DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
3. Results
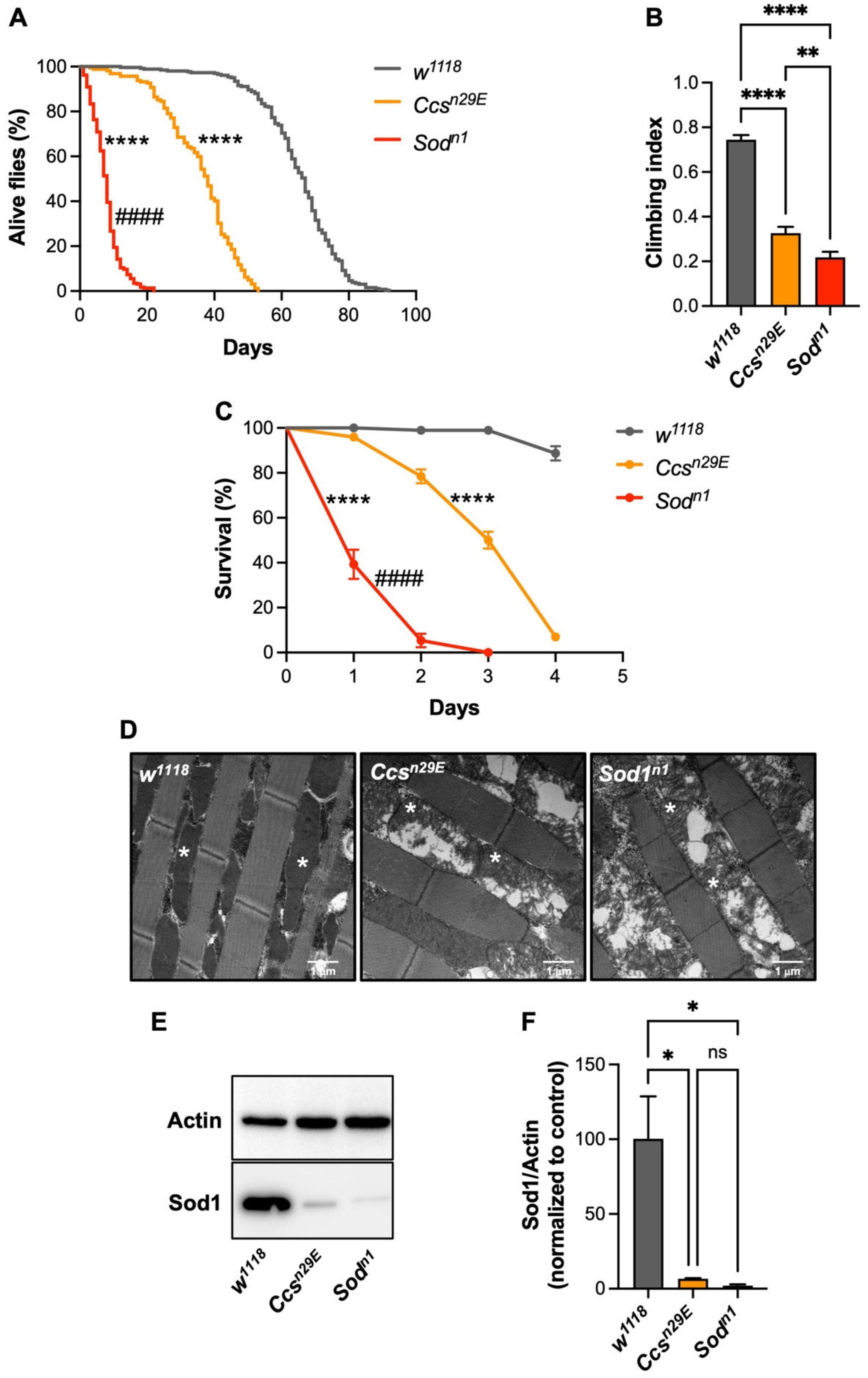
3.1. Ccs-Dependent and Ccs-Independent Sod1 Maturation Modulate D. melanogaster Life Expectancy, and Capability to Cope with Oxidative Stress Conditions
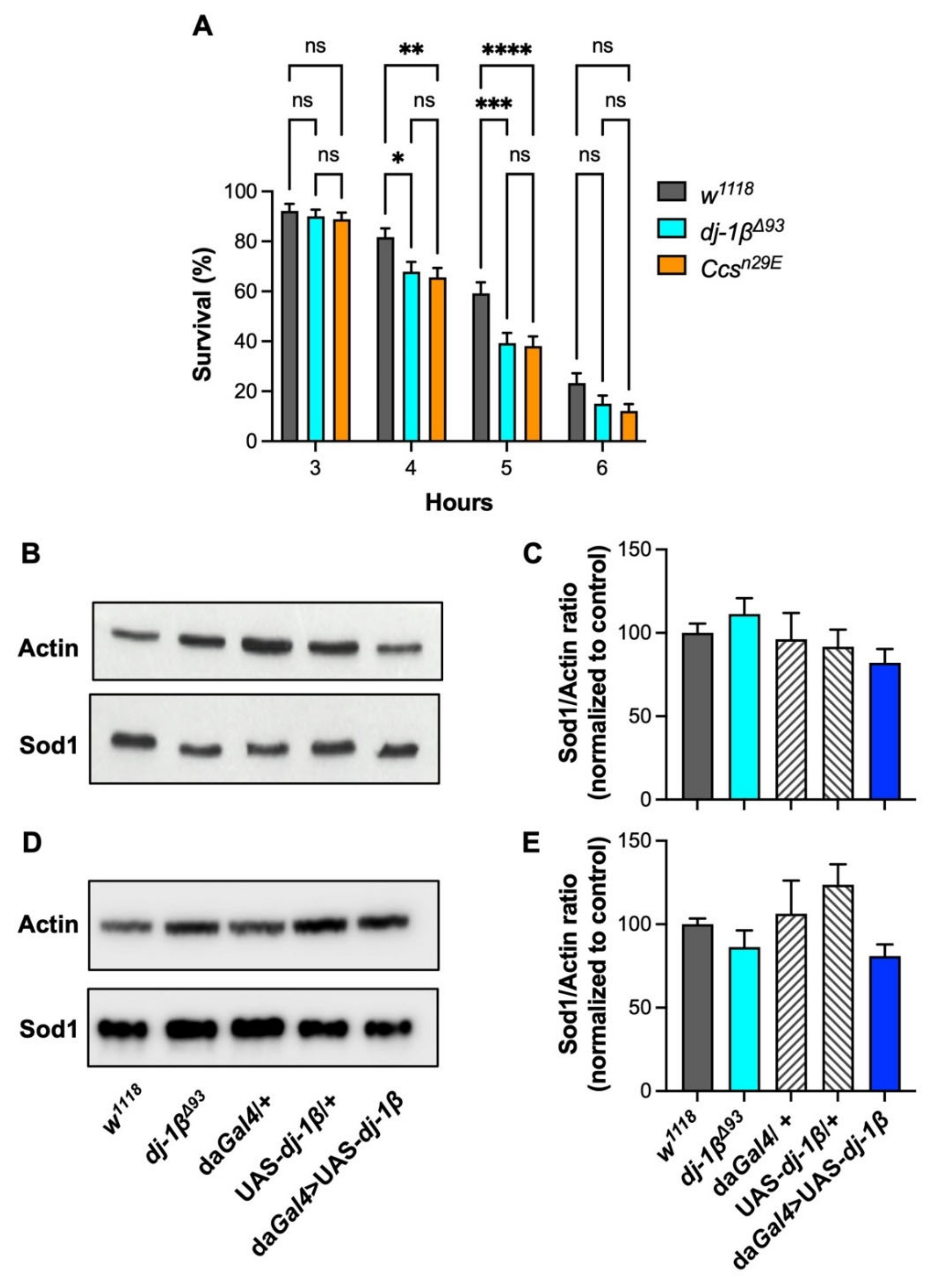
3.2. Drosophila dj-1β Participates in the Protection against Oxygen Deprivation without Affecting Sod1 Expression
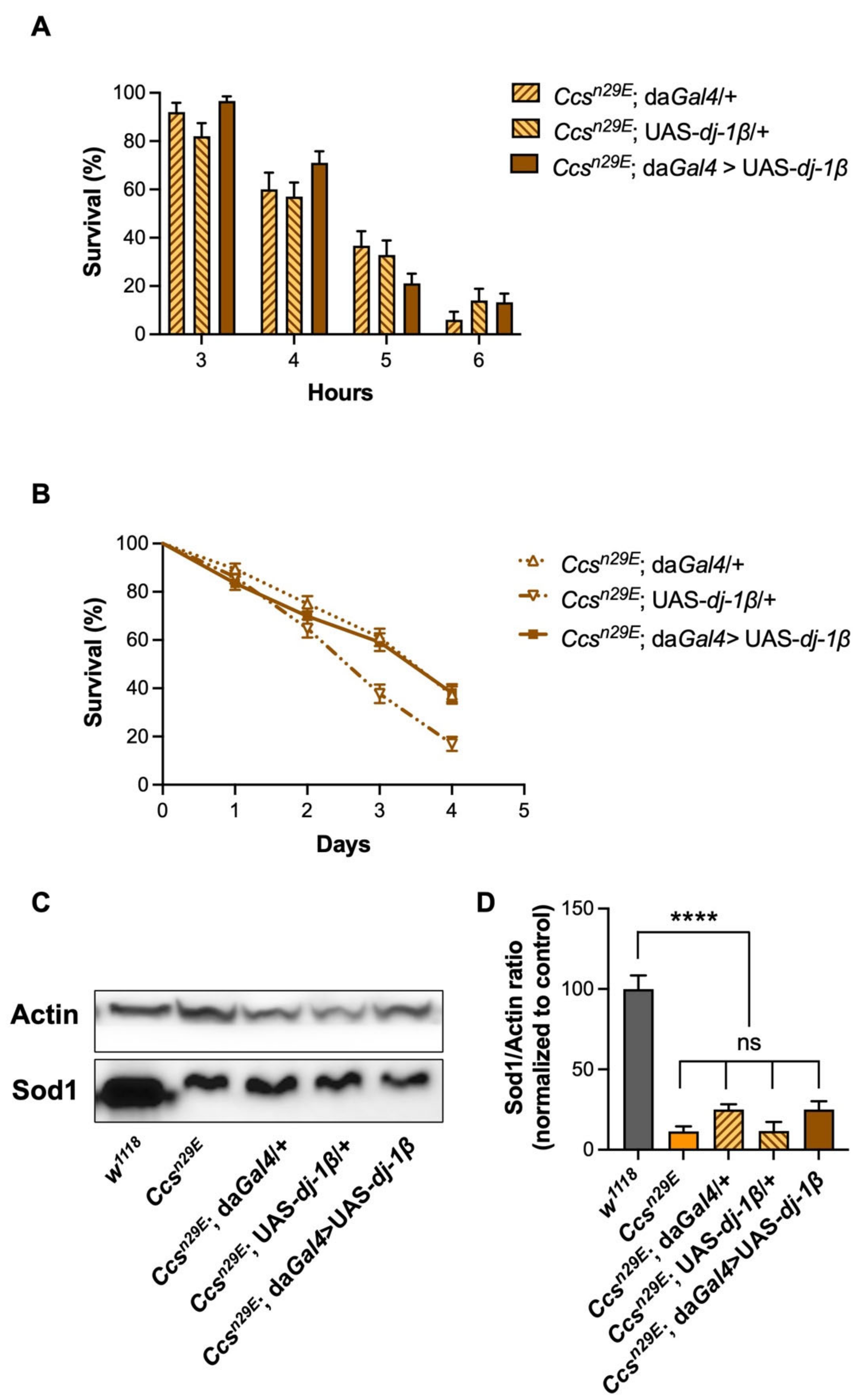
3.3. The Overexpression of dj-1β Does Not Rescue the Effects Induced by Ccs Depletion
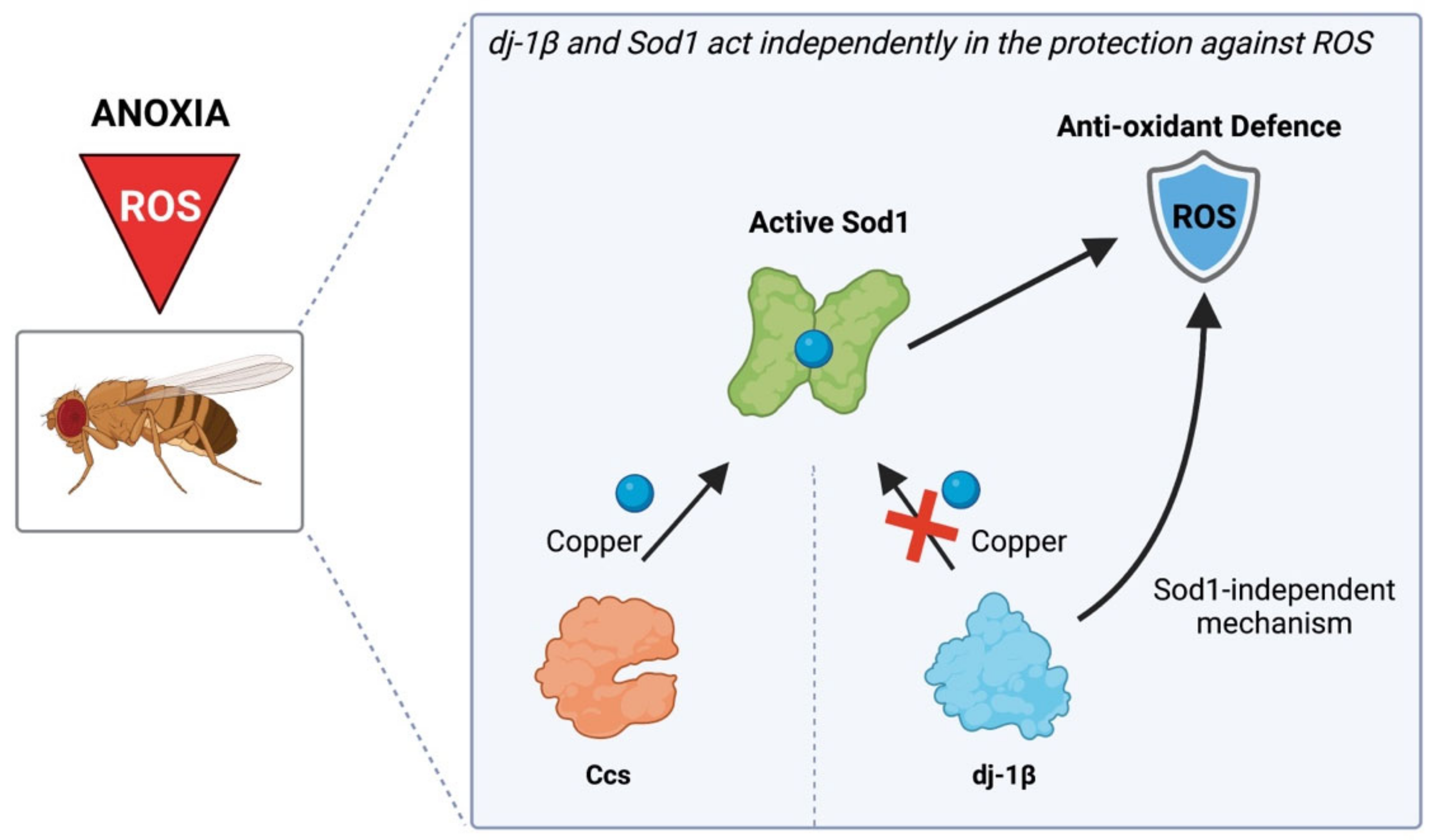
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. Engl. 2021, 60, 9215–9246. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.S.A.; Antonyuk, S.v.; Hasnain, S.S. The Biophysics of Superoxide Dismutase-1 and Amyotrophic Lateral Sclerosis. Q. Rev. Biophys. 2019, 52, e12. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.R.; Lim, N.K.H.; McAllum, E.J.; Donnelly, P.S.; Hare, D.J.; Doble, P.A.; Turner, B.J.; Price, K.A.; Lim, S.C.; Paterson, B.M.; et al. Oral Treatment with Cu (II) (Atsm) Increases Mutant SOD1 in Vivo but Protects Motor Neurons and Improves the Phenotype of a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurosci. 2014, 34, 8021–8031. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Trias, E.; Beilby, P.R.; Lopez, N.I.; Labut, E.M.; Bradford, C.S.; Roberts, B.R.; McAllum, E.J.; Crouch, P.J.; Rhoads, T.W.; et al. Copper Delivery to the CNS by Cu ATSM Effectively Treats Motor Neuron Disease in SOD (G93A) Mice Co-Expressing the Copper-Chaperone-for-SOD. Neurobiol. Dis. 