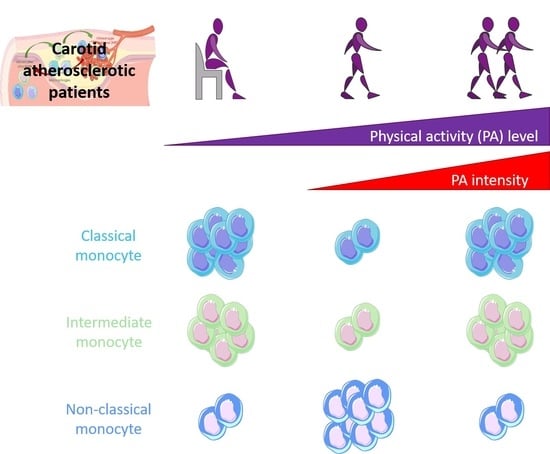
Monocyte Phenotypes and Physical Activity in Patients with Carotid Atherosclerosis
Abstract
:1. Introduction
2. Methods
2.1. Ethics and Population
2.2. Data Collection
2.2.1. Questionnaires
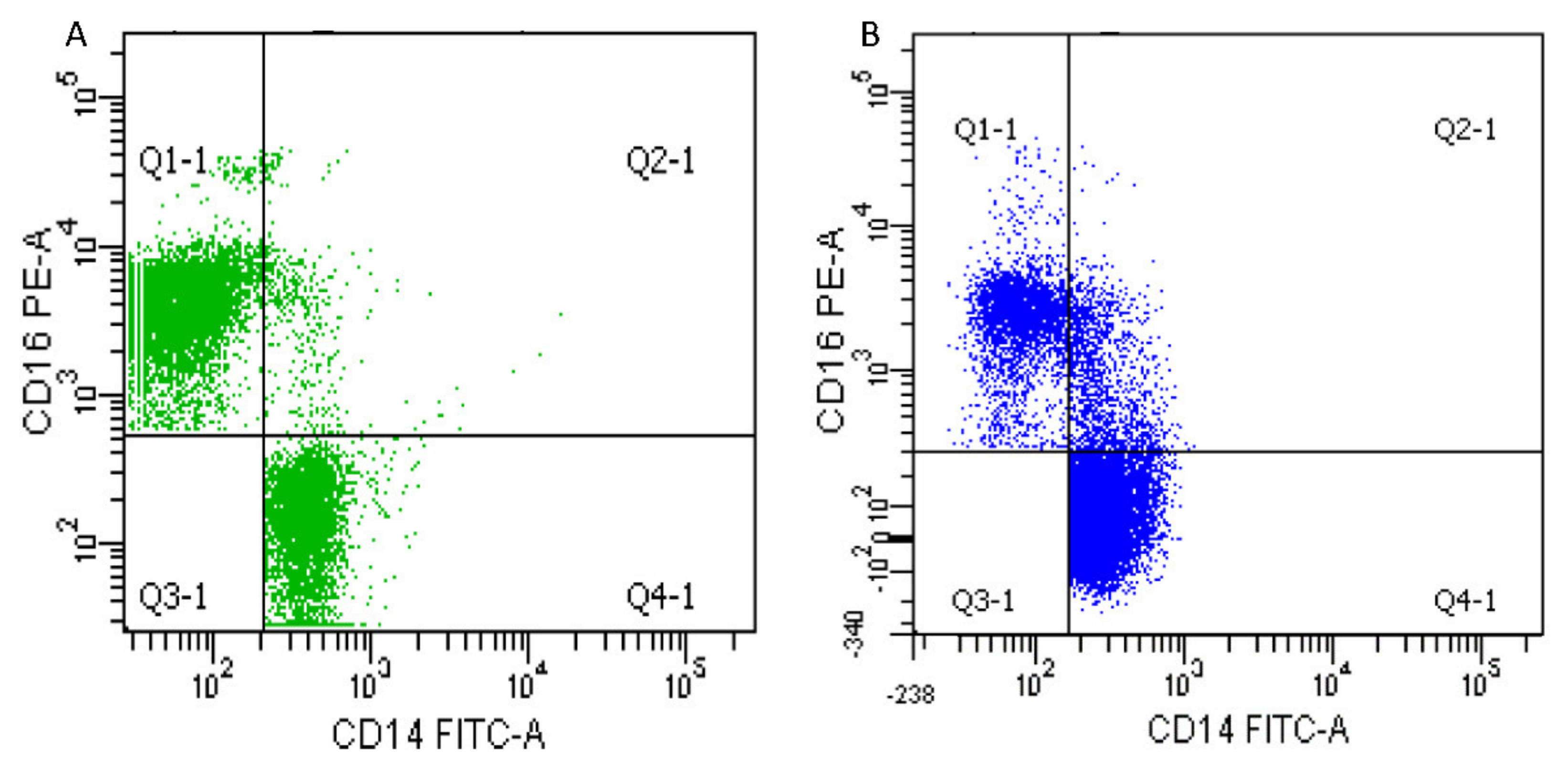
2.2.2. Monocytes Analysis
2.2.3. Oxidative Stress and Antioxidants Assays
2.2.4. Clinical Data
2.3. Statistics
3. Results
3.1. Populations Characteristics
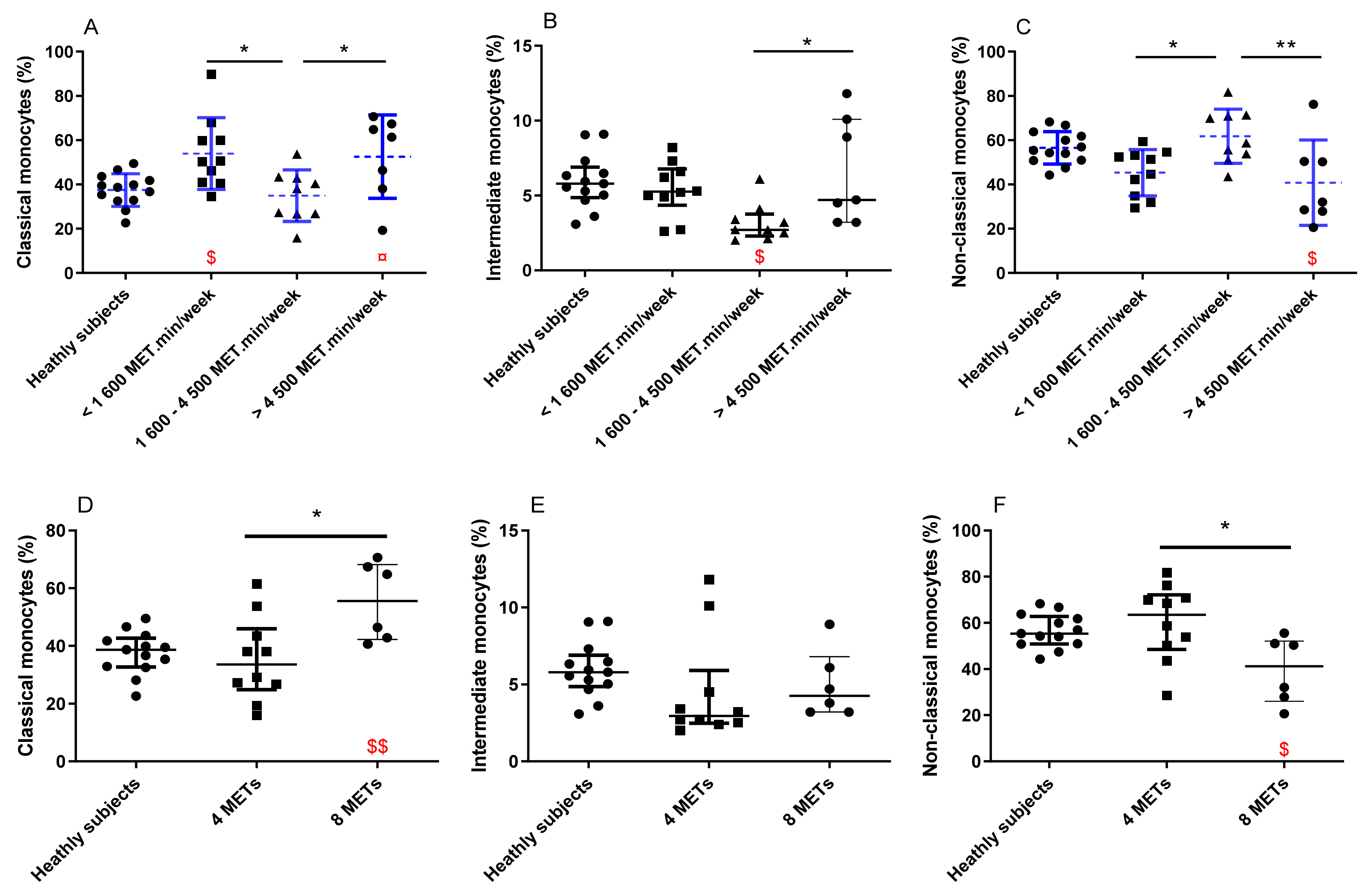
3.2. Comparison between PA Groups and with the Healthy Subject Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, R.A.; Rockman, C.B.; Guo, Y.; Adelman, M.A.; Riles, T.; Hiatt, W.R.; Berger, J.S. Association Between Physical Activity and Peripheral Artery Disease and Carotid Artery Stenosis in a Self-Referred Population of 3 Million Adults. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018, 48, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol. 2015, 213, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis as an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Miller, Y.I.; Hedrick, C.C. Monocyte and Macrophage Dynamics During Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1506–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaipersad, A.S.; Lip, G.Y.; Silverman, S.; Shantsila, E. The Role of Monocytes in Angiogenesis and Atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aw, N.H.; Canetti, E.; Suzuki, K.; Goh, J. Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans. Antioxidants 2018, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Moroni, F.; Ammirati, E.; Norata, G.D.; Magnoni, M.; Camici, P.G. The Role of Monocytes and Macrophages in Human Atherosclerosis, Plaque Neoangiogenesis, and Atherothrombosis. Mediat. Inflamm. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, M.; Weber, C. Differential role of monocyte subsets in atherosclerosis. Thromb. Haemost. 2011, 106, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Arnold, K.A.; Blair, J.E.; Paul, J.D.; Shah, A.P.; Nathan, S.; Alenghat, F.J. Monocyte and macrophage subtypes as paired cell biomarkers for coronary artery disease. Exp. Physiol. 2019, 104, 1343. [Google Scholar] [CrossRef]
