Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Interventional Study Design
2.3. Saliva Sampling and Hormone Analysis
2.4. Oxidative Stress Markers
2.5. Statistical Analyses
3. Results
3.1. Baseline Data
3.2. Comparison of Salivary Hormone Concentrations in Patients and Healthy Subjects
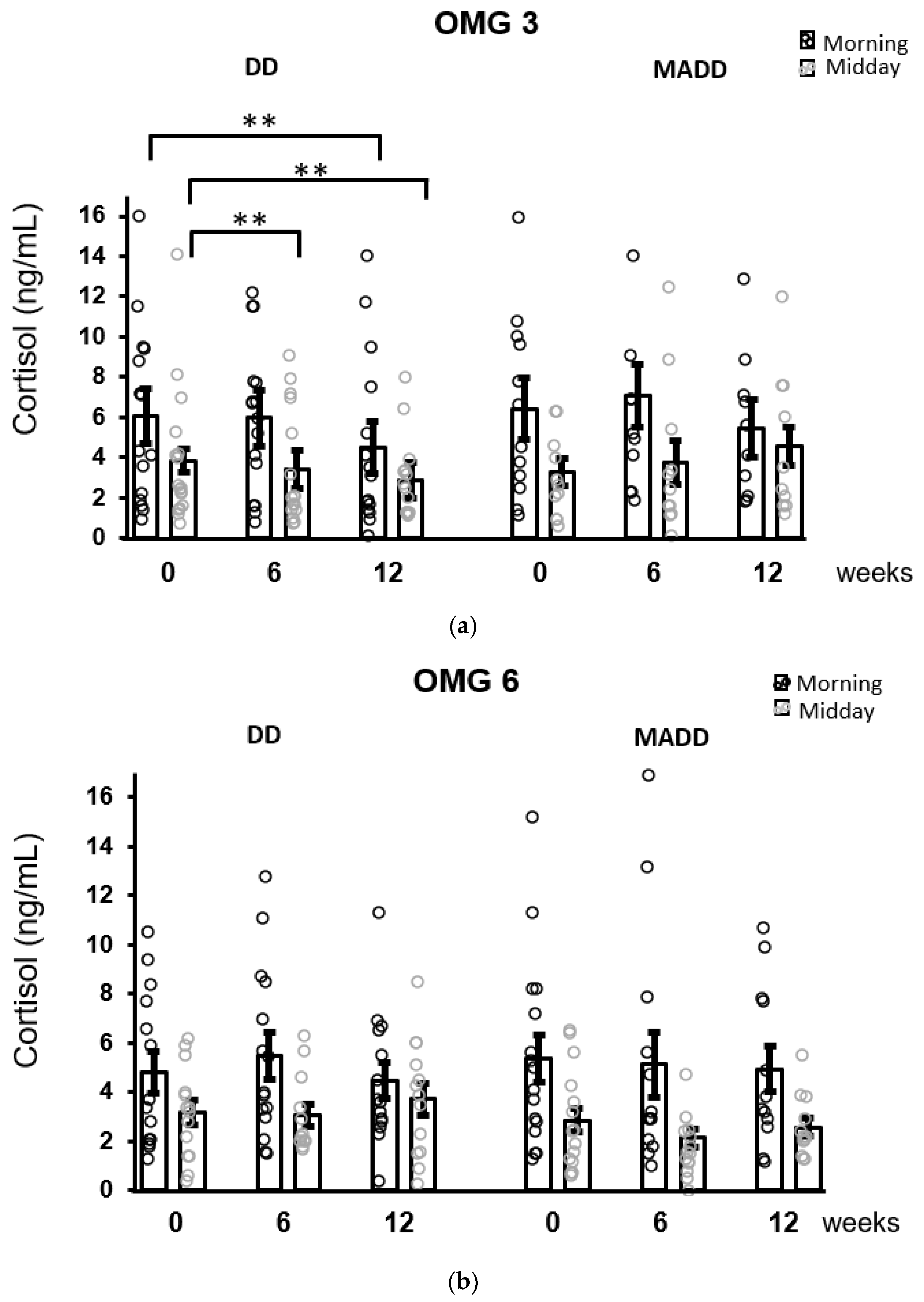
3.3. Effect of Fatty Acids Supplementation in Patients with Depression
3.4. Correlations between Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selye, H. A Syndrome produced by Diverse Nocuous Agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Jezova, D.; Jurankova, E.; Mosnarova, A.; Kriska, M.; Skultétyová, I. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol. Exp. 1996, 56, 779–785. [Google Scholar]
- Jezova, D.; Herman, J.P. Stress and stress-related disease states as topics of multi-approach research. Stress 2020, 23, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Romanova, Z.; Hlavacova, N.; Jezova, D. Psychotropic Drug Effects on Steroid Stress Hormone Release and Possible Mechanisms Involved. Int. J. Mol. Sci. 2022, 23, 908. [Google Scholar] [CrossRef]
- Gospodaryov, D.V.; Lushchak, V. Oxidative stress: Cause and consequence of diseases. In Oxidative Stress and Diseases 2012, 1st ed.; Lushchak, V., Ed.; InTech: Rijeka, Croatia, 2012; Volume 1, pp. 13–38. [Google Scholar]
- Vavakova, M.; Durackova, Z.; Trebaticka, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Jezova, D.; Hlavacova, N. Endocrine factors in stress and psychiatric disorders: Focus on anxiety and salivary steroids. Ann. N. Y. Acad. Sci. 2008, 1148, 495–503. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Maripuu, M.; Wikgren, M.; Karling, P.; Adolfsson, R.; Norrback, K.F. Relative hypo- and hypercortisolism are both associated with depression and lower quality of life in bipolar disorder: A cross-sectional study. PLoS ONE 2014, 9, e98682. [Google Scholar] [CrossRef]
- Izakova, L.; Hlavacova, N.; Segeda, V.; Kapsdorfer, D.; Morovicsova, E.; Jezova, D. Salivary aldosterone, cortisol and their morning to evening slopes in patients with depressive disorder and healthy subjects: Acute episode and follow up six months after reaching remission. Neuroendocrinology 2020, 110, 1001–1009. [Google Scholar] [CrossRef]
- Zajkowska, Z.; Gullett, N.; Walsh, A.; Zonca, V.; Pedersen, G.A.; Souza, L.; Kieling, C.; Fisher, H.L.; Kohrt, B.A.; Mondelli, V. Cortisol and development of depression in adolescence and young adulthood—A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 136, 205625. [Google Scholar] [CrossRef]
- Khoury, J.E.; Jamieson, B.; Gonzalez, A.; Atkinson, L. Child depressive symptoms: Associations with salivary cortisol and alpha amylase in two distinct challenges. Biol. Psychol. 2020, 149, 107808. [Google Scholar] [CrossRef]