2016, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.B.; Mercer, S.W.; Lim, N.K.H.; Faux, N.G.; Buncic, G.; Beckman, J.S.; Roberts, B.R.; Donnelly, P.S.; White, A.R.; Crouch, P.J. Cu II (Atsm) Improves the Neurological Phenotype and Survival of SOD1 G93A Mice and Selectively Increases Enzymatically Active SOD1 in the Spinal Cord. Sci. Rep. 2017, 7, 42292. [Google Scholar] [CrossRef]
- Trist, B.G.; Davies, K.M.; Cottam, V.; Genoud, S.; Ortega, R.; Roudeau, S.; Carmona, A.; de Silva, K.; Wasinger, V.; Lewis, S.J.G.; et al. Amyotrophic Lateral Sclerosis-like Superoxide Dismutase 1 Proteinopathy Is Associated with Neuronal Loss in Parkinson’s Disease Brain. Acta Neuropathol. 2017, 134, 113–127. [Google Scholar] [CrossRef]
- Davies, K.M.; Bohic, S.; Carmona, A.; Ortega, R.; Cottam, V.; Hare, D.J.; Finberg, J.P.M.; Reyes, S.; Halliday, G.M.; Mercer, J.F.B.; et al. Copper Pathology in Vulnerable Brain Regions in Parkinson’s Disease. Neurobiol. Aging. 2014, 35, 858–866. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. A Proposed Mechanism for Neurodegeneration in Movement Disorders Characterized by Metal Dyshomeostasis and Oxidative Stress. Cell Chem. Biol. 2018, 25, 807–816. [Google Scholar] [CrossRef]
- Culotta, V.C.; Klomp, L.W.J.; Strain, J.; Casareno, R.L.B.; Krems, B.; Gitlin, J.D. The Copper Chaperone for Superoxide Dismutase. J. Biol. Chem. 1997, 272, 23469–23472. [Google Scholar] [CrossRef]
- Leitch, J.M.; Jensen, L.T.; Bouldin, S.D.; Outten, C.E.; Hart, P.J.; Culotta, V.C. Activation of Cu, Zn-Superoxide Dismutase in the Absence of Oxygen and the Copper Chaperone CCS. J. Biol. Chem. 2009, 284, 21863–21871. [Google Scholar] [CrossRef]
- Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper Sources for Sod1 Activation. Antioxidants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Waggoner, D.; Subramaniam, J.R.; Tessarollo, L.; Bartnikas, T.B.; Culotta, V.C.; Price, D.L.; Rothstein, J.; Gitlin, J.D. Copper Chaperone for Superoxide Dismutase Is Essential to Activate Mammalian Cu/Zn Superoxide Dismutase. Proc. Natl. Acad. Sci. USA 2000, 97, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Björkblom, B.; Adilbayeva, A.; Maple-Grødem, J.; Piston, D.; Ökvist, M.; Xu, X.M.; Brede, C.; Larsen, J.P.; Møller, S.G. Parkinson Disease Protein DJ-1 Binds Metals and Protects against Metal-Induced Cytotoxicity. J. Biol. Chem. 2013, 288, 22809–22820. [Google Scholar] [CrossRef]
- Puno, M.R.; Patel, N.A.; Møller, S.G.; Robinson, C.v.; Moody, P.C.E.; Odell, M. Structure of Cu (I)-Bound DJ-1 Reveals a Biscysteinate Metal Binding Site at the Homodimer Interface: Insights into Mutational Inactivation of DJ-1 in Parkinsonism. J. Am. Chem. Soc. 2013, 135, 15974–15977. [Google Scholar] [CrossRef]
- Girotto, S.; Cendron, L.; Bisaglia, M.; Tessari, I.; Mammi, S.; Zanotti, G.; Bubacco, L. DJ-1 Is a Copper Chaperone Acting on SOD1 Activation. J. Biol. Chem. 2014, 289, 10887–10899. