, A.; van Kuijk, S.; Melo-Durán, D.; Solà-Oriol, D. Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J. Anim. Sci. 2020, 98, skaa117. [Google Scholar] [CrossRef]
- Khajeh Bami, M.; Afsharmanesh, M.; Ebrahimnejad, H. Effect of dietary Bacillus coagulans and different forms of zinc on performance, intestinal microbiota, carcass and meat quality of broiler chickens. Probiotics Antimicrob. Proteins 2020, 12, 461–472. [Google Scholar] [CrossRef]
- Aktas, M.; Ciger, O.; Genc, E.; Genc, M.A.; Cavdar, N. Effects of mannan oligosaccharide and serotonin on molting, growth, body composition and hepatopancreas histology of white leg shrimp Litopenaeus vannamei (Boone 1931). Turk. J. Fish Aquat. Sci. 2014, 14, 205–211. [Google Scholar] [CrossRef]
- Patil, P.K.; Muralidhar, M.; Solanki, H.G.; Patel, P.P.; Patel, K.; Gopla, C. Effect of culture intensity and probiotics application on microbiological and environmental parameters in Litopenaeus vannamei culture ponds. J. Environ. Biol. 2016, 37, 21–29. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Vargas-Albores, F.; Guzmán, M.-A.; Ochoa, J.-L. An anticoagulant solution for haemolymph collection and prophenoloxidase studies of penaeid shrimp (Penaeus californiensis). Comp. Biochem. Physiol. Part A Physiol. 1993, 106, 299–303. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-pcr data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef] [PubMed]
- Shahpar, Z.; Johari, S.A. Effects of dietary organic, inorganic, and nanoparticulate zinc on rainbow trout, Oncorhynchus mykiss larvae. Biol. Trace Elem. Res. 2019, 190, 535–540. [Google Scholar] [CrossRef]
- Maage, A.; Julshamn, K.; Berge, G.E. Zinc gluconate and zinc sulphate as dietary zinc sources for Atlantic salmon. Aquac. Nutr. 2001, 7, 183–187. [Google Scholar] [CrossRef]
- Fountoulaki, E.; Morgane, H.; Rigos, G.; Antigoni, V.; Mente, E.; Sweetman, J.; Nengas, I. Evaluation of zinc supplementation in European sea bass (Dicentrarchus labrax) juvenile diets. Aquac. Res. 2010, 41, 208–216. [Google Scholar] [CrossRef]
- Sekler, I.; Sensi, S.L.; Hershfinkel, M.; Silverman, W.F. Mechanism and regulation of cellular zinc transport. Mol. Med. 2007, 13, 337–343. [Google Scholar] [CrossRef]
- Kambe, T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci. Biotechnol. Biochem. 2011, 75, 1036–1043. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef]
- Hamer, D.H. Metallothionein. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar] [CrossRef]
- Pourang, N.; Dennis, J.H. Distribution of trace elements in tissues of two shrimp species from the Persian Gulf and roles of metallothionein in their redistribution. Environ. Int. 2005, 31, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Hogstrand, C. Zinc. In Fish Physiology; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Waltham, MA, USA, 2011; pp. 135–200. [Google Scholar]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Wang, G.; Zhou, S.; Wang, J.; Zhao, J.; Hoare, R.; Monaghan, S.J.; Wang, Z.; Sun, C. Survival and immune response of white shrimp Litopenaeus vannamei following single and concurrent infections with WSSV and Vibrio parahaemolyticus. Fish Shellfish Immunol. 2019, 92, 712–718. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Boonchuen, P.; Jaree, P.; Somboonviwat, K.; Somboonwiwat, K. Regulation of shrimp prophenoloxidase activating system by lva-miR-4850 during bacterial infection. Sci. Rep. 2021, 11, 3821. [Google Scholar] [CrossRef]
- De-la-Re-Vega, E.; García-Galaz, A.; Díaz-Cinco, M.E.; Sotelo-Mundo, R.R. White shrimp (Litopenaeus vannamei) recombinant lysozyme has antibacterial activity against Gram negative bacteria: Vibrio alginolyticus, Vibrio parahemolyticus and Vibrio cholerae. Fish Shellfish Immunol. 2006, 20, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, F.; Hu, Z.; Zhao, X.; Min, S.; Du, Z.; Zhao, S.; Ye, X.; Li, Y. Hemocyanin from shrimp Litopenaeus vannamei shows hemolytic activity. Fish Shellfish Immunol. 2009, 27, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef]