- Moore, X.-L.; Fang, L.; Sviridov, D.; Chin-Dusting, J.; Andrews, K.L.; Al-Sharea, A.; Lee, M.K.S.; Murphy, A.J. Native LDL promotes differentiation of human monocytes to macrophages with an inflammatory phenotype. Thromb. Haemost. 2016, 115, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Stöger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Leitinger, N.; Schulman, I.G. Phenotypic Polarization of Macrophages in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1120–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmerman, K.L.; Flynn, M.G.; Coen, P.M.; Markofski, M.M.; Pence, B.D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 2008, 84, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Child, M.; Leggate, M.; Gleeson, M. Effects of Two Weeks of High-intensity Interval Training (HIIT) on Monocyte TLR2 and TLR4 Expression in High BMI Sedentary men. Int. J. Exerc. Sci. 2013, 6, 10. [Google Scholar]
- Gano, L.B.; Donato, A.J.; Pierce, G.L.; Pasha, H.M.; Magerko, K.A.; Roeca, C.; Seals, D.R. Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: Amelioration by habitual exercise. Physiol. Genom. 2011, 43, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Suzuki, K. Toll-like Receptor 4: Target of Lipotoxicity and Exercise- Induced Anti-inflammatory Effect? Ann. Nutr. Food Sci. 2018, 2, 1027. [Google Scholar]
- Stewart, L.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.P.; McFarlin, B.K.; Timmerman, K.L.; Coen, P.M.; Felker, J.; Talbert, E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav. Immun. 2005, 19, 389–397. [Google Scholar] [CrossRef]
- Mury, P.; Mura, M.; Della-Schiava, N.; Chanon, S.; Vieille-Marchiset, A.; Nicaise, V.; Chirico, E.N.; Collet-Benzaquen, D.; Lermusiaux, P.; Connes, P.; et al. Association between physical activity and sedentary behaviour on carotid atherosclerotic plaques: An epidemiological and histological study in 90 asymptomatic patients. Br. J. Sports Med. 2019, 54, 469–474. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA 1993, 269, 2386. [Google Scholar] [CrossRef] [PubMed]
- Gusto, G.; Vol, S.; Bedouet, M.; Leglu, C.; Decou, P.; Beslin, E.; Guillaud, C.; Copin, N.; Lantieri, O.; Tichet, J. Good reproducibility and validity of a self-administered questionnaire evaluating adherence to the French national nutrition and health program recommendations. Press Med. 2013, 42, e245–e258. [Google Scholar] [CrossRef] [PubMed]