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Lin, H.; Maple, J.; Björkblom, B.; Alves, G.; Larsen, J.P.; Møller, S.G. The Arabidopsis DJ-1a Protein Confers Stress Protection through Cytosolic SOD Activation. J. Cell Sci. 2010, 123, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Biosa, A.; Sandrelli, F.; Beltramini, M.; Greggio, E.; Bubacco, L.; Bisaglia, M. Recent Findings on the Physiological Function of DJ-1: Beyond Parkinson’s Disease. Neurobiol. Dis. 2017, 108, 65–72. [Google Scholar] [CrossRef]
- De Lazzari, F.; Prag, H.A.; Gruszczyk, A.V.; Whitworth, A.J.; Bisaglia, M. DJ-1: A Promising Therapeutic Candidate for Ischemia-Reperfusion Injury. Redox Biol. 2021, 41, 101884. [Google Scholar] [CrossRef]
- Huang, M.; Chen, S. DJ-1 in Neurodegenerative Diseases: Pathogenesis and Clinical Application. Prog. Neurobiol. 2021, 204, 102114. [Google Scholar] [CrossRef]
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377. [Google Scholar] [CrossRef]
- Annesi, G.; Savettieri, G.; Pugliese, P.; D’Amelio, M.; Tarantino, P.; Ragonese, P.; la Bella, V.; Piccoli, T.; Civitelli, D.; Annesi, F.; et al. DJ-1 Mutations and Parkinsonism-Dementia-Amyotrophic Lateral Sclerosis Complex. Ann. Neurol. 2005, 58, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Hanagasi, H.A.; Giri, A.; Kartal, E.; Guven, G.; Bilgiç, B.; Hauser, A.K.; Emre, M.; Heutink, P.; Basak, N.; Gasser, T.; et al. A Novel Homozygous DJ1 Mutation Causes Parkinsonism and ALS in a Turkish Family. Parkinsonism Relat. Disord. 2016, 29, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Mori, A.; Kimura, E.; Mita, S.; Maeda, Y.; Hirano, T.; Uchino, M. DJ-1 Forms Complexes with Mutant SOD1 and Ameliorates Its Toxicity. J. Neurochem. 