The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction
Abstract
:1. Introduction
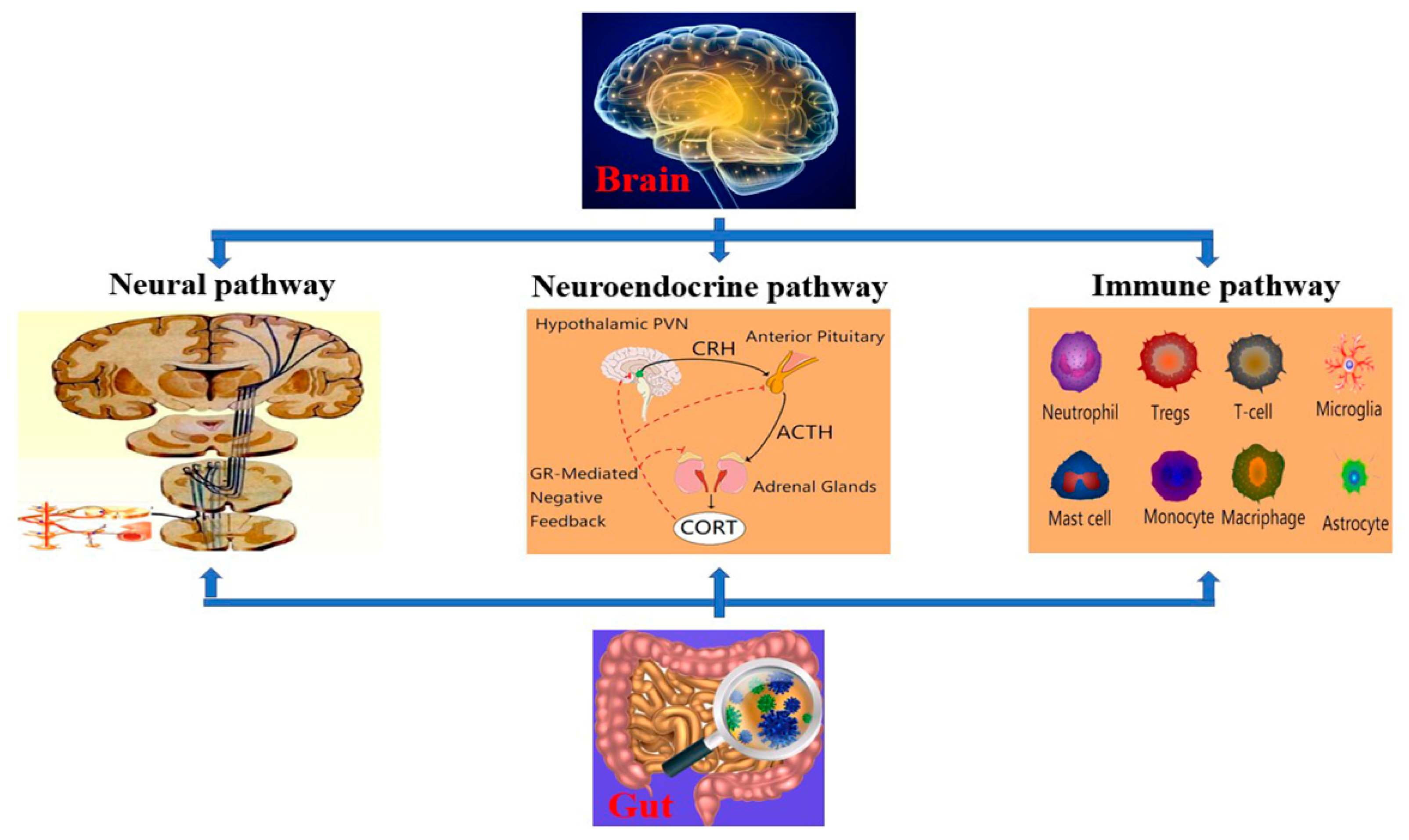
2. BGA
3. TBI and BGA
4. m6A RNA Modification and BGA
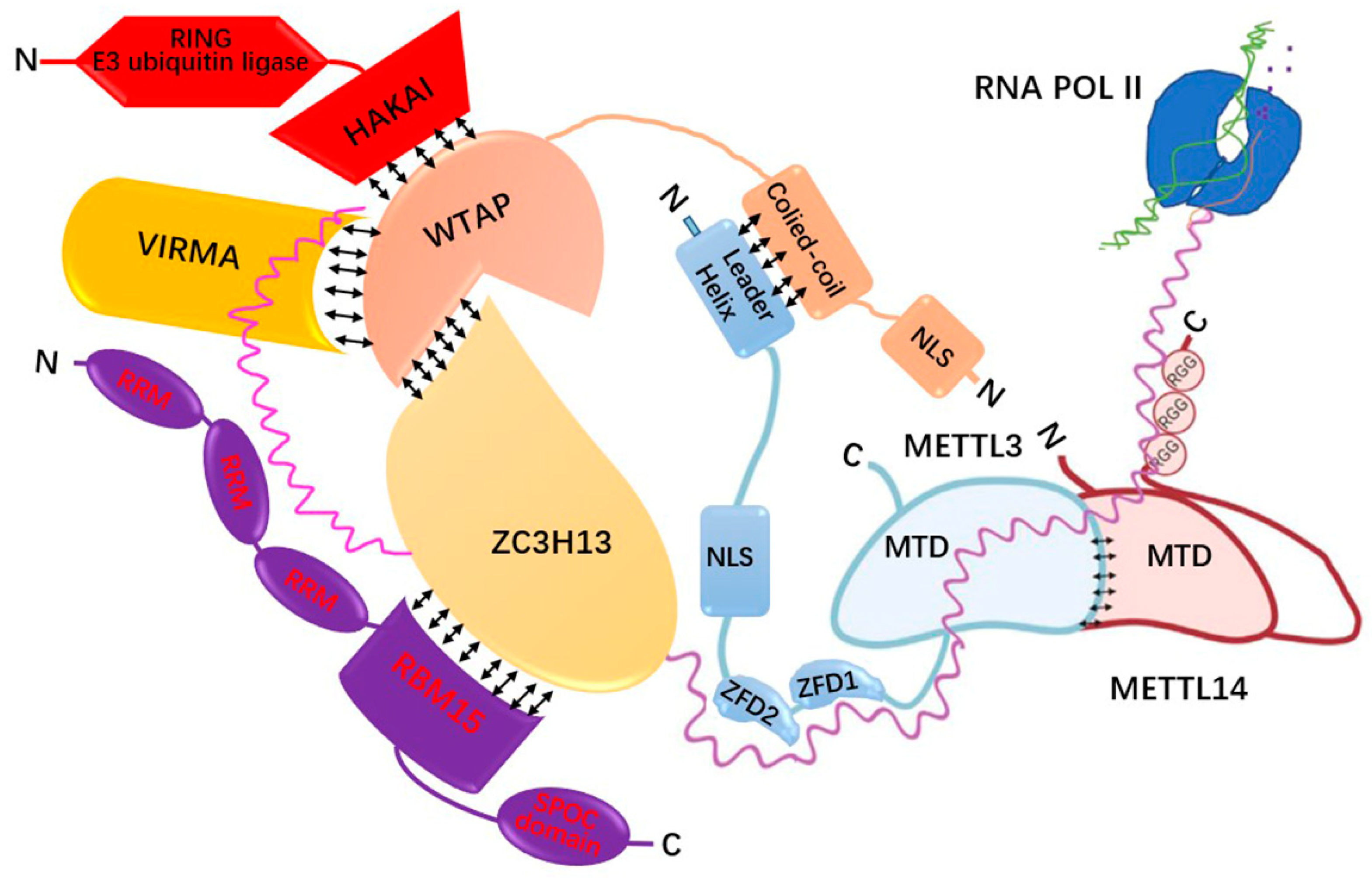
4.1. m6A Related Genes and the Regulatory Mechanism of m6A Modification
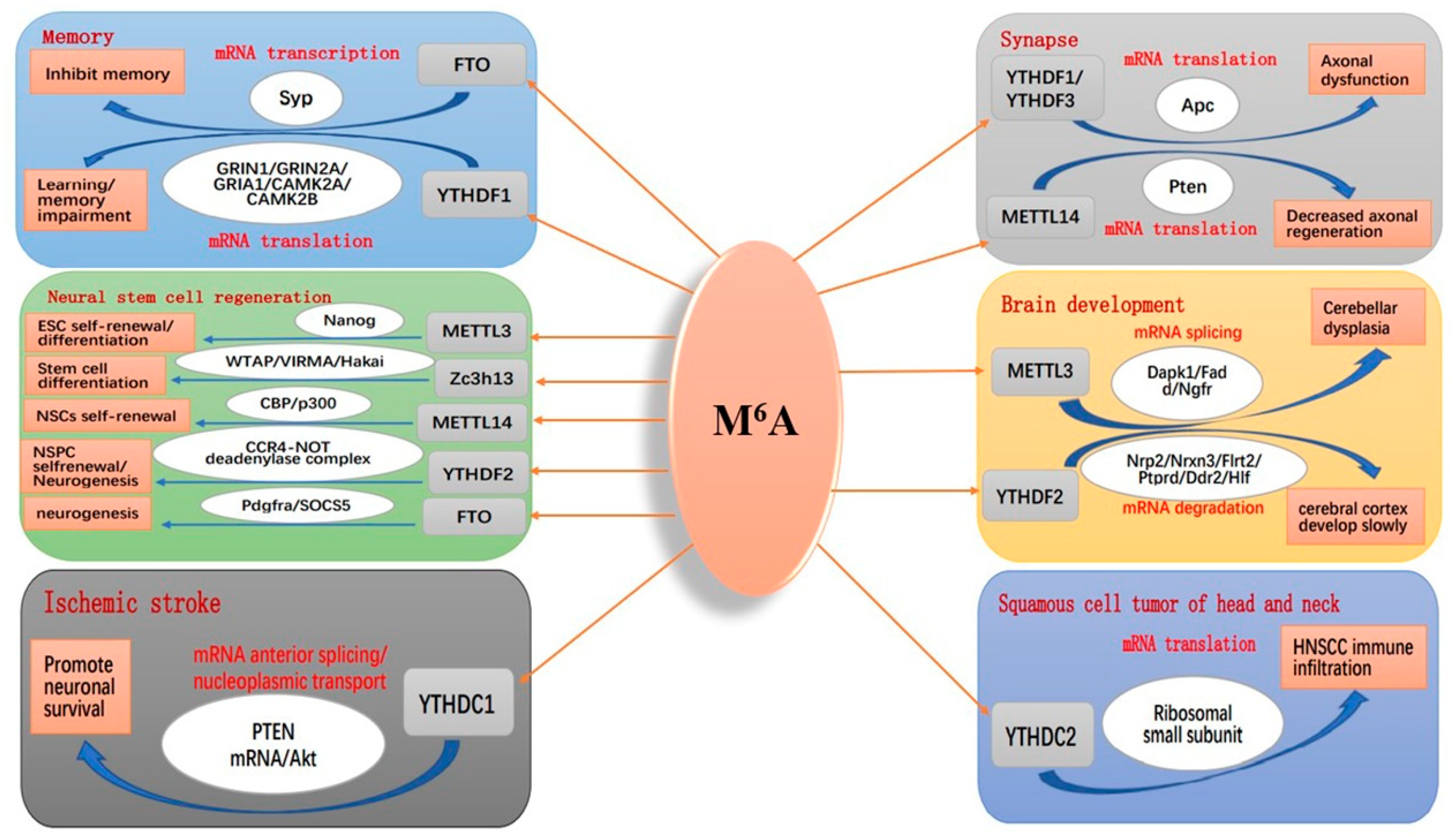
4.2. m6A Modification and Brain
4.3. m6A Modification and Gut Microbiome
5. The Role of m6A RNA Modification in TBI-Mediated BGA
5.1. m6A Modification and TBI
5.2. The Role of m6A in TBI-Mediated BGA
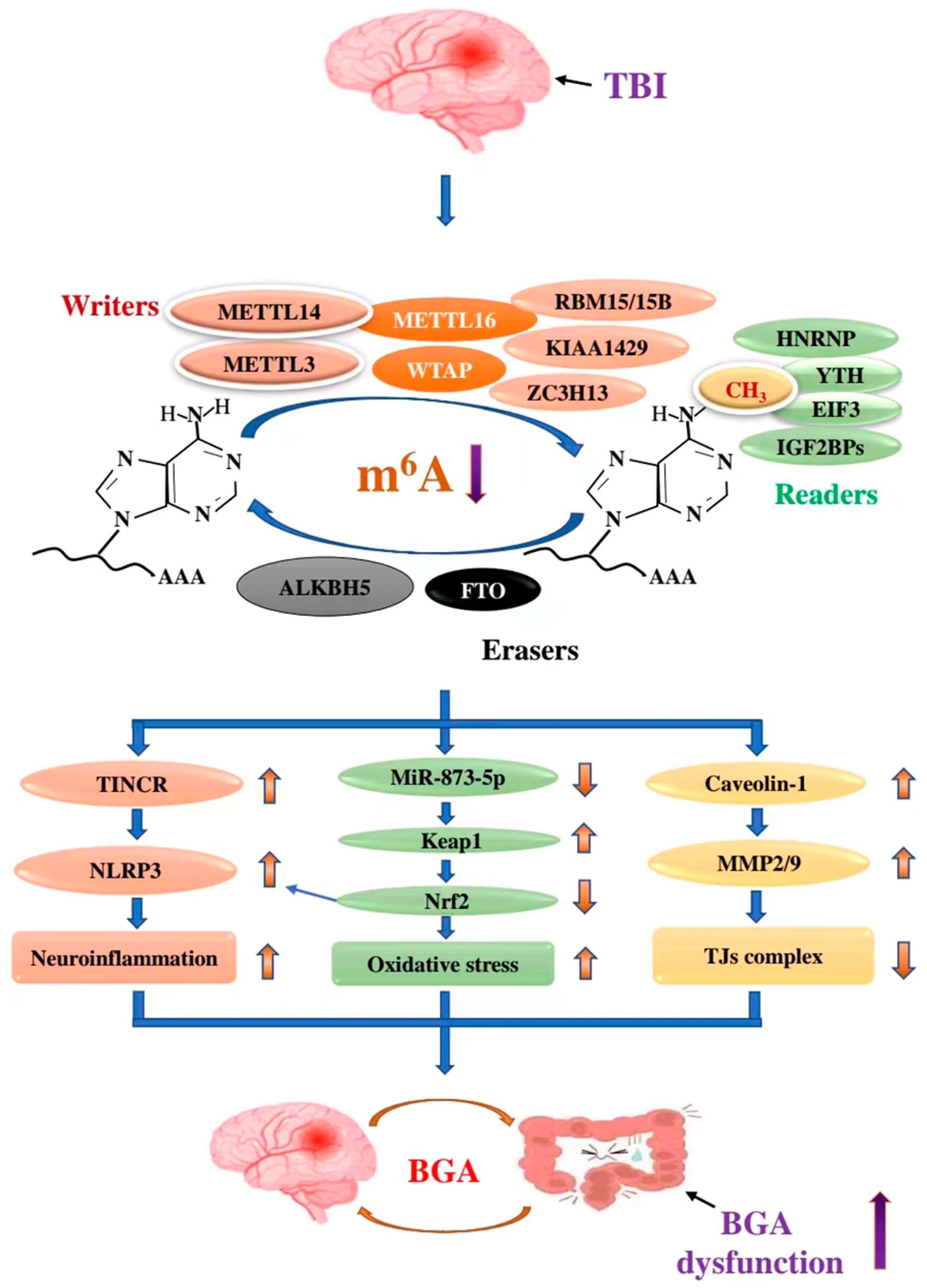
5.2.1. METTL14/TINCR/NLRP3 Axis
5.2.2. METLL3/miR-873-5p/Keap1/Nrf2 Signalling Pathway
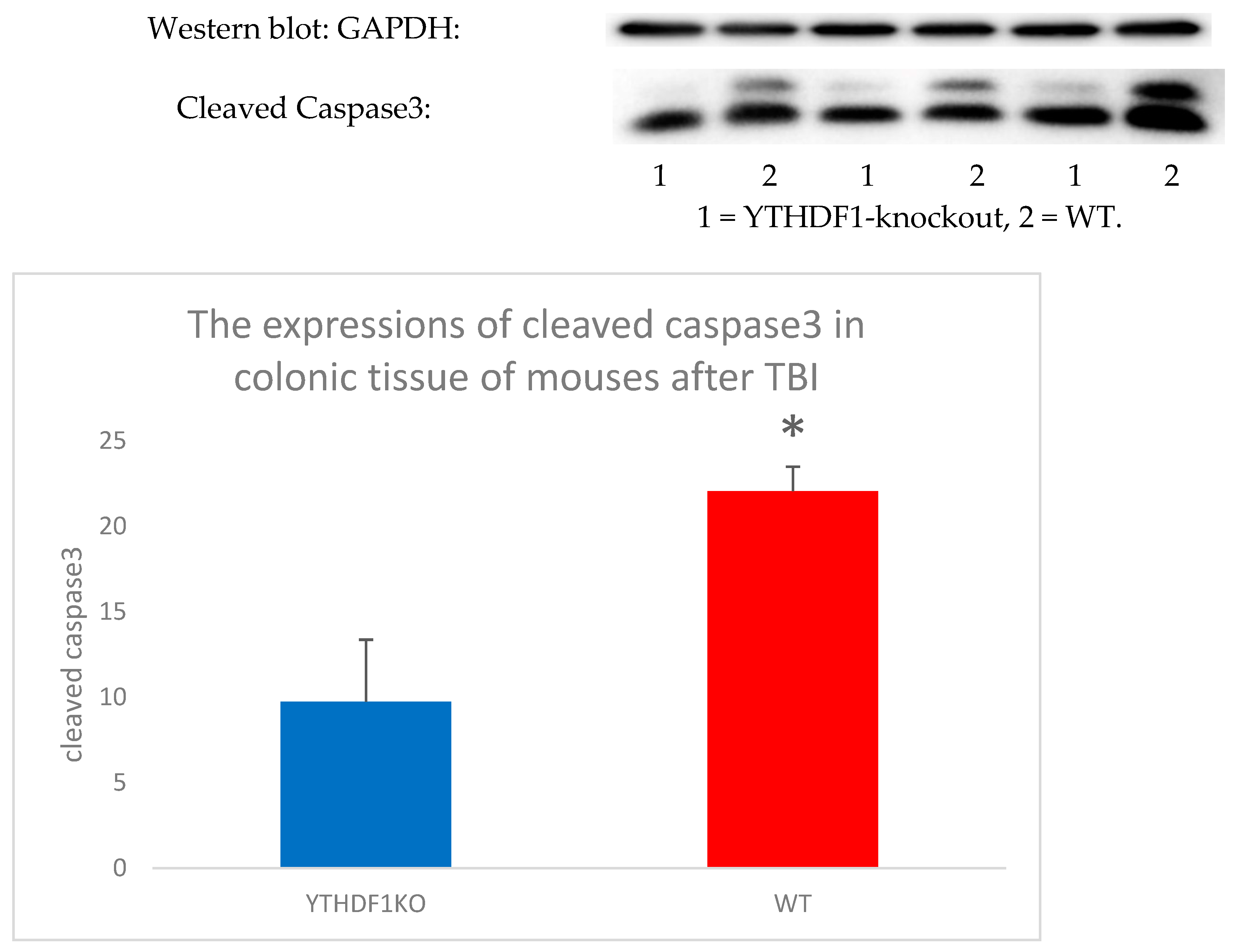
5.2.3. FTO/Caveolin-1/MMP2/9 Pathway
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| m6A | N6-methyladenosine |
| TBI | Traumatic brain injury |
| BGA | Brain-gut axis |
| IBD | Inflammatory bowel disease |
| CNS | Central nervous system |
| ENS | Enteric nervous system |
| ANS | Autonomic nervous system |
| HPA | Hypothalamus-pituitary-adrenal |
| TNF-α | Tumour necrosis factor-alpha |
| IL-1β/6/1/8/17 | Interleukin-1 beta/6/1/8/17 |
| BBB | Blood–brain barrier |
| TJs | Tight junctions |
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| MS | Multiple sclerosis |
| BCAAs | Branched chain amino acids |
| LPS | Lipopolysaccharides |
| BFLPS | Bacteroides fragilis lipopolysaccharides |
| iNOS | Inducible nitric oxide synthase |
| p-tau | Phosphorylated tau |
| NFκB | Nuclear factor kappaB |
| COX-2 | Cyclooxygenase-2 |
| CCR9+ | C-C chemokine receptor type 9+ |
| IFNγ | Interferon-γ |
| c-Maf | C-musculoaponeurotic-fibrosarcoma |
| RORγt | Retinoic acid-related orphan receptor gamma t |
| SPMS | Secondary progression multiple sclerosis |
| ZO | Zonula occludens |
| VE-cadherin | Vascular endothelial–cadherin |
| JAM-1 | Junction adhesion molecule-1 |
| GCs | Glucocorticoids |
| ICAM-1 | Intracellular adhesion molecule-1 |
| IEC | Intestinal epithelial cell |
| SAE | Sepsis-associated encephalopathy |
| YTHDF1/2/3 | YTH domain family 1/2/3 |
| WT | Wild type |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| METTL3/14/16/5 | Methyltransferase-like 3/14/16/5 |
| WTAP | Wilms’tumor 1-associating protein |
| SAM | S-adenosylmethionine |
| NLSs | Nuclear localisation signals |
| ZC3H13 | Zinc finger CCCH-type containing 13 |
| MTC | Methyltransferase cpmplex |
| RBM15 | RNA binding motif protein 15 |
| ZCCHC4 | Zinc finger CCHC domain containing 4 |
| MACOM | m6A-METTL Associated Complex |
| RNA POL II | RNA polymerase II |
| ZFD1/2 | Zinc finger domain1/2 |
| RRM | RNA recognition motif |
| SPOC domain | SpenParalog and OrthologsC-terminal domain |
| ssRNA | Single-stranded RNA |
| snRNA | Small nuclear RNA |
| lncRNA | Long non-coding RNA, |
| CPSF5/6 | Cleavage and polyadenylation specific factor 5/6 |
| FTO | Fat mass and obesity-associated protein |
| ALKBH5 | AlkB homolog 5 |
| m6Am | N6, 2′-O-dimethyladenosine |
| M1A | N1-methyladenosine |
| YTHDC1/2 | YTH domain containing 1/2 |
| HNRNPA2B1 | Heterogenous nuclear ribonucleoprotein A2B1 |
| HNRNPC | Heterogenous nuclear ribonucleoprotein C |
| hn-RNPG | Heterogenous nuclear ribonucleoprotein G |
| EIF3 | Eukaryotic initiation factor 3 |
| IGF2BP1/2/3 | Insulin-like growth factor 2 mRNA-binding protein 1/2/3 |
| hm6A | N6-hydroxymethyladenosine |
| f6A | N6-formyladenosine |
| A | Adenosine |
| DCP2 | Decapping RNA2 |
| dCasRx-M3 | Dead RfxCas13d-Methyltransferase-like 3 |
| dCasRx-A5 | Dead RfxCas13d-AlkB homolog 5 |
| SRSF3/10 | Serine/arginine-rich splicing Factor 3/10 |
| MCM4/5/2 | Minichromosome maintenance deficient 4/5/2 |
| AML | Acute myeloid leukaemia |
| HIF-1α | Hypoxia-inducible factor 1α |
| Dgcr8 | DiGeorge syndrome critical region gene 8 |
| 3′/5′UTR | 3′/5′untranslated region |
| G3BP1/2 | Ras GTPase-activating protein-binding protein 1/2 |
| Dapk1 | Death Associated Protein Kinase 1 |
| Fadd | Fas-associated death domain |
| Ngfr | Nerve growth factor receptor |
| mESC | Mouse embryonic stem cell |
| SYP | Synaptophysin |
| CXCL2 | C-X-C motif chemokine ligand 2 |
| IFN-CD4 | Interferon-CD4 |
| GRIN1/2A | Glutamate receptor, ionotropic,N-methyl D-aspartate 1/2A |
| GRIA1 | Glutamate Receptor AMPA Type Subunit 1 |
| CAMK2A/2B | Calcium/Calmodulin Dependent Protein Kinase 2A/2B |
| NSPCs | Neural stem/progenitor cells |
| Apc | Adenomatous polyposis coli |
| PTEN | Phosphatase and tensin homolog deleted on chromosome ten |
| OGD | Oxygen-glucose deprivation |
| HNSCC | Head and neck squamous cell carcinoma |
| CBP | CREB binding proteins |
| SOCSS | Suppressors of cytokine signaling |
| H/R | Hypoxia/reoxygenation |
| MCAO/R | Middle cerebral arteryocclusion/reperfusion |
| OGD/R | Oxygen-glucose deprivation/reoxygenation |
| CCI | Controlled cortical impact |
| 6-OHDA | 6-hydroxydop amine |
| SCFAs | Short-chain fatty acids |
| MERIP-Seq | Methylated RNA immunoprecipitation sequencing |
| GF | Germ free |
| SPF | Specific pathogen free |
| NSPs | Nonstarch polysaccharides |
| ILC3 | Innate lymphoid cells |
| Nr4a1 | Nuclear receptor subfamily 4 group A member1 |
| TRAF6 | Tumour necrosis factor receptor-associated factor 6 |
| DDX60 | DEXD/H box helicase 60 |
| XPO1 | Exportin 1 |
| TCGA | The Cancer Genome Atlas |
| COAD | Colonic adenocarcinoma |
| NMDA | N-methyl-D-aspartate |
| ROS | Reactive oxygen stke |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 |
| p75NTR | P75-neurotrophin receptor |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| TINCR | Non-protein coding RNA |
| GSDMD | Gasdermin D |
| HO-1 | Heme oxygenase-1 |
| MMPs/2/9 | matrix metalloproteinases/2/9 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| ARE | Antioxidant response element |
References
- Gu, D.; Ou, S.; Liu, G. Traumatic Brain Injury and Risk of Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 4–16. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Wilson, J.R.; Fehlings, M.G. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019, 18, 24–25. [Google Scholar] [CrossRef] [Green Version]
- Singaravelu Jaganathan, K.; Sullivan, K.A. Traumatic Brain Injury Rehabilitation: An Exercise Immunology Perspective. Exerc. Immunol. Rev. 2022, 28, 90–97. [Google Scholar]
- Jiang, J.Y.; Gao, G.Y.; Feng, J.F.; Mao, Q.; Chen, L.G.; Yang, X.F.; Liu, J.F.; Wang, Y.H.; Qiu, B.H.; Huang, X.J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Peng, J.; Gerner, S.T.; Yin, S.; Jiang, Y. Gut microbiota-brain interaction: An emerging immunotherapy for traumatic brain injury. Exp. Neurol. 2021, 337, 113585. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524.e1. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Investig. 2021, 131, e143777. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Hall, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef]