- England, C.Y.; Andrews, R.C.; Jago, R.; Thompson, J.L. A systematic review of brief dietary questionnaires suitable for clinical use in the prevention and management of obesity, cardiovascular disease and type 2 diabetes. Eur. J. Clin. Nutr. 2015, 69, 977–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, D.E.; Norman, G.; Wagner, N.; Patrick, K.; Calfas, K.J.; Sallis, J.F. Reliability and Validity of the Sedentary Behavior Questionnaire (SBQ) for Adults. J. Phys. Act. Health 2010, 7, 697–705. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global Physical Activity Questionnaire (GPAQ): Nine Country Reliability and Validity Study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Pialoux, V.; Mounier, R.; Ponsot, E.; Rock, E.; Mazur, A.; Dufour, S.P.; Richard, R.L.; Richalet, J.-P.; Coudert, J.D.; Fellmann, N. Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur. J. Clin. Nutr. 2006, 60, 1345–1354. [Google Scholar] [CrossRef] [Green Version]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggio, R.; Gutierrez, L.; Irazola, V.; Rubinstein, A.L.; Danaei, G. Preventable Ischaemic Heart Disease and Stroke Deaths Attributable to Insufficient Physical Activity: A Comparative Risk Assessment Analysis in the Argentinian Population. Int. J. Sports Exerc. Med. 2017, 3, 056. [Google Scholar] [CrossRef] [Green Version]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; De Gonzalez, A.B.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure Time Physical Activity and Mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Raftery, A.E.; Lalic, N.; Gerland, P.; Li, N.; Heilig, G. Joint probabilistic projection of female and male life expectancy. Demogr. Res. 2014, 30, 795–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Naylor, A.R. Time to rethink management strategies in asymptomatic carotid artery disease. Nat. Rev. Cardiol. 2011, 9, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Kim, K.-W.; Ivanov, S.; Williams, J.W. Monocyte Recruitment, Specification, and Function in Atherosclerosis. Cells 2020, 10, 15. [Google Scholar] [CrossRef]
- Singh, U.; Jialal, I. Oxidative stress and atherosclerosis. Pathophysiology 2006, 13, 129–142. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, C.; Shantsila, E.; Hristov, M.; Caligiuri, G.; Guzik, T.; Heine, G.H.; Lip, G.Y. Role and analysis of monocyte subsets in cardiovascular disease joint consensus document of the european society of cardiology (Esc) working groups “atherosclerosis & vascular biology” and “thrombosis”. Thromb. Haemost. 