2010, 113, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Sipos, J.; Thau-Habermann, N.; Körner, S.; Rath, K.J.; Dengler, R.; Petri, S. Altered Expression of DJ-1 and PINK1 in Sporadic ALS and in the SOD1 (G93A) ALS Mouse Model. J. Neuropathol. Exp. Neurol. 2013, 72, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.; Jensen, L.T.; Binnington, J.; Hilliker, A.J.; Ulloa, J.; Culotta, V.C.; Phillips, J.P. Instability of Superoxide Dismutase 1 of Drosophila in Mutants Deficient for Its Cognate Copper Chaperone. J. Biol. Chem. 2008, 283, 35393–35401. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.P.; Tainer, J.A.; Getzoff, E.D.; Boulianne, G.L.; Kirby, K.; Hilliker, A.J. Subunit-Destabilizing Mutations in Drosophila Copper/Zinc Superoxide Dismutase: Neuropathology and a Model of Dimer Dysequilibrium. Proc. Natl. Acad. Sci. USA 1995, 92, 8574–8578. [Google Scholar] [CrossRef]
- Rodriguez-Rocha, H.; Garcia-Garcia, A.; Pickett, C.; Li, S.; Jones, J.; Chen, H.; Webb, B.; Choi, J.; Zhou, Y.; Zimmerman, M.C.; et al. Compartmentalized Oxidative Stress in Dopaminergic Cell Death Induced by Pesticides and Complex I Inhibitors: Distinct Roles of Superoxide Anion and Superoxide Dismutases. Free Radic. Biol. Med. 2013, 61, 370–383. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Chaouhan, H.; Li, X.; Sun, K.; Wang, I.; Yu, T.; Yu, S.; Chen, K.; Lin, W.; Li, C. Calycosin Alleviates Paraquat-Induced Neurodegeneration by Improving Mitochondrial Functions and Regulating Autophagy in a Drosophila Model of Parkinson’s Disease. Antioxidants 2022, 11, 222. [Google Scholar] [CrossRef]