- Ezaki, O. IIb group metal ions (Zn2+, Cd2+, Hg2+) stimulate glucose transport activity by post-insulin receptor kinase mechanism in rat adipocytes. J. Biol. Chem. 1989, 264, 16118–16122. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Zinc and diabetes mellitus: Understanding molecular mechanisms and clinical implications. Daru 2015, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Quintana, J.A.; Yepiz-Plascencia, G. Glucose and other hexoses transporters in marine invertebrates: A mini review. Electron. J. Biotechnol. 2012, 15, 16. [Google Scholar]
- Kumari, A. Glycolysis. In Sweet Biochemistry; Academic Press: Waltham, MA, USA, 2018; pp. 1–5. [Google Scholar]
- Yetkin-Arik, B.; Vogels, I.M.C.; Nowak-Sliwinska, P.; Weiss, A.; Houtkooper, R.H.; Van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. The role of glycolysis and mitochondrial respiration in the formation and functioning of endothelial tip cells during angiogenesis. Sci. Rep. 2019, 9, 12608. [Google Scholar] [CrossRef]
- McKerrecher, D.; Waring, M.J. Property-based design in the optimisation of benzamide glucokinase activators. In Progress in Medicinal Chemistry; Lawton, G., Witty, D.R., Eds.; Elsevier: Oxford, UK, 2013; pp. 1–43. [Google Scholar]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Kechrid, Z.; Demýr, N.; Abdennour, C.; Bouzerna, N. Effect of low dietary zinc intake and experimental diabetes on the zinc and carbohydrate metabolism in rats. Turk. J. Med. Sci. 2002, 32, 101–105. [Google Scholar]
- Xiong, J.; Wang, K.; Wu, J.; Qiuqian, L.; Yang, K.; Qian, Y.; Zhang, D. Changes in intestinal bacterial communities are closely associated with shrimp disease severity. Appl. Microbiol. Biotechnol. 2015, 99, 6911–6919. [Google Scholar] [CrossRef]
- Wang, T.; Yang, J.; Lin, G.; Li, M.; Zhu, R.; Zhang, Y.; Mai, K. Effects of dietary mannan oligosaccharides on non-specific immunity, intestinal health, and antibiotic resistance genes in pacific white shrimp Litopenaeus vannamei. Front. Immunol. 2021, 12, 772570. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Qazi, J.I. Probiotic antagonism of Sphingomonas sp. against Vibrio anguillarum exposed Labeo rohita fingerlings. Adv. Life Sci. 2014, 4, 156–165. [Google Scholar]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Farzanfar, A. The use of probiotics in shrimp aquaculture. FEMS Immunol. Med. Microbiol. 2006, 48, 149–158. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B. Short-chain fatty acid production and functional aspects on host metabolism. In Human Microbiota in Health and Disease; Tungland, B., Ed.; Academic Press: Waltham, MA, USA, 2018; pp. 37–106. [Google Scholar]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 9–17. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef] [PubMed]
- Schink, B.; Pfennig, N. Propionigenium modestum gen. nov. sp. nov. a new strictly anaerobic, nonsporing bacterium growing on succinate. Arch. Microbiol. 1982, 133, 209–216. [Google Scholar] [CrossRef]
- Madhana, S.; Kanimozhi, G.; Panneerselvam, A. Probiotics in shrimp aquaculture. In Advances in Probiotics; Dhanasekaran, D., Sankaranarayanan, A., Eds.; Academic Press: Waltham, MA, USA, 2021; pp. 309–325. [Google Scholar]
- Panthee, S.; Hamamoto, H.; Paudel, A.; Sekimizu, K. Lysobacter species: A potential source of novel antibiotics. Arch. Microbiol. 2016, 198, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Brescia, F.; Pertot, I.; Puopolo, G. Lysobacter. In Benef Microbes Agro-Ecology; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Waltham, MA, USA, 2020; pp. 313–338. [Google Scholar]
- Al-Daghistani, H.I.; Mohammad, B.T.; Kurniawan, T.A.; Singh, D.; Rabadi, A.D.; Xue, W.; Avtar, R.; Othman, M.H.D.; Shirazian, S. Characterization and applications of Thermomonas hydrothermalis isolated from Jordan’s hot springs for biotechnological and medical purposes. Process. Biochem. 2021, 104, 171–181. [Google Scholar] [CrossRef]
- Hazarika, S.N.; Thakur, D. Actinobacteria. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Waltham, MA, USA, 2020; pp. 443–476. [Google Scholar]