2016, 116, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.K.; Dykes, R.; Douglas, J.E.; Krishnaswamy, G.; Berk, S. Long-term Exercise and Atherogenic Activity of Blood Mononuclear Cells in Persons at Risk of Developing Ischemic Heart Disease. JAMA 1999, 281, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Schrijvers, D.; De Meyer, G. Necrotic cell death in atherosclerosis. Basic Res. Cardiol. 2011, 106, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, V.; Brown, A.D.; Leigh, R.; Friedenreich, C.M.; Poulin, M.J. Effect of Cardiorespiratory Fitness on Vascular Regulation and Oxidative Stress in Postmenopausal Women. Hypertension 2009, 54, 1014–1020. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients With Cardiovascular Disease—Is It Safe? A Systematic Review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef] [Green Version]
- Bzowska, M.; Nogieć, A.; Skrzeczyńska-Moncznik, J.; Mickowska, B.; Guzik, K.; Pryjma, J. Oxidized LDLs Inhibit TLR-induced IL-10 Production by Monocytes: A New Aspect of Pathogen-Accelerated Atherosclerosis. Inflammation 2012, 35, 1567–1584. [Google Scholar] [CrossRef] [Green Version]
- Cathcart, M.K. Regulation of Superoxide Anion Production by NADPH Oxidase in Monocytes/Macrophages. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, Y.; Lagache, S.M.M.; Schnack, L.; Godfrey, R.; Kahles, F.; Bruemmer, D.; Waltenberger, J.; Findeisen, H.M. mTOR-Dependent Oxidative Stress Regulates oxLDL-Induced Trained Innate Immunity in Human Monocytes. Front. Immunol. 2019, 9, 3155. [Google Scholar] [CrossRef] [Green Version]
- Kasapis, C.; Thompson, P.D. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers: A Systematic Review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuan, T.-C.; Hsu, T.-G.; Fong, M.-C.; Hsu, C.-F.; Tsai, K.K.C.; Lee, C.-Y.; Kong, C.-W. Deleterious effects of short-term, high-intensity exercise on immune function: Evidence from leucocyte mitochondrial alterations and apoptosis. Br. J. Sports Med. 2008, 42, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Tedgui, A.; Mallat, Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef] [PubMed]
| Healthy Control Group (n = 14) | All Patients (n = 29) | Group 1 (n = 10) | Group 2 (n = 9) | Group 3 (n = 7) | MPA (n = 10) | IPA (n = 6) | |
|---|---|---|---|---|---|---|---|
| Antioxidant enzymes activity | |||||||