- Meulener, M.; Whitworth, A.J.; Armstrong-Gold, C.E.; Rizzu, P.; Heutink, P.; Wes, P.D.; Pallanck, L.J.; Bonini, N.M. Drosophila DJ-1 Mutants Are Selectively Sensitive to Environmental Toxins Associated with Parkinson’s Disease. Curr. Biol. 2005, 15, 1572–1577. [Google Scholar] [CrossRef]
- Meulener, M.C.; Xu, K.; Thompson, L.; Ischiropoulos, H.; Bonini, N.M. Mutational Analysis of DJ-1 in Drosophila Implicates Functional Inactivation by Oxidative Damage and Aging. Proc. Natl. Acad. Sci. USA 2006, 103, 12517–12522. [Google Scholar] [CrossRef] [PubMed]
- Callier, V.; Hand, S.C.; Campbell, J.B.; Biddulph, T.; Harrison, J.F. Developmental Changes in Hypoxic Exposure and Responses to Anoxia in Drosophila Melanogaster. J. Exp. Biol. 2015, 218, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Chen, S.; Wang, Y.; Cao, L.; Zhang, Y.; Kang, W.; Li, H.; Gui, Y.; Chen, S.; et al. DJ-1 Modulates the Expression of Cu/Zn-Superoxide Dismutase-1 through the Erk1/2-Elk1 Pathway in Neuroprotection. Ann. Neurol. 2011, 70, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Luchinat, E.; Banci, L. Intracellular Metal Binding and Redox Behavior of Human DJ-1. J. Biol. Inorg. Chem. 2018, 23, 61–69. [Google Scholar] [CrossRef]
- Chin, T.Y.; Wang, C.C.; Ma, K.H.; Kuo, C.W.; Hu, M.K.; Chueh, S.H. Antioxidative Effect of DJ-1 Is Enhanced in NG108-15 Cells by DPMQ-Induced Copper Influx. Am. J. Physiol. Cell Physiol. 2021, 320, C635–C651. [Google Scholar] [CrossRef]
- Srivastava, S.; Blower, P.J.; Aubdool, A.A.; Hider, R.C.; Mann, G.E.; Siow, R.C. Cardioprotective Effects of Cu (II) ATSM in Human Vascular Smooth Muscle Cells and Cardiomyocytes Mediated by Nrf2 and DJ-1. Sci. Rep. 2016, 6, 7. [Google Scholar] [CrossRef]