- Xiang, Z.-B.; Xu, R.-S.; Zhu, Y.; Yuan, M.; Liu, Y.; Yang, F.; Chen, W.-Z.; Xu, Z.-Z. Association between inflammatory bowel diseases and Parkinson’s disease: Systematic review and meta-analysis. Neural Regen. Res. 2022, 17, 344–353. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, W.; Zhang, X.; Pei, Y.; Zhang, H.; Li, Y. The role of gut microbiota in patients with benign and malignant brain tumors: A pilot study. Bioengineered 2022, 13, 7847–7859. [Google Scholar] [CrossRef]
- Herbreteau, A.; Aubert, P.; Croyal, M.; Naveilhan, P.; Billon-Crossouard, S.; Neunlist, M.; Delneste, Y.; Couez, D.; Aymeric, L. Late-Stage Glioma Is Associated with Deleterious Alteration of Gut Bacterial Metabolites in Mice. Metabolites 2022, 12, 290. [Google Scholar] [CrossRef]
- Weaver, J.L.; Eliceiri, B.; Costantini, T.W. Fluoxetine reduces organ injury and improves motor function after traumatic brain injury in mice. J. Trauma Acute Care Surg. 2022, 93, 38–42. [Google Scholar] [CrossRef]
- Taraskina, A.; Ignatyeva, O.; Lisovaya, D.; Ivanov, M.; Ivanova, L.; Golovicheva, V.; Baydakova, G.; Silachev, D.; Popkov, V.; Ivanets, T.; et al. Effects of Traumatic Brain Injury on the Gut Microbiota Composition and Serum Amino Acid Profile in Rats. Cells 2022, 11, 1409. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Ren, H.; Ma, L.; Guo, J.; Mao, D.; Lu, Z.; Lu, L.; Yan, D. Role of Hakai in m6A modification pathway in Drosophila. Nat. Commun. 2021, 12, 2159. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Zhang, Y.; Liang, M.; Du, Z.; Li, H.; Fan, C.; Zhang, H.; Jiang, Y.; Bi, X. N6-methyladenosine (m6A) modification and its clinical relevance in cognitive dysfunctions. Aging 2021, 13, 20716–20737. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, H.; Xiao, L.; Liu, C.; Liu, Y.-L.; Gao, W. Identification of the function and mechanism of m6A reader IGF2BP2 in Alzheimer’s disease. Aging 2021, 13, 24086–24100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ding, C.; Zuo, Y.; Peng, Y.; Zuo, L. N6-methyladenosine and Neurological Diseases. Mol. Neurobiol. 2022, 59, 1925–1937. [Google Scholar] [CrossRef]
- Zhuo, R.; Xu, M.; Wang, X.; Zhou, B.; Wu, X.; Leone, V.; Chang, E.B.; Zhong, X. The regulatory role of N6-methyladenosine modification in the interaction between host and microbes. Wiley Interdiscip. Rev. RNA 2022, e1725. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Wang, X.; Kong, L.; Johnston, L.J.; Lu, L.; Ma, X. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit. Rev. Food Sci. Nutr. 2020, 62, 783–797. [Google Scholar] [CrossRef]
- Sun, L.; Wan, A.; Zhou, Z.; Chen, D.; Liang, H.; Liu, C.; Yan, S.; Niu, Y.; Lin, Z.; Zhan, S.; et al. RNA-binding protein RALY reprogrammes mitochondrial metabolism via mediating miRNA processing in colorectal cancer. Gut 2021, 70, 1698–1712. [Google Scholar] [CrossRef]
- Jabs, S.; Biton, A.; Bécavin, C.; Nahori, M.-A.; Ghozlane, A.; Pagliuso, A.; Spanò, G.; Guérineau, V.; Touboul, D.; Gianetto, Q.G.; et al. Impact of the gut microbiota on the m6A epitranscriptome of mouse cecum and liver. Nat. Commun. 2020, 11, 1344. [Google Scholar] [CrossRef]
- Tait, C.; Sayuk, G.S. The Brain-Gut-Microbiotal Axis: A framework for understanding functional GI illness and their therapeutic interventions. Eur. J. Intern. Med. 2021, 84, 1–9. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef]
- Beopoulos, A.; Gea, M.; Fasano, A.; Iris, F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome–Host Interaction Mechanistic Hypothesis. Nutrients 2021, 14, 65. [Google Scholar] [CrossRef]
- Sudo, N. Microbiome, HPA Axis and Production of Endocrine Hormones in the Gut. Adv. Exp. Med. Biol. 2014, 817, 177–194. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocr. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Renteria, M.A.; Gillett, S.R.; McClure, L.A.; Wadley, V.G.; Glasser, S.P.; Howard, V.J.; Kissela, B.M.; Unverzagt, F.W.; Jenny, N.S.; Manly, J.J.; et al. C-reactive protein and risk of cognitive decline: The REGARDS study. PLoS ONE 2020, 15, e0244612. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Aubretch, T.; Leser, J.; Molesworth, K.; Hedgekar, N.; Ritzel, R.M.; Abulwerdi, G.; Shea-Donohue, T.; Faden, A.I. Acute colitis during chronic experimental traumatic brain injury in mice induces dysautonomia and persistent extraintestinal, systemic, and CNS inflammation with exacerbated neurological deficits. J. Neuroinflamm. 2021, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson’s Disease. Neuroscience 2020, 432, 160–173. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoner-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, D.; Cionci, N.B.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.L.; Fournier, C.; Houser, M.; Tansey, M.; Glass, J.; Hertzberg, V.S. Potential Role of the Gut Microbiome in ALS: A Systematic Review. Biol. Res. Nurs. 2018, 20, 513–521. [Google Scholar] [CrossRef]
- Kadowaki, A.; Saga, R.; Lin, Y.; Sato, W.; Yamamura, T. Gut microbiota-dependent CCR9+CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain 2019, 142, 916–931. [Google Scholar] [CrossRef] [Green Version]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.-F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in Multiple Sclerosis: Where Are We, What We Know and Do Not Know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Reynders, T.; Devolder, L.; Valles-Colomer, M.; Van Remoortel, A.; Joossens, M.; De Keyser, J.; Nagels, G.; D’Hooghe, M.; Raes, J. Gut microbiome variation is associated to Multiple Sclerosis phenotypic subtypes. Ann. Clin. Transl. Neurol. 2020, 7, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Villaran, R.F.; Espinosa-Oliva, A.M.; Sarmiento, M.; De Pablos, R.M.; Argüelles, S.; Delgado-Cortés, M.J.; Sobrino, V.; Van Rooijen, N.; Venero, J.L.; Herrera, A.J.; et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: Potential risk factor in Parkinson’s disease. J. Neurochem. 2010, 114, 1687–1700. [Google Scholar] [CrossRef]
- Banerjee, A.; Pradhan, L.K.; Sahoo, P.K.; Jena, K.K.; Chauhan, N.R.; Chauhan, S.; Das, S.K. Unravelling the potential of gut microbiota in sustaining brain health and their current prospective towards development of neurotherapeutics. Arch. Microbiol. 2021, 203, 2895–2910. [Google Scholar] [CrossRef]
- Hung, C.C.; Chang, C.-C.; Huang, C.-W.; Nouchi, R.; Cheng, C.-H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Hernandez-Ontiveros, D.G.; Rodrigues, M.C.; Haller, E.; Frisina-Deyo, A.; Mirtyl, S.; Sallot, S.; Saporta, S.; Borlongan, C.; Sanberg, P.R. Impaired blood–brain/spinal cord barrier in ALS patients. Brain Res. 2012, 1469, 114–128. [Google Scholar] [CrossRef]
- Paray, B.A.; Albeshr, M.F.; Jan, A.T.; Rather, I.A. Leaky Gut and Autoimmunity: An Intricate Balance in Individuals Health and the Diseased State. Int. J. Mol. Sci. 2020, 21, 9770. [Google Scholar] [CrossRef]
- Dabrowski, W.; Siwicka-Gieroba, D.; Kotfis, K.; Zaid, S.; Terpilowska, S.; Robba, C.; Siwicki, A.K. The Brain-gut Axis-where are we now and how can we Modulate these Connections? Curr. Neuropharmacol. 2021, 19, 1164–1177. [Google Scholar] [CrossRef]