| SOD (µmol/mL/min) | 34.6 ± 6.2 | 32.8 ± 6.9 | 31.0 ± 5.4 | 33.3 ± 7.3 | 34.2 ± 8.4 | ||
| 36.9 ± 10.7 | 36.1 ± 13.1 | 31.1 ± 9.8 | |||||
| CAT (µmol/mL/min) | 1.7 ± 0.5 | 0.9 ± 0.4 **** | 0.9 ± 0.5 µµ | 1.1 ± 0.2 µ | 0.7 ± 0.3 µµµ | ||
| 1.8 ± 0.8 | 0.9 ± 0.5 ¤¤¤ | 0.9 ± 0.9 ¤¤ | |||||
| GPX (µmol/mL/min) | 7.6 ± 1.3 | 7.7 ± 1.6 | 7.8 ± 1.5 | 7.5 ± 0.6 | 7.3 ± 1.6 | ||
| 7.4 ± 1.7 | 7.7 ± 1.2 | 7.4 ± 2.1 | |||||
| Oxidative damage | |||||||
| MDA (µmol/mL) | 46.0 ± 8.7 | 59.0 ± 9.5 *** | 59.2 ± 10.8 | 60.7 ± 9.2 µ | 64.0 ± 21.2 µµ | ||
| 47.0 ± 8.7 | 59.4 ± 16.6 ¤ | 65.5 ± 37.3 ¤¤ | |||||
| AOPP (µmol/mL) | 91.1 ± 34.6 | 165.6 ± 54.9 *** | 185.0 ± 55.6 µµµ | 182.8 ± 32.3 µµµ | 128.6 ± 56.2 | ||
| 95.7 ± 68.5 | 161.8 ± 117.4 ¤¤ | 150.6 ± 82.1 |
| Group 1 (n = 10) | Group 2 (n = 9) | Group 3 (n = 7) | p- Value | |
|---|---|---|---|---|
| Age (years old) | 70.1 ± 6.8 | 71.6 ± 10.0 | 70.4 ± 12.6 | ns |
| BMI | 24.3 ± 3.2 | 26.6 ± 2.7 | 24.6 ± 4.3 | ns |
| Asymptomatic/Symptomatic | 5/7 | 5/4 | 5/3 | ns |
| Stenosis (%) | 73.4 ± 14.5 | 77.3 ± 7.6 | 67.9 ± 12.5 | ns |
| Type 2 diabetes (n) | 2 | 2 | 3 | ns |
| Dyslipidaemia (n) | 2 | 4 | 3 | ns |
| Hypertension (n) | 5 | 8 | 6 | ns |
| Statin use (n) | 7 | 8 | 5 | ns |
| Anti-agregant use (n) | 8 | 8 | 5 | ns |
| Intense PA practice (min/week) | 0 ± 0 | 80.0 ± 158.8 | 120.8 ± 149.0 | p < 0.01 |
| Sedentary behaviour (min/week) | 525.7 ± 144.0 | 494.2 ± 161.8 | 485.4 ± 129.0 | ns |
| Smoking habit (pack-year) | 20.7 ± 30.7 | 33.1 ± 34.6 | 29.7 ± 24.0 | ns |
| Nutrition score (UA) | 1.5 ± 0.4 | 1.3 ± 0.2 | 1.3 ± 0.3 | ns |
| Leucocytes (×109) | 8.9 ± 3.5 | 7.7 ± 2.1 | 7.5 ± 2.4 | ns |
| Monocytes (×109) | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.3 | ns |
| Neutrophils (×109) | 5.6 ± 2.7 | 5.1 ± 1.6 | 4.6 ± 1.6 | ns |
| Lymphocytes (×109) | 2.2 ± 0.8 | 1.7 ± 0.6 | 1.9 ± 0.8 | ns |
| Fibrinogen (g/L) | 3.6 ± 0.9 | 4.0 ± 0.9 | 3.7 ± 0.8 | ns |
| Total cholestérol (mmol/L) | 5.7 ± 1.5 | 4.4 ± 1.7 | 4.0 ± 1.1 | ns |
| HDL cholesterol (mmol/L) | 1.1 ± 0.3 | 0.9 ± 0.3 | 1.1 ± 0.3 | ns |
| LDL cholesterol (mmol/L) | 3.9 ± 1.2 | 2.7 ± 1.2 | 2.3 ± 0.9 | ns |
| Triglycerides (mmol/L) | 1.5 ± 1.0 | 1.8 ± 0.8 | 1.4 ± 0.4 | ns |
| Moderate PA Group (n = 10) | Intense PA Group (n = 6) | p-Value | |
|---|---|---|---|
| Age (years old) | 75.4 ± 10.6 | 71.5 ± 5.7 | ns |