- Waśkiewicz, A.; Irzykowska, L. Flavobacterium spp.—Characteristics, occurrence, and toxicity. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Waltham, MA, USA, 2014; pp. 938–942. [Google Scholar]
- Wu, Z.; Zhang, Q.; Lin, Y.; Hao, J.; Wang, S.; Zhang, J.; Li, A. Taxonomic and functional characteristics of the gill and gastrointestinal microbiota and its correlation with intestinal metabolites in NEW GIFT strain of farmed adult nile tilapia (Oreochromis niloticus). Microorganisms 2021, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Juniper, S.K.; Perez, M.; Ju, S.; Kim, S. Diversity and characterization of bacterial communities of five co-occurring species at a hydrothermal vent on the Tonga Arc. Ecol. Evol. 2021, 11, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D. The impact of pathogens on exploited populations of decapod crustaceans. J. Invertebr. Pathol. 2012, 110, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.J. Actinobacteria. In Taxonomic Guide to Infectious Diseases; Berman, J.J., Ed.; Academic Press: Waltham, MA, USA, 2012; pp. 77–84. [Google Scholar]
- Coretti, L.; Cuomo, M.; Florio, E.; Palumbo, D.; Keller, S.; Pero, R.; Chiariotti, L.; Lembo, F.; Cafiero, C. Subgingival dysbiosis in smoker and non-smoker patients with chronic periodontitis. Mol. Med. Rep. 2017, 15, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.J. Gamma Proteobacteria. In Taxonomic Guide to Infectious Diseases; Berman, J.J., Ed.; Academic Press: Waltham, MA, USA, 2012; pp. 37–47. [Google Scholar]
- Sękowska, A. Raoultella spp.—Clinical significance, infections and susceptibility to antibiotics. Folia Microbiol. 2017, 62, 221–227. [Google Scholar] [CrossRef]
- Ghosh, R.; Chatterjee, S.; Mandal, N.C. Stenotrophomonas. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Waltham, MA, USA, 2020; pp. 427–442. [Google Scholar]
- Mori, F.; Umezawa, Y.; Kondo, R.; Wada, M. Dynamics of sulfate-reducing bacteria community structure in surface sediment of a seasonally hypoxic enclosed bay. Microbes Environ. 2018, 33, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Drancourt, M.; Prebet, T.; Aghnatios, R.; Edouard, S.; Cayrou, C.; Henry, M.; Blaise, D.; Raoult, D. Planctomycetes DNA in febrile aplastic patients with leukemia, rash, diarrhea, and micronodular pneumonia. J. Clin. Microbiol. 2014, 52, 3453–3455. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Bronowicki, J.P.; Pereira, I.A.C.; Mougenel, J.L.; Le Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, P.; Yu, X.; Ding, H.; Wang, Z.; Feng, J. Effect of long-term and short-term imbalanced Zn manipulation on gut microbiota and screening for microbial markers sensitive to zinc status. Microbiol. Spectr. 2021, 9, e0048321. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wolf, P.G.; Guo, S.; Guo, Y.; Gaskins, H.R.; Zhang, B. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 2017, 43, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Lian, S.; Wu, Y.; Yan, L.; Quan, G.; Zhu, G. Zinc is an important inter-kingdom signal between the host and microbe. Vet. Res. 2021, 52, 39. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (%) | Diets | |||||
|---|---|---|---|---|---|---|
| Control | S120 | O30 | O60 | O90 | O120 | |
| Fish meal 1 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Shrimp shell meal 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Brewer yeast 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean meal 1 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Cottonseed protein 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Peanut meal 1 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Wheat flour 1 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 |