- Ma, E.L.; Smith, A.D.; Desai, N.; Cheung, L.; Hanscom, M.; Stoica, B.A.; Loane, D.J.; Shea-Donohue, T.; Faden, A.I. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. 2017, 66, 56–69. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Du, J.; Wang, F.; Fang, R.; Yu, C.; Xiong, J.; Chen, W.; Lu, Z.; Liu, J. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol. Motil. 2017, 30, e13260. [Google Scholar] [CrossRef]
- George, A.K.; Behera, J.; Homme, R.P.; Tyagi, N.; Tyagi, S.C.; Singh, M. Rebuilding Microbiome for Mitigating Traumatic Brain Injury: Importance of Restructuring the Gut-Microbiome-Brain Axis. Mol. Neurobiol. 2021, 58, 3614–3627. [Google Scholar] [CrossRef] [PubMed]
- Kharrazian, D. Traumatic Brain Injury and the Effect on the Brain-Gut Axis. Altern. Ther. Health Med. 2015, 21 (Suppl. S3), 28–32. [Google Scholar] [PubMed]
- Toklu, H.Z.; Sakarya, Y.; Tumer, N. A Proteomic Evaluation of Sympathetic Activity Biomarkers of the Hypothalamus-Pituitary-Adrenal Axis by Western Blotting Technique Following Experimental Traumatic Brain Injury. Methods Mol. Biol. 2017, 1598, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Tapp, Z.M.; Godbout, J.P.; Kokiko-Cochran, O.N. A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front. Neurol. 2019, 10, 345. [Google Scholar] [CrossRef]
- McDonald, S.; Sharkey, J.M.; Sun, M.; Kaukas, L.M.; Shultz, S.R.; Turner, R.J.; Leonard, A.V.; Brady, R.D.; Corrigan, F. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 770–781. [Google Scholar] [CrossRef]
- Taylor, A.N.; Rahman, S.U.; Sanders, N.C.; Tio, D.L.; Prolo, P.; Sutton, R.L. Injury Severity Differentially Affects Short- and Long-Term Neuroendocrine Outcomes of Traumatic Brain Injury. J. Neurotrauma 2008, 25, 311–323. [Google Scholar] [CrossRef]
- Jassam, Y.N.; Izzy, S.; Whalen, M.; McGavern, D.B.; El Khoury, J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 2017, 95, 1246–1265. [Google Scholar] [CrossRef] [Green Version]
- Hang, C.H.; Shi, J.-X.; Li, J.-S.; Li, W.-Q.; Yin, H.-X. Up-regulation of intestinal nuclear factor kappa B and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J. Gastroenterol. 2005, 11, 1149–1154. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Li, Z.; Tang, J.; Huang, B.; Zhi, F.; Zhao, X. Epithelial PBLD attenuates intestinal inflammatory response and improves intestinal barrier function by inhibiting NF-kappaB signaling. Cell Death Dis. 2021, 12, 563. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.H.; Shi, J.-X.; Li, J.-S.; Li, W.-Q.; Wu, W. Expressions of intestinal NF-κB, TNF-α, and IL-6 following traumatic brain injury in rats. J. Surg. Res. 2005, 123, 188–193. [Google Scholar] [CrossRef]
- Bansal, V.; Costantini, T.; Kroll, L.; Peterson, C.; Loomis, W.; Eliceiri, B.; Baird, A.; Wolf, P.; Coimbra, R. Traumatic Brain Injury and Intestinal Dysfunction: Uncovering the Neuro-Enteric AXIS. J. Neurotrauma 2009, 26, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.J.; Pyles, R.B.; Stewart, C.J.; Ajami, N.; Randolph, K.M.; Durham, W.J.; Danesi, C.P.; Dillon, E.L.; Summons, M.J.R.; Singh, C.K.; et al. Altered Fecal Microbiome Years after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Dong, Z.; Li, J.; Yu, Y.; Chen, X.; Ren, F.; Cui, G.; Sun, R. Expression patterns and prognostic value of m6A-related genes in colorectal cancer. Am. J. Transl. Res. 2019, 11, 3972–3991. [Google Scholar]
- Coker, H.; Wei, G.; Brockdorff, N. m6A modification of non-coding RNA and the control of mammalian gene expression. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Roignant, J.Y.; Soller, M. m6A in mRNA: An Ancient Mechanism for Fine-Tuning Gene Expression. Trends Genet. 2017, 33, 380–390. [Google Scholar] [CrossRef]
- Sun, T.; Wu, R.; Ming, L. The role of m6A RNA methylation in cancer. Biomed. Pharmacother. 2019, 112, 108613. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X.; Li, Q.; Xu, J.; Tan, Y.; Xiao, M.; Song, J.; Liu, F.; Fang, C.; Wang, H. m6A modification in RNA: Biogenesis, functions and roles in gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 192. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, P.; Liu, Y.; Li, W.; Shu, Y. Multiple functions of m6A RNA methylation in cancer. J. Hematol. Oncol. 2018, 11, 48. [Google Scholar] [CrossRef]
- Ma, S.; Chen, C.; Ji, X.; Liu, J.; Zhou, Q.; Wang, G.; Yuan, W.; Kan, Q.; Sun, Z. The interplay between m6A RNA methylation and noncoding RNA in cancer. J. Hematol. Oncol. 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Zhang, X.; Weng, Y.-L.; Lu, Z.; Liu, Y.; Lu, Z.; Li, J.; Hao, P.; Zhang, Y.; Zhang, F.; et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 2018, 563, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sha, Y.; Sun, T. m6A Modifications Play Crucial Roles in Glial Cell Development and Brain Tumorigenesis. Front. Oncol. 2021, 11, 611660. [Google Scholar] [CrossRef]
- Lu, T.X.; Zheng, Z.; Zhang, L.; Sun, H.-L.; Bissonnette, M.; Huang, H.; He, C. A New Model of Spontaneous Colitis in Mice Induced by Deletion of an RNA m6A Methyltransferase Component METTL14 in T Cells. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shao, J.; Song, H.; Wang, J. The YTH Domain Family of N6-Methyladenosine “Readers” in the Diagnosis and Prognosis of Colonic Adenocarcinoma. BioMed Res. Int. 2020, 2020, 9502560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Chen, J.; Chen, C. Association Among the Gut Microbiome, the Serum Metabolomic Profile and RNA m6A Methylation in Sepsis-Associated Encephalopathy. Front. Genet. 2022, 13, 859727. [Google Scholar] [CrossRef]
- Wu, J.; Frazier, K.; Zhang, J.; Gan, Z.; Wang, T.; Zhong, X. Emerging role of m6A RNA methylation in nutritional physiology and metabolism. Obes. Rev. 2020, 21, e12942. [Google Scholar] [CrossRef] [PubMed]
- Bedi, R.K.; Huang, D.; Eberle, S.A.; Wiedmer, L.; Śledź, P.; Caflisch, A. Small-Molecule Inhibitors of METTL3, the Major Human Epitranscriptomic Writer. ChemMedChem 2020, 15, 744–748. [Google Scholar] [CrossRef]
- Tuck, M.T. Partial purification of a 6-methyladenine mRNA methyltransferase which modifies internal adenine residues. Biochem. J. 1992, 288 Pt 1, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Scholler, E.; Weichmann, F.; Treiber, T.; Ringle, S.; Treiber, N.; Flatley, A.; Feederle, R.; Bruckmann, A.; Meister, G. Interactions, localization, and phosphorylation of the m6A generating METTL3–METTL14–WTAP complex. RNA 2018, 24, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Morales, D.G.; Reyes, J.L. A birds’-eye view of the activity and specificity of themRNA m6Amethyltransferase complex. Wiley Interdiscip. Rev. RNA 2021, 12, e1618. [Google Scholar] [CrossRef]
- Balacco, D.L.; Soller, M. The m6A Writer: Rise of a Machine for Growing Tasks. Biochemistry 2019, 58, 363–378. [Google Scholar] [CrossRef]