- Jezova, D.; Trebaticka, J.; Buzgoova, K.; Durackova, Z.; Hlavacova, N. Lower activity of salivary alpha-amylase in youths with depression. Stress 2020, 23, 688–693. [Google Scholar] [CrossRef]
- Peters, K.Z.; Zlebnik, N.E.; Cheer, J.F. Cannabis exposure during adolescence: A uniquely sensitive period for neurobiological effects. Int. Rev. Neurobiol. 2022, 161, 95–120. [Google Scholar]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhe, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef]
- Trebaticka, J.; Hradecna, Z.; Surovcova, A.; Katrencikova, B.; Gushina, I.; Waczulikova, I.; Susienkova, K.; Garaiova, I.; Suba, J.; Durackova, Z. Omega-3 fatty-acids modulate symptoms of depressive disorder, serum levels of omega-3 fatty acids and omega-6/omega-3 ratio in children. A randomized, double-blind and controlled trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef]
- Katrencikova, B.; Vavakova, M.; Paduchova, Z.; Nagyova, Z.; Garaiova, I.; Muchova, J.; Durackova, Z.; Trebaticka, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef]
- Katrencikova, B.; Vavakova, M.; Waczulikova, I.; Oravec, S.; Garaiova, I.; Nagyova, Z.; Hlavacova, N.; Durackova, Z.; Trebaticka, J. Lipid profile, lipoprotein subfractions, and fluidity of membranes in children and adolescents with depressive disorder: Effect of omega-3 fatty acids in a double-blind randomized controlled study. Biomolecules 2020, 10, 1427. [Google Scholar] [CrossRef]
- Paduchova, Z.; Katrencikova, B.; Vavakova, M.; Laubertova, L.; Nagyova, Z.; Garaiova, I.; Durackova, Z.; Trebaticka, J. The effect of omega-3 fatty acids on thromboxane, brain-derived neurotrophic factor, homocysteine, and vitamin D in depressive children and adolescents: Randomized controlled trial. Nutrients 2021, 13, 1095. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Henricus, G.R.; Assies, J.; Lok, A.; Koeter, M.W.J.; Visser, L.; Bockting, C.L.H.; Schene, A.H. Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology 2013, 38, 1607–1617. [Google Scholar] [CrossRef]
- Thesing, C.S.; Bot, M.; Milaneschi, Y.; Giltay, E.J.; Penninx, B.W.J.H. Omega-3 polyunsaturated fatty acid levels and dysregulations in biological stress systems. Psychoneuroendocrinology 2018, 97, 206–215. [Google Scholar] [CrossRef]
- Song, C.; Phillips, A.G.; Leonard, B.E.; Horrobin, D.E. Ethyl-eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of interleukin-1beta in rats. Mol. Psychiatry 2004, 9, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Delarue, J.; Matzinger, O.; Binnert, C.; Schneiter, P.; Chiolero, R.; Tappy, L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003, 29, 289–295. [Google Scholar] [CrossRef]