| Fish oil 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Phospholipid 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Monocalcium phosphate 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride 2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin mix 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral mix (Zn Free) 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Lysine hydrochloride 1 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Methionine 2 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Threonine 2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin C-35 phosphate 1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| ZnSO4·7H2O (22.74%) 2 | - | 0.0528 | - | - | - | - |
| Bioplex Zn® (15%) 4 | - | - | 0.02 | 0.04 | 0.06 | 0.08 |
| Astaxanthin (10%) 1 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Y2O3 2 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Carrier 1 | 1.24 | 1.1872 | 1.22 | 1.20 | 1.18 | 1.16 |
| Analyzed Nutrient Compositions (% Dry Matter) | ||||||
| Crude protein | 45.70 | 45.76 | 45.96 | 46.22 | 45.89 | 46.46 |
| Crude lipid | 4.32 | 4.48 | 4.41 | 4.41 | 4.72 | 4.67 |
| Ash | 8.92 | 9.16 | 9.36 | 9.13 | 9.23 | 9.25 |
| Zinc Analysis (mg·kg−1) | ||||||
| Zn (formulated value) | 0 | 120 | 30 | 60 | 90 | 120 |
| Zn (analyzed value) | 53 | 133 | 86 | 106 | 138 | 171 |
| Diets | Control | S120 | O30 | O60 | O90 | O120 |
|---|---|---|---|---|---|---|
| IBW (g) | 2.34 ± 0.02 | 2.38 ± 0.02 | 2.38 ± 0.03 | 2.36 ± 0.02 | 2.35 ± 0.02 | 2.38 ± 0.01 |
| FBW (g) | 10.02 ± 0.32 a | 11.25 ± 0.43 ab | 10.56 ± 0.18 ab | 12.41 ± 0.83 b | 10.50 ± 0.32 ab | 10.73 ± 0.39 ab |
| WGR (%) | 328.3 ± 13.1 a | 374.0 ± 20.1 ab | 343.9 ± 12.0 ab | 427.2 ± 36.2 b | 348.0 ± 16.2 ab | 351.6 ± 16.0 ab |
| SGR (%·day−1) | 2.59 ± 0.06 a | 2.77 ± 0.08 ab | 2.66 ± 0.05 ab | 2.96 ± 0.12 b | 2.67 ± 0.07 ab | 2.69 ± 0.06 ab |
| FI (%·day−1) | 1.56 ± 0.04 | 1.43 ± 0.05 | 1.47 ± 0.04 | 1.33 ± 0.07 | 1.44 ± 0.09 | 1.54 ± 0.08 |
| FE | 0.357 ± 0.014 | 0.409 ± 0.022 | 0.384 ± 0.013 | 0.463 ± 0.040 | 0.399 ± 0.029 | 0.372 ± 0.024 |
| CF (100 g·cm−3) | 0.652 ± 0.004 | 0.658 ± 0.010 | 0.650 ± 0.007 | 0.650 ± 0.003 | 0.648 ± 0.007 | 0.637 ± 0.011 |
| Total hemocyte (×106) | 27.14 ± 1.35 | 24.18 ± 1.90 | 26.25 ± 2.02 | 28.64 ± 2.11 | 24.42 ± 2.26 | 22.51 ± 2.42 |
| Diets | Control | S120 | O30 | O60 | O90 | O120 |
|---|---|---|---|---|---|---|
| OTUs | 281 ± 25 | 346 ± 33 | 386 ± 47 | 336 ± 17 | 253 ± 39 | 281 ± 21 |
| Chao1 | 310.7 ± 22.9 ab | 385.4 ± 35.7 ab | 422.0 ± 48.2 b | 379.1 ± 16.9 ab | 278.1 ± 40.0 a | 303.1 ± 21.4 ab |
| ACE | 332.8 ± 21.3 ab | 404.8 ± 36.7 ab | 445.7 ± 47.5 b | 403.5 ± 15.7 ab | 292.6 ± 41.3 a | 319.8 ± 21.0 ab |
| Shannon | 2.66 ± 0.18 | 3.05 ± 0.20 | 3.35 ± 0.30 | 2.99 ± 0.10 | 2.70 ± 0.16 | 3.24 ± 0.10 |
| Simpson | 0.693 ± 0.045 a | 0.757 ± 0.026 ab | 0.801 ± 0.023 ab | 0.768 ± 0.016 ab | 0.695 ± 0.028 a | 0.812 ± 0.012 b |
| PD whole tree | 18.61 ± 1.00 | 22.64 ± 1.45 | 24.10 ± 1.89 | 22.60 ± 0.81 | 19.07 ± 2.33 | 20.67 ± 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, T.; Lin, G.; Li, M.; Zhang, Y.; Mai, K. The Assessment of Dietary Organic Zinc on Zinc Homeostasis, Antioxidant Capacity, Immune Response, Glycolysis and Intestinal Microbiota in White Shrimp (Litopenaeus vannamei Boone, 1931). Antioxidants 2022, 11, 1492. https://doi.org/10.3390/antiox11081492
Yang J, Wang T, Lin G, Li M, Zhang Y, Mai K. The Assessment of Dietary Organic Zinc on Zinc Homeostasis, Antioxidant Capacity, Immune Response, Glycolysis and Intestinal Microbiota in White Shrimp (Litopenaeus vannamei Boone, 1931). Antioxidants. 2022; 11(8):1492. https://doi.org/10.3390/antiox11081492
Chicago/Turabian StyleYang, Jinzhu, Tiantian Wang, Gang Lin, Mingzhu Li, Yanjiao Zhang, and Kangsen Mai. 2022. "The Assessment of Dietary Organic Zinc on Zinc Homeostasis, Antioxidant Capacity, Immune Response, Glycolysis and Intestinal Microbiota in White Shrimp (Litopenaeus vannamei Boone, 1931)" Antioxidants 11, no. 8: 1492. https://doi.org/10.3390/antiox11081492
APA StyleYang, J., Wang, T., Lin, G., Li, M., Zhang, Y., & Mai, K. (2022). The Assessment of Dietary Organic Zinc on Zinc Homeostasis, Antioxidant Capacity, Immune Response, Glycolysis and Intestinal Microbiota in White Shrimp (Litopenaeus vannamei Boone, 1931). Antioxidants, 11(8), 1492. https://doi.org/10.3390/antiox11081492