- Warda, A.S.; Kretschmer, J.; Hackert, P.; Lenz, C.; Urlaub, H.; Höbartner, C.; Sloan, K.E.; Bohnsack, M.T. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017, 18, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Bawankar, P.; Lence, T.; Paolantoni, C.; Haussmann, I.U.; Kazlauskiene, M.; Jacob, D.; Heidelberger, J.B.; Richter, F.M.; Nallasivan, M.P.; Morin, V.; et al. Hakai is required for stabilization of core components of the m6A mRNA methylation machinery. Nat. Commun. 2021, 12, 3778. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Tang, M.; Ma, J.; Zhang, H.; Gimple, R.C.; Prager, B.C.; Tang, H.; Sun, C.; Liu, F.; Lin, P.; et al. Epitranscriptomic editing of the RNA N6-methyladenosine modification by dCasRx conjugated methyltransferase and demethylase. Nucleic Acids Res. 2021, 49, 7361–7374. [Google Scholar] [CrossRef]
- Ping, X.L.; Sun, B.-F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.-J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.-S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Toth, J.I.; Petroski, M.D.; Zhang, Z.; Zhao, J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014, 16, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Huang, W.; Li, Y.; Weng, H. Roles of METTL3 in cancer: Mechanisms and therapeutic targeting. J. Hematol. Oncol. 2020, 13, 117. [Google Scholar] [CrossRef]
- Mendel, M.; Chen, K.-M.; Homolka, D.; Gos, P.; Pandey, R.R.; McCarthy, A.A.; Pillai, R.S. Methylation of Structured RNA by the m6A Writer METTL16 Is Essential for Mouse Embryonic Development. Mol. Cell 2018, 71, 986–1000.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendleton, K.E.; Chen, B.; Liu, K.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 snRNA m6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 2017, 169, 824–835.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, D.P.; Chen, C.-K.; Pickering, B.F.; Chow, A.; Jackson, C.; Guttman, M.; Jaffrey, S.R. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016, 537, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Liu, J.; Cui, X.; Cao, J.; Luo, G.; Zhang, Z.; Cheng, T.; Gao, M.; Shu, X.; Ma, H.; et al. VIRMA mediates preferential m6A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Lv, R.; Ma, H.; Shen, H.; He, C.; Wang, J.; Jiao, F.; Liu, H.; Yang, P.; Tan, L.; et al. Zc3h13 Regulates Nuclear RNA m6A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell 2018, 69, 1028–1038.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatova, V.V.; Stolz, P.; Kaiser, S.; Gustafsson, T.H.; Lastres, P.R.; Sanz-Moreno, A.; Cho, Y.-L.; Amarie, O.V.; Aguilar-Pimentel, A.; Klein-Rodewald, T.; et al. The rRNA m6A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 2020, 34, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, N.; Ernst, F.G.M.; Hawley, B.R.; Zorbas, C.; Ulryck, N.; Hackert, P.; Bohnsack, K.E.; Bohnsack, M.T.; Jaffrey, S.R.; Graille, M.; et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019, 47, 7719–7733. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.; Vågbø, C.B.; Jakobsson, M.E.; Kim, Y.; Baltissen, M.P.; O’Donohue, M.-F.; Guzmán, U.H.; Małecki, J.M.; Wu, J.; Kirpekar, F.; et al. The human methyltransferase ZCCHC4 catalyses N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Res. 2020, 48, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Lu, J.; Huang, M.; Gao, L.; Li, D.; Wang, G.G.; Song, J. Structure and regulation of ZCCHC4 in m6A-methylation of 28S rRNA. Nat. Commun. 2019, 10, 5042. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Wang, X.; Cai, J.; Dai, Q.; Natchiar, S.K.; Lv, R.; Chen, K.; Lu, Z.; Chen, H.; Shi, Y.G.; et al. N6-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 2019, 15, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, F.; Lu, Z.; Fei, Q.; Ai, Y.; He, P.C.; Shi, H.; Cui, X.; Su, R.; Klungland, A.; et al. Differential m6A, m6Am, and m1A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol. Cell 2018, 71, 973–985.e5. [Google Scholar] [CrossRef] [Green Version]
- Mauer, J.; Sindelar, M.; Despic, V.; Guez, T.; Hawley, B.R.; Vasseur, J.-J.; Rentmeister, A.; Gross, S.S.; Pellizzoni, L.; Debart, F.; et al. FTO controls reversible m6Am RNA methylation during snRNA biogenesis. Nat. Chem. Biol. 2019, 15, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Bartosovic, M.; Molares, H.C.; Gregorova, P.; Hrossova, D.; Kudla, G.; Vanacova, S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res. 2017, 45, 11356–11370. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; Luo, G.-Z.; Zhang, Z.; Wang, X.; Zhou, T.; Cui, Y.; Sha, J.; Huang, X.; Guerrero, L.; Xie, P.; et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife 2017, 6, e31311. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.-S.; Hao, Y.-J.; Sun, B.-F.; Sun, H.-Y.; Li, A.; Ping, X.-L.; Lai, W.-Y.; et al. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccara, S.; Jaffrey, S.R. A Unified Model for the Function of YTHDF Proteins in Regulating m6A-Modified mRNA. Cell 2020, 181, 1582–1595.e18. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Dong, L.; Liu, X.-M.; Guo, J.; Ma, H.; Shen, B.; Qian, S.-B. m6A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat. Commun. 2019, 10, 5332. [Google Scholar] [CrossRef]
- Kretschmer, J.; Rao, H.; Hackert, P.; Sloan, K.E.; Höbartner, C.; Bohnsack, M.T. The m6A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5′–3′ exoribonuclease XRN1. RNA 2018, 24, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, C.R.; Goodarzi, H.; Lee, H.; Liu, X.; Tavazoie, S.; Tavazoie, S.F. HNRNPA2B1 Is a Mediator of m6A-Dependent Nuclear RNA Processing Events. Cell 2015, 162, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Zhou, K.I.; Parisien, M.; Dai, Q.; Diatchenko, L.; Pan, T. N 6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017, 45, 6051–6063. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Dai, Q.; Zheng, G.; He, C.; Parisien, M.; Pan, T. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.-B.; Jaffrey, S.R. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Publisher Correction: Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2020, 22, 1288. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, G.; Pang, X.; Wang, R.N.; Wang, X.; Li, C.J.; Smemo, S.; Dai, Q.; Bailey, K.A.; Nobrega, M.A.; et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 2013, 4, 1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.-J.; Chen, Q.; et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Campos, M.A.; Edelheit, S.; Toth, U.; Safra, M.; Shachar, R.; Viukov, S.; Winkler, R.; Nir, R.; Lasman, L.; Brandis, A.; et al. Deciphering the “m6A Code” via Antibody-Independent Quantitative Profiling. Cell 2019, 178, 731–747.e16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Jin, F.; Wang, B.-Y.; Yin, X.-J.; Hong, W.; Tian, F.-J. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 2019, 9, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Zhang, X.; Wang, J.; Ma, Y.; Zhang, L.; Cao, X. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc. Natl. Acad. Sci. USA 2019, 116, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, Q.; Zhao, X.; Shao, L.; Liu, G.; Zheng, X.; Xie, L.; Zhang, Y.; Sun, C.; Xu, R. YTHDC1 mitigates ischemic stroke by promoting Akt phosphorylation through destabilizing PTEN mRNA. Cell Death Dis. 2020, 11, 977. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, J.; Yu, F.; Xu, H.; Yu, C.; Wu, Q.; Liu, Y.; Li, L.; Cui, X.-L.; Gu, X.; et al. A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex–mediated DNA replication. Blood 2021, 138, 2838–2852. [Google Scholar] [CrossRef]