- Jazayeri, S.; Keshavarz, S.A.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Amini, H.; Djazayery, A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010, 178, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Teisen, M.N.; Vuholm, S.; Rantanen, J.M.; Christensen, J.H.; Damsgaard, C.T.; Lauritzen, L. Exploring the effects of oily fish consumption on measures of acute and long-term stress in healthy 8–9-year-old children: The FiSK Junior randomised trial. Br. J. Nutr. 2020, 126, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kapsdorfer, D.; Hlavacova, N.; Vondrova, D.; Argalasova, L.; Sevcikova, L.; Jezova, D. Neuroendocrine Response to School Load in Prepubertal Children: Focus on Trait Anxiety. Cell. Mol. Neurobiol. 2018, 38, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Segeda, V.; Izakova, L.; Hlavacova, N.; Bednarova, A.; Jezova, D. Aldosterone concentrations in saliva reflect the duration and severity of depressive episode in a sex dependent manner. J. Psychiatr. Res. 2017, 91, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhang, Q. Biological Rhythms Advance in Depressive Disorder. Adv. Exp. Med. Biol. 2019, 1180, 117–133. [Google Scholar]
- Van den Bergh, B.R.H.; Van Calster, B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology 2009, 34, 791–794. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]





| Cortisol and | n | r | p | Aldosteron and | n | r | p |
|---|---|---|---|---|---|---|---|
| CDI | 57 | 0.301 | 0.015 | CDI | 48 | −0.091 | 0.269 |
| LP | 52 | 0.295 | 0.017 | LP | 43 | 0.055 | 0.363 |
| 8-IsoP | 56 | 0.179 | 0.093 | 8-IsoP | 47 | 0.342 | 0.019 |
| NT | 55 | −0.198 | 0.074 | NT | 45 | −0.06 | 0.692 |
| AOPP | 59 | 0.027 | 0.055 | AOPP | 48 | 0.022 | 0.441 |
| TEAC | 59 | 0.004 | 0.486 | TEAC | 49 | −0.027 | 0.425 |
| SOD | 58 | −0.182 | 0.086 | SOD | 49 | 0.135 | 0.177 |
| GPx | 59 | 0.101 | 0.222 | GPx | 49 | −0.118 | 0.416 |
| CAT | 59 | −0.061 | 0.323 | CAT | 48 | 0.012 | 0.935 |
| TXB | 55 | −0.109 | 0.215 | TXB | 44 | −0.057 | 0.711 |
| HCy | 58 | −0.007 | 0.479 | HCy | 47 | −0.001 | 0.497 |
| BDNF | 52 | −0.067 | 0.319 | BDNF | 41 | 0.182 | 0.253 |
| DHA | 55 | −0.197 | 0.075 | DHA | 47 | 0.163 | 0.137 |
| EPA | 55 | −0.164 | 0.115 | EPA | 47 | 0.24 | 0.052 |
| Patients accordig to diagnosis | |||||||
| Cortisol and | n | r | p | Aldosteron and | n | r | p |
| CDI-DD | 33 | 0.265 | 0.068 | CDI-DD | 25 | −0.091 | 0.269 |
| CDI-MADD | 24 | 0.33 | 0.057 | CDI-MADD | 23 | −0.175 | 0.212 |
| LP-DD | 28 | 0.173 | 0.188 | 8-IsoP-DD | 25 | 0.222 | 0.284 |
| LP-MADD | 24 | 0.468 | 0.011 | 8-IsoP-MADD | 22 | 0.516 | 0.015 |
| NT-DD | 32 | 0.101 | 0.291 | GPx-DD | 25 | −0.006 | 0.978 |
| NT-MADD | 23 | −0.591 | 0.002 | GPx-MADD | 23 | −0.499 | 0.016 |
| DHA-DD | 31 | −0.225 | 0.111 | ||||
| DHA-MADD | 23 | −0.815 | 0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oravcova, H.; Katrencikova, B.; Garaiova, I.; Durackova, Z.; Trebaticka, J.; Jezova, D. Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression. Antioxidants 2022, 11, 1546. https://doi.org/10.3390/antiox11081546
Oravcova H, Katrencikova B, Garaiova I, Durackova Z, Trebaticka