| BMI | 26.0 ± 3.3 | 24.4 ± 2.9 | ns |
| Asymptomatic/Symptomatic | 6/4 | 3/2 | ns |
| Stenosis (%) | 75.2 ± 10.5 | 68.6 ± 11.2 | ns |
| Type 2 diabetes (n) | 3 | 1 | ns |
| Dyslipidaemia (n) | 3 | 2 | ns |
| Hypertension (n) | 7 | 4 | ns |
| Statin use (n) | 9 | 4 | ns |
| Anti-platelets use (n) | 9 | 3 | ns |
| Intense PA practice (min/week) | 1.8 ± 6.0 | 285.0 ± 113.2 | p < 0.01 |
| Sedentary behaviour (min/week) | 485.3 ± 159.4 | 468.0 ± 113.9 | ns |
| Smoking habit (pack-year) | 28.6 ± 35.28 | 31.6 ± 22.2 | ns |
| Nutrition score (UA) | 1.3 ± 0.2 | 1.3 ± 0.1 | ns |
| Leucocytes (×109) | 7.7 ± 1.9 | 7.8 ± 2.9 | ns |
| Monocytes (×109) | 0.6 ± 0.2 | 0.8 ± 0.3 | ns |
| Neutrophils (×109) | 5.0 ± 1.4 | 4.9 ± 2.0 | ns |
| Lymphocytes (×109) | 1.9 ± 0.7 | 1.8 ± 0.5 | ns |
| Fibrinogen (g/L) | 1.9 ± 0.7 | 1.8 ± 0.5 | ns |
| Total cholestérol (mmol/L) | 4.4 ± 1.5 | 3.8 ± 1.0 | ns |
| HDL cholesterol (mmol/L) | 1.0 ± 0.3 | 0.9 ± 0.3 | ns |
| LDL cholesterol (mmol/L) | 2.6 ± 1.2 | 2.2 ± 0.7 | ns |
| Triglycerides (mmol/L) | 1.6 ± 0.7 | 1.5 ± 0.6 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mura, M.; Weiss-Gayet, M.; Della-Schiava, N.; Chirico, E.; Lermusiaux, P.; Chambion-Diaz, M.; Faes, C.; Boreau, A.; Chazaud, B.; Millon, A.; et al. Monocyte Phenotypes and Physical Activity in Patients with Carotid Atherosclerosis. Antioxidants 2022, 11, 1529. https://doi.org/10.3390/antiox11081529
Mura M, Weiss-Gayet M, Della-Schiava N, Chirico E, Lermusiaux P, Chambion-Diaz M, Faes C, Boreau A, Chazaud B, Millon A, et al. Monocyte Phenotypes and Physical Activity in Patients with Carotid Atherosclerosis. Antioxidants. 2022; 11(8):1529. https://doi.org/10.3390/antiox11081529
Chicago/Turabian StyleMura, Mathilde, Michèle Weiss-Gayet, Nellie Della-Schiava, Erica Chirico, Patrick Lermusiaux, Marie Chambion-Diaz, Camille Faes, Anaelle Boreau, Bénédicte Chazaud, Antoine Millon, and et al. 2022. "Monocyte Phenotypes and Physical Activity in Patients with Carotid Atherosclerosis" Antioxidants 11, no. 8: 1529. https://doi.org/10.3390/antiox11081529
APA StyleMura, M., Weiss-Gayet, M., Della-Schiava, N., Chirico, E., Lermusiaux, P., Chambion-Diaz, M., Faes, C., Boreau, A., Chazaud, B., Millon, A., & Pialoux, V. (2022). Monocyte Phenotypes and Physical Activity in Patients with Carotid Atherosclerosis. Antioxidants, 11(8), 1529. https://doi.org/10.3390/antiox11081529