- Roundtree, I.A.; He, C. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Trends Genet. 2016, 32, 320–321. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.-N.; Wang, E.-H.; Gong, C.-J.; Lan, K.-F.; Ding, X. The m6A reader protein YTHDC2 is a potential biomarker and associated with immune infiltration in head and neck squamous cell carcinoma. PeerJ 2020, 8, e10385. [Google Scholar] [CrossRef]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Des Georges, A.; Dhote, V.; Kuhn, L.; Hellen, C.U.; Pestova, T.V.; Frank, J.; Hashem, Y. Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature 2015, 525, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Livneh, I.; Moshitch-Moshkovitz, S.; Amariglio, N.; Rechavi, G.; Dominissini, D. The m6A epitranscriptome: Transcriptome plasticity in brain development and function. Nat. Rev. Neurosci. 2019, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Arguello, A.E.; DeLiberto, A.N.; Kleiner, R.E. RNA Chemical Proteomics Reveals the N6-Methyladenosine (m6A)-Regulated Protein–RNA Interactome. J. Am. Chem. Soc. 2017, 139, 17249–17252. [Google Scholar] [CrossRef] [PubMed]
- Edupuganti, R.R.; Geiger, S.; Lu, Z.; Wang, S.-Y.; Baltissen, M.P.A.; Jansen, P.W.T.C.; Rossa, M.; Müller, M.; Stunnenberg, H.G.; He, C.; et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol. 2017, 24, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Wang, Z.; Gao, J.; Yang, C.; Feng, M.; Niu, Y.; Tong, W.-M.; Bao, X.; Wang, R. METTL3-mediated RNA m6A Hypermethylation Promotes Tumorigenesis and GH Secretion of Pituitary Somatotroph Adenomas. J. Clin. Endocrinol. Metab. 2022, 107, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xu, T.; Li, R.; Zheng, D.; Li, Y.; Li, W.; Yang, Y.; Hao, Y. Downregulation of m6A Methyltransferase in the Hippocampus of Tyrobp–/– Mice and Implications for Learning and Memory Deficits. Front. Neurosci. 2022, 16, 739201. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Q. The Progression of N6-methyladenosine Study and Its Role in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 5922. [Google Scholar] [CrossRef]
- Wang, C.X.; Cui, G.-S.; Liu, X.; Xu, K.; Wang, M.; Zhang, X.-X.; Jiang, L.-Y.; Li, A.; Yang, Y.; Lai, W.-Y.; et al. METTL3-mediated m6A modification is required for cerebellar development. PLoS Biol. 2018, 16, e2004880. [Google Scholar] [CrossRef]
- Weng, Y.L.; Wang, X.; An, R.; Cassin, J.; Vissers, C.; Liu, Y.; Liu, Y.; Xu, T.; Wang, X.; Wong, S.Z.H.; et al. Epitranscriptomic m6A Regulation of Axon Regeneration in the Adult Mammalian Nervous System. Neuron 2018, 97, 313–325.e6. [Google Scholar] [CrossRef] [Green Version]
- Walters, B.J.; Mercaldo, V.; Gillon, C.; Yip, M.; Neve, R.L.; Boyce, F.M.; Frankland, P.W.; Josselyn, S.A. The Role of The RNA Demethylase FTO (Fat Mass and Obesity-Associated) and mRNA Methylation in Hippocampal Memory Formation. Neuropsychopharmacology 2017, 42, 1502–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhang, X.; Hu, J.; Qu, R.; Yu, Z.; Xu, H.; Chen, H.; Yan, L.; Ding, C.; Zou, Q.; et al. m6A demethylase ALKBH5 controls CD4 + T cell pathogenicity and promotes autoimmunity. Sci. Adv. 2021, 7, eabg0470. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, X.; Wang, W.; Shi, H.; Pan, Q.; Lu, Z.; Perez, S.P.; Suganthan, R.; He, C.; Bjørås, M.; et al. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 2018, 19, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkurjev, D.; Hong, W.-T.; Iida, K.; Oomoto, I.; Goldie, B.J.; Yamaguti, H.; Ohara, T.; Kawaguchi, S.-Y.; Hirano, T.; Martin, K.C.; et al. Author Correction: Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat. Neurosci. 2018, 21, 1493. [Google Scholar] [CrossRef]
- Quan, W.; Li, J.; Liu, L.; Zhang, Q.; Qin, Y.; Pei, X.; Chen, J. Influence of N6-Methyladenosine Modification Gene HNRNPC on Cell Phenotype in Parkinson’s Disease. Park. Dis. 2021, 2021, 9919129. [Google Scholar] [CrossRef]
- Qiu, X.; He, H.; Huang, Y.; Wang, J.; Xiao, Y. Genome-wide identification of m6A-associated single-nucleotide polymorphisms in Parkinson’s disease. Neurosci. Lett. 2020, 737, 135315. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Guo, M.; Zheng, X.; Ali, S.; Huang, H.; Zhang, L.; Wang, S.; Huang, Y.; Qie, S.; et al. Down-Regulation of m6A mRNA Methylation Is Involved in Dopaminergic Neuronal Death. ACS Chem. Neurosci. 2019, 10, 2355–2363. [Google Scholar] [CrossRef]
- Xu, C.; Huang, H.; Zhang, M.; Zhang, P.; Li, Z.; Liu, X.; Fang, M. Methyltransferase-Like 3 Rescues the Amyloid-beta protein-Induced Reduction of Activity-Regulated Cytoskeleton Associated Protein Expression via YTHDF1-Dependent N6-Methyladenosine Modification. Front. Aging Neurosci. 2022, 14, 890134. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, F.; Tan, M.; Wang, T.; Chen, Y.; Xu, W.; Li, B.; Wang, X.; Deng, X.; He, M.-L. Targeting m6A modification inhibits herpes virus 1 infection. Genes Dis. 2022, 9, 1114–1128. [Google Scholar] [CrossRef]
- Cockova, Z.; Honc, O.; Telensky, P.; Olsen, M.J.; Novotny, J. Streptozotocin-Induced Astrocyte Mitochondrial Dysfunction Is Ameliorated by FTO Inhibitor MO-I-500. ACS Chem. Neurosci. 2021, 12, 3818–3828. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Gao, S.; Qin, L.; Austria, Q.; Siedlak, S.L.; Pajdzik, K.; Dai, Q.; He, C.; Wang, W.; et al. METTL3-dependent RNA m6A dysregulation contributes to neurodegeneration in Alzheimer’s disease through aberrant cell cycle events. Mol. Neurodegener. 2021, 16, 70. [Google Scholar] [CrossRef]
- Shafik, A.M.; Zhang, F.; Guo, Z.; Dai, Q.; Pajdzik, K.; Li, Y.; Kang, Y.; Yao, B.; Wu, H.; He, C.; et al. N6-methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer’s disease. Genome Biol. 2021, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wang, T.; Wu, X.; Liang, J.; Li, J.; Sheng, W. N6-Methyladenosine RNA modification in cerebrospinal fluid as a novel potential diagnostic biomarker for progressive multiple sclerosis. J. Transl. Med. 2021, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, F.; Wang, N.; Hou, K.; Du, J. Identification of Implications of Angiogenesis and m6A Modification on Immunosuppression and Therapeutic Sensitivity in Low-Grade Glioma by Network Computational Analysis of Subtypes and Signatures. Front. Immunol. 2022, 13, 871564. [Google Scholar] [CrossRef]
- Miao, Y.Q.; Chen, W.; Zhou, J.; Shen, Q.; Sun, Y.; Li, T.; Wang, S.-C. N(6)-adenosine-methyltransferase-14 promotes glioma tumorigenesis by repressing argininosuccinate synthase 1 expression in an m6A-dependent manner. Bioengineered 2022, 13, 1858–1871. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, P.; Lv, Y.; Shi, Z.; Shi, F. m6A Regulatory Gene-Mediated Methylation Modification in Glioma Survival Prediction. Front. Genet. 2022, 13, 873764. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.N.; Tang, H.-L.; Lin, G.-W.; Chen, Y.-H.; Lu, P.; Li, H.-J.; Gao, M.-M.; Zhao, Q.-H.; Yi, Y.-H.; Liao, W.-P.; et al. Epigenetic Downregulation of Scn3a Expression by Valproate: A Possible Role in Its Anticonvulsant Activity. Mol. Neurobiol. 2017, 54, 2831–2842. [Google Scholar] [CrossRef]