- Mazza, M.C.; Shuck, S.C.; Lin, J.; Moxley, M.A.; Termini, J.; Cookson, M.R.; Wilson, M.A. DJ-1 Is Not a Deglycase and Makes a Modest Contribution to Cellular Defense against Methylglyoxal Damage in Neurons. J. Neurochem. 2022, 162, 245–261. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity Gradients Drive Copper to Cellular Destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lazzari, F.; Agostini, F.; Doni, D.; Malacrida, S.; Zordan, M.A.; Costantini, P.; Bubacco, L.; Sandrelli, F.; Bisaglia, M. DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster. Antioxidants 2022, 11, 1527. https://doi.org/10.3390/antiox11081527
De Lazzari F, Agostini F, Doni D, Malacrida S, Zordan MA, Costantini P, Bubacco L, Sandrelli F, Bisaglia M. DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster. Antioxidants. 2022; 11(8):1527. https://doi.org/10.3390/antiox11081527
Chicago/Turabian StyleDe Lazzari, Federica, Francesco Agostini, Davide Doni, Sandro Malacrida, Mauro A. Zordan, Paola Costantini, Luigi Bubacco, Federica Sandrelli, and Marco Bisaglia. 2022. "DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster" Antioxidants 11, no. 8: 1527. https://doi.org/10.3390/antiox11081527
APA StyleDe Lazzari, F., Agostini, F., Doni, D., Malacrida, S., Zordan, M. A., Costantini, P., Bubacco, L., Sandrelli, F., & Bisaglia, M. (2022). DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster. Antioxidants, 11(8), 1527. https://doi.org/10.3390/antiox11081527