- Wang, X.L.; Wei, X.; Yuan, J.-J.; Mao, Y.-Y.; Wang, Z.-Y.; Xing, N.; Gu, H.-W.; Lin, C.-H.; Wang, W.-T.; Zhang, W.; et al. Downregulation of Fat Mass and Obesity-Related Protein in the Anterior Cingulate Cortex Participates in Anxiety- and Depression-Like Behaviors Induced by Neuropathic Pain. Front. Cell. Neurosci. 2022, 16, 884296. [Google Scholar] [CrossRef]
- Anderson, K.S.; Gosselin, N.; Sadikot, A.F.; Laguë-Beauvais, M.; Kang, E.S.H.; Fogarty, A.E.; Marcoux, J.; Dagher, J.; de Guise, E. Pitch and Rhythm Perception and Verbal Short-Term Memory in Acute Traumatic Brain Injury. Brain Sci. 2021, 11, 1173. [Google Scholar] [CrossRef]
- Chokkalla, A.K.; Mehta, S.L.; Kim, T.; Chelluboina, B.; Kim, J.; Vemuganti, R. Transient Focal Ischemia Significantly Alters the m6A Epitranscriptomic Tagging of RNAs in the Brain. Stroke 2019, 50, 2912–2921. [Google Scholar] [CrossRef]
- Diao, M.Y.; Zhu, Y.; Yang, J.; Xi, S.-S.; Wen, X.; Gu, Q.; Hu, W. Hypothermia protects neurons against ischemia/reperfusion-induced pyroptosis via m6A-mediated activation of PTEN and the PI3K/Akt/GSK-3β signaling pathway. Brain Res. Bull. 2020, 159, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Li, Y.; Ye, S.; Li, Z.; Liu, Y.; Kuang, W.; Chen, D.; Zhu, M. Methyltransferase 3 Mediated miRNA m6A Methylation Promotes Stress Granule Formation in the Early Stage of Acute Ischemic Stroke. Front. Mol. Neurosci. 2020, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Ma, H.; Zeng, R.; Liu, R.; Wang, P.; Jin, X.; Zhao, Y. Epitranscriptomic profiling of N6-methyladenosine-related RNA methylation in rat cerebral cortex following traumatic brain injury. Mol. Brain 2020, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mao, J.; Wang, X.; Lin, Y.; Hou, G.; Zhu, J.; Xie, B. Genome-wide screening of altered m6A-tagged transcript profiles in the hippocampus after traumatic brain injury in mice. Epigenomics 2019, 11, 805–819. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Y.; Luo, X.; Liu, Y.; Zhang, X.; Gao, L. N6-methyladenosine RNA modification: A promising regulator in central nervous system injury. Exp. Neurol. 2021, 345, 113829. [Google Scholar] [CrossRef] [PubMed]
- He, P.C.; He, C. m6A RNA methylation: From mechanisms to therapeutic potential. EMBO J. 2021, 40, e105977. [Google Scholar] [CrossRef]
- Sommer, F.; Backhed, F. Know your neighbor: Microbiota and host epithelial cells interact locally to control intestinal function and physiology. BioEssays 2016, 38, 455–464. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Heaver, S.L.; Johnson, E.L.; Ley, R.E. Sphingolipids in host–microbial interactions. Curr. Opin. Microbiol. 2018, 43, 92–99. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Y.; Chen, W.; Shi, H.; Eren, A.M.; Morozov, A.; He, C.; Luo, G.-Z.; Pan, T. Transcriptome-wide reprogramming of N6-methyladenosine modification by the mouse microbiome. Cell Res. 2019, 29, 167–170. [Google Scholar] [CrossRef]
- Luo, J.; Yu, J.; Peng, X. Could partial nonstarch polysaccharides ameliorate cancer by altering m6A RNA methylation in hosts through intestinal microbiota? Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Cao, G.; Zhang, T.; Sefik, E.; Vesely, M.C.A.; Broughton, J.P.; Zhu, S.; Li, H.-B.; Li, B.; Chen, L.; et al. m6A mRNA methylation sustains Treg suppressive functions. Cell Res. 2018, 28, 253–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Liu, N.; Zhu, X.; Yang, L.; Ye, B.; Li, H.; Zhu, P.; Lu, T.; Tian, Y.; Fan, Z. Circular RNA circZbtb20 maintains ILC3 homeostasis and function via Alkbh5-dependent m6A demethylation of Nr4a1 mRNA. Cell. Mol. Immunol. 2021, 18, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Xiao, X.; Shen, B.; Jiang, Q.; Wang, H.; Lu, Z.; Wang, F.; Jin, M.; Min, J.; Wang, F.; et al. The N 6-methyladenosine RNA-binding protein YTHDF1 modulates the translation of TRAF6 to mediate the intestinal immune response. Nucleic Acids Res. 2021, 49, 5537–5552. [Google Scholar] [CrossRef]
- Olazagoitia-Garmendia, A.; Zhang, L.; Mera, P.; Godbout, J.K.; Sebastian-DelaCruz, M.; Garcia-Santisteban, I.; Mendoza, L.M.; Huerta, A.; Irastorza, I.; Bhagat, G.; et al. Gluten-induced RNA methylation changes regulate intestinal inflammation via allele-specific XPO1 translation in epithelial cells. Gut 2022, 71, 68–76. [Google Scholar] [CrossRef]
- Tanabe, A.; Tanikawa, K.; Tsunetomi, M.; Takai, K.; Ikeda, H.; Konno, J.; Torigoe, T.; Maeda, H.; Kutomi, G.; Okita, K.; et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016, 376, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Shakkour, Z.; Tabet, M.; Abdelhady, S.; Kobaisi, A.; Abedi, R.; Nasrallah, L.; Pintus, G.; Al-Dhaheri, Y.; Mondello, S.; et al. Traumatic Brain Injury: Oxidative Stress and Novel Anti-Oxidants Such as Mitoquinone and Edaravone. Antioxidants 2020, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The Molecular Pathophysiology of Concussive Brain Injury—An Update. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Tarudji, A.W.; Gee, C.C.; Romereim, S.M.; Convertine, A.J.; Kievit, F.M. Antioxidant thioether core-crosslinked nanoparticles prevent the bilateral spread of secondary injury to protect spatial learning and memory in a controlled cortical impact mouse model of traumatic brain injury. Biomaterials 2021, 272, 120766. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, N.; Agarwal, V.; Nune, M. Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches. Antioxidants 2021, 11, 72. [Google Scholar] [CrossRef]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef]
- Ding, H.; Li, Z.; Li, X.; Yang, X.; Zhao, J.; Guo, J.; Lu, W.; Liu, H.; Wang, J. FTO Alleviates CdCl2-Induced Apoptosis and Oxidative Stress via the AKT/Nrf2 Pathway in Bovine Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 4948. [Google Scholar] [CrossRef]
- Chen, P.-B.; Shi, G.-X.; Liu, T.; Li, B.; Jiang, S.-D.; Zheng, X.-F.; Jiang, L.-S. Oxidative Stress Aggravates Apoptosis of Nucleus Pulposus Cells through m6A Modification of MAT2A Pre-mRNA by METTL16. Oxidative Med. Cell. Longev. 2022, 2022, 4036274. [Google Scholar] [CrossRef]
- Xia, C.; Wang, J.; Wu, Z.; Miao, Y.; Chen, C.; Li, R.; Li, J.; Xing, H. METTL3-mediated m6A methylation modification is involved in colistin-induced nephrotoxicity through apoptosis mediated by Keap1/Nrf2 signaling pathway. Toxicology 2021, 462, 152961. [Google Scholar] [CrossRef]
- Sun, R.; Tian, X.; Li, Y.; Zhao, Y.; Wang, Z.; Hu, Y.; Zhang, L.; Wang, Y.; Gao, D.; Zheng, S.; et al. The m6A reader YTHDF3-mediated PRDX3 translation alleviates liver fibrosis. Redox Biol. 2022, 54, 102378. [Google Scholar] [CrossRef] [PubMed]
- Anders, M.; Chelysheva, I.; Goebel, I.; Trenkner, T.; Zhou, J.; Mao, Y.; Verzini, S.; Qian, S.-B.; Ignatova, Z. Dynamic m6A methylation facilitates mRNA triaging to stress granules. Life Sci. Alliance 2018, 1, e201800113. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhuang, X. m6A-binding YTHDF proteins promote stress granule formation. Nat. Chem. Biol. 2020, 16, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ishfaq, M.; Xu, L.; Xia, C.; Chen, C.; Li, J. METTL3/m6A/miRNA-873-5p Attenuated Oxidative Stress and Apoptosis in Colistin-Induced Kidney Injury by Modulating Keap1/Nrf2 Pathway. Front. Pharmacol. 2019, 10, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.X.J.; Zhu, M.H.-Y.; Wang, X.-L.; Lu, X.-W.; Pan, M.C.-L.; Xu, L.; Liu, M.X.; Xu, N.; Zhang, Z.-Y. Oridonin Ameliorates Traumatic Brain Injury-Induced Neurological Damage by Improving Mitochondrial Function and Antioxidant Capacity and Suppressing Neuroinflammation through the Nrf2 Pathway. J. Neurotrauma 2022, 39, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Ni, H.; Lin, X.; Zhu, X.; Li, D.; Liu, H.; Chen, G. Astrocyte-derived fatty acid-binding protein 7 protects blood-brain barrier integrity through a caveolin-1/MMP signaling pathway following traumatic brain injury. Exp. Neurol. 2019, 322, 113044. [Google Scholar] [CrossRef]
- Hausburg, M.A.; Banton, K.L.; Roman, P.E.; Salgado, F.; Baek, P.; Waxman, M.J.; Tanner, A.; Yoder, J.; Bar-Or, D. Effects of propofol on ischemia-reperfusion and traumatic brain injury. J. Crit. Care 2020, 56, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, L.; Liu, H.; Lu, W.; Ling, X.; Gong, M. Rutaecarpine Attenuates Oxidative Stress-Induced Traumatic Brain Injury and Reduces Secondary Injury via the PGK1/KEAP1/NRF2 Signaling Pathway. Front. Pharmacol. 2022, 13, 807125. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Zhao, Y.; Li, P.; Ning, Y.-L.; Huang, Z.-Z.; Yang, N.; Liu, D.; Zhou, Y.-G. HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury. J. Neuroinflamm. 2021, 18, 241. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, C.; Jin, F.; Yang, M.; Kong, L.; Han, W.; Jiang, P. Calcitriol confers neuroprotective effects in traumatic brain injury by activating Nrf2 signaling through an autophagy-mediated mechanism. Mol. Med. 2021, 27, 118. [Google Scholar] [CrossRef]
- Yi, W.; Cheng, J.; Wei, Q.; Pan, R.; Song, S.; He, Y.; Tang, C.; Liu, X.; Zhou, Y.; Su, H. Effect of temperature stress on gut-brain axis in mice: Regulation of intestinal microbiome and central NLRP3 inflammasomes. Sci. Total Environ. 2021, 772, 144568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.; Moon, Y. Caveolar communication with xenobiotic-stalled ribosomes compromises gut barrier integrity. Commun. Biol. 2020, 3, 270. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Xin, R.; Cui, D.; He, J.; Zhang, S.; Sun, Y. The gut microbiota attenuate neuroinflammation in manganese exposure by inhibiting cerebral NLRP3 inflammasome. Biomed. Pharmacother. 2020, 129, 110449. [Google Scholar] [CrossRef]
- Sadovnikova, I.; Gureev, A.; Ignatyeva, D.; Gryaznova, M.; Chernyshova, E.; Krutskikh, E.; Novikova, A.; Popov, V. Nrf2/ARE Activators Improve Memory in Aged Mice via Maintaining of Mitochondrial Quality Control of Brain and the Modulation of Gut Microbiome. Pharmaceuticals 2021, 14, 607. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lin, H.; Huang, X.; Weng, J.; Peng, F.; Wu, S. METTL14 suppresses pyroptosis and diabetic cardiomyopathy by downregulating TINCR lncRNA. Cell Death Dis. 2022, 13, 38. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Deng, H.; Xu, B.; Zhou, Y.; Liu, J.; Liu, Y.; Shi, Y.; Zheng, X.; Jiang, J. N6-methyladenosine demethylase FTO promotes growth and metastasis of gastric cancer via m6A modification of caveolin-1 and metabolic regulation of mitochondrial dynamics. Cell Death Dis. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, J.; Li, Z.; Xu, B.; Miyata, M. Importance of NLRP3 Inflammasome in Abdominal Aortic Aneurysms. J. Atheroscler. Thromb. 2021, 28, 454–466. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.T.; Pham, L.; Symons, G.F.; Monif, M.; Shultz, S.R.; McDonald, S.J. The NLRP3 inflammasome in traumatic brain injury: Potential as a biomarker and therapeutic target. J. Neuroinflamm. 2020, 17, 104. [Google Scholar] [CrossRef]
- Irrera, N.; Russo, M.; Pallio, G.; Bitto, A.; Mannino, F.; Minutoli, L.; Altavilla, D.; Squadrito, F. The Role of NLRP3 Inflammasome in the Pathogenesis of Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 6204. [Google Scholar] [CrossRef]
- Liu, H.D.; Li, W.; Chen, Z.-R.; Hu, Y.-C.; Zhang, D.-D.; Shen, W.; Zhou, M.-L.; Zhu, L.; Hang, C.-H. Expression of the NLRP3 Inflammasome in Cerebral Cortex After Traumatic Brain Injury in a Rat Model. Neurochem. Res. 2013, 38, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Nasoohi, S.; Ishrat, T. MCC950, the Selective Inhibitor of Nucleotide Oligomerization Domain-Like Receptor Protein-3 Inflammasome, Protects Mice against Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1294–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yin, D.; Ren, H.; Gao, W.; Li, F.; Sun, D.; Wu, Y.; Zhou, S.; Lyu, L.; Yang, M.; et al. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol. Dis. 2018, 117, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Bao, Z.; Hou, L.; Liu, Y.; Wang, X.; You, Y.; et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp. Neurol. 2017, 290, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wallisch, J.; Simon, D.W.; Bayır, H.; Bell, M.J.; Kochanek, P.M.; Clark, R.S.B. Cerebrospinal Fluid NLRP3 is Increased After Severe Traumatic Brain Injury in Infants and Children. Neurocrit. Care 2017, 27, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Antonioli, L.; Calderone, V.; Colucci, R.; Fornai, M.; Blandizzi, C. Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog. Neurobiol. 2020, 191, 101806. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Venugopalan, R.; Stewart, D.J. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood–brain barrier breakdown. Acta Neuropathol. 2007, 114, 459–469. [Google Scholar] [CrossRef]
- Nag, S.; Manias, J.L.; Stewart, D.J. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol. Appl. Neurobiol. 2009, 35, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zheng, G.-Q.; Xu, M.; Li, Y.; Chen, X.; Zhu, W.; Tong, Y.; Chung, S.K.; Liu, K.J.; Shen, J. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 2012, 120, 147–156. [Google Scholar] [CrossRef]
- Gu, Y.; Dee, C.M.; Shen, J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. 2011, 3, 1216–1231. [Google Scholar] [CrossRef]
- Blochet, C.; Buscemi, L.; Clément, T.; Gehri, S.; Badaut, J.; Hirt, L. Involvement of caveolin-1 in neurovascular unit remodeling after stroke: Effects on neovascularization and astrogliosis. J. Cereb. Blood Flow Metab. 2020, 40, 163–176. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Liang, J.; Pan, R.; Dong, W.; Shen, J.; Yang, Y.; Zhao, Y.; Shi, W.; Luo, Y.; Ji, X.; et al. Zinc contributes to acute cerebral ischemia-induced blood–brain barrier disruption. Neurobiol. Dis. 2016, 95, 12–21. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Rizzarelli, E.; Owen, J.B.; Dinkova-Kostova, A.T.; Butterfield, D.A. Nitric Oxide in Cell Survival: A Janus Molecule. Antioxid. Redox Signal. 2009, 11, 2717–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Guagliano, E.; Sapienza, M.; Panebianco, M.; Calafato, S.; Puleo, E.; Pennisi, G.; Mancuso, C.; Butterfield, D.A.; Stella, A.G. Redox Regulation of Cellular Stress Response in Aging and Neurodegenerative Disorders: Role of Vitagenes. Neurochem. Res. 2007, 32, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: Evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res. 1997, 49, 681–697. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.; Calabrese, E.J.; Mattson, M.P. Cellular Stress Responses, The Hormesis Paradigm, and Vitagenes: Novel Targets for Therapeutic Intervention in Neurodegenerative Disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Pani, G.; Calabrese, V. Bilirubin: An endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006, 11, 207–213. [Google Scholar] [CrossRef]
- Woronyczova, J.; Nováková, M.; Leníček, M.; Bátovský, M.; Bolek, E.; Cífková, R.; Vítek, L. Serum Bilirubin Concentrations and the Prevalence of Gilbert Syndrome in Elite Athletes. Sports Med. Open 2022, 8, 84. [Google Scholar] [CrossRef]
- Mancuso, C.; Capone, C.; Ranieri, S.C.; Fusco, S.; Calabrese, V.; Eboli, M.L.; Preziosi, P.; Galeotti, T.; Pani, G. Bilirubin as an endogenous modulator of neurotrophin redox signaling. J. Neurosci. Res. 2008, 86, 2235–2249. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear Factor E2-Related Factor 2-Dependent Antioxidant Response Element Activation by tert-Butylhydroquinone and Sulforaphane Occurring Preferentially in Astrocytes Conditions Neurons against Oxidative Insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Calkins, M.J.; Jakel, R.J.; Johnson, D.A.; Chan, K.; Kan, Y.W.; Johnson, J.A. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.G.; Yong, Y.-Y.; Pan, Y.-R.; Zhang, L.; Wu, J.-M.; Zhang, Y.; Tang, Y.; Wei, J.; Yu, L.; Law, B.Y.-K.; et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxidative Med. Cell. Longev. 2022, 2022, 1015791. [Google Scholar] [CrossRef]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A.A. Fumonisin B1 alters global m6A RNA methylation and epigenetically regulates Keap1-Nrf2 signaling in human hepatoma (HepG2) cells. Arch. Toxicol. 2021, 95, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.X.; Wang, J.-K.; Shen, L.-J.; Long, C.-L.; Liu, B.; Wei, Y.; Han, L.-D.; Wei, Y.-X.; Wu, S.-D.; Wei, G.-H. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ. Pollut. 2020, 259, 113911. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Z.; Zhuo, R.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates Aflatoxin B1-Induced ROS Formation and Increase of m6A RNA Methylation. Animals 2020, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Wang, T.; Zhong, X. Modification of N6-methyladenosine RNA methylation on heat shock protein expression. PLoS ONE 2018, 13, e0198604. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Yu, J.; Gan, Z.; Wei, W.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates High-Fat Diet Induced Hepatic Lipid Homeostasis Disorder and Decreases m6A RNA Methylation. Front. Pharmacol. 2020, 11, 568006. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Wei, W.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol and Curcumin Improve Intestinal Mucosal Integrity and Decrease m6A RNA Methylation in the Intestine of Weaning Piglets. ACS Omega 2019, 4, 17438–17446. [Google Scholar] [CrossRef]
- Lu, N.; Li, X.; Yu, J.; Li, Y.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Curcumin Attenuates Lipopolysaccharide-Induced Hepatic Lipid Metabolism Disorder by Modification of m6A RNA Methylation in Piglets. Lipids 2018, 53, 53–63. [Google Scholar] [CrossRef]



| Types | Regulators | Functions | References |
|---|---|---|---|
| m6A writers | METTL3 | Catalyses the transfer of the methyl in single-stranded RNA (ssRNA) sequence motif DRACH (D = A, G or U; R = A or G; H = A, C or U) from S-Adenosyl methionine (SAM) to adenine. | [87,93] |
| METTL14 | Recognizes the RNA substrate that activates METTL3 and offers RNA binding sites as scaffolds to form a stable heterodimer with METTLL3. | [84,87,93] | |
| WTAP | The first one binds to METTL3-METTL14 heterodimer and recruits it to target RNA. The three proteins form together a conservative complex located in nuclear spot. | [84,94,95] | |
| METTL16 | Responsible for m6A modification of lncRNAs, U6 snRNA, and introns of pre-mRNAs. | [91,96,97,98] | |
| RBM15 | Binds m6A complex and recruits it to a special RNA site. | [84,99] | |
| VIRMA | Recruits m6A complex to a special RNA site and interacts with polyadenosine cleavage factors CPSF5 and CPSF6. | [89,100] | |
| ZC3H13 | Bridges WTAP to mRNA binding factor Nito. | [84,101] | |
| METTL5 | Responsible for m6A modification of 18s rRNA. | [96,102,103] | |
| ZCCHC4 | Responsible for m6A modification of 28s rRNA. | [96,104,105,106] | |
| HAKAI | Exerts effects on gender determination and mediates lethal splicing, maintains the functions of m6A writers by ensuring the stability of MACOM components via the Hakai ubiquitin domain. | [92] | |
| m6A erasers | FTO | Demethylates m6A, also harbours activity towards m6Am and m1A. | [96,107,108,109] |
| ALKBH5 | Mainly demethylates m6A. | [96,110,111] | |
| m6A readers | YTHDF1/2/3 | Highly similar to m6A sites bound by YTHDF1, YTHDF2 or YTHDF3, and these three analogues together exerts effects on mediating mRNA degradation. | [93,112] |
| YTHDC1 | Promotes alternative splicing and RNA output. | [96,110,111] | |
| YTHDC2 | Boosts target RNA translation and reduces its abundance. | [96,113,114] | |
| HNRNPA2B1 | Mediates mRNA splicing and major microRNA processing. | [84,115] | |
| HNRNPC/ hn-RNPG | Regulates mRNA structure and alternative splicing. | [96,116,117] | |
| EIF3 | Facilitates mRNA translation. | [84,118] | |
| IGF2BP1/2/3 | Enhances mRNA stability, storage capacity and translation. | [96,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Liu, M.; Zhang, J.; Zhong, X.; Zhong, C. The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction. Antioxidants 2022, 11, 1521. https://doi.org/10.3390/antiox11081521
Huang P, Liu M, Zhang J, Zhong X, Zhong C. The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction. Antioxidants. 2022; 11(8):1521. https://doi.org/10.3390/antiox11081521
Chicago/Turabian StyleHuang, Peizan, Min Liu, Jing Zhang, Xiang Zhong, and Chunlong Zhong. 2022. "The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction" Antioxidants 11, no. 8: 1521. https://doi.org/10.3390/antiox11081521
APA StyleHuang, P., Liu, M., Zhang, J., Zhong, X., & Zhong, C. (2022). The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction. Antioxidants, 11(8), 1521. https://doi.org/10.3390/antiox11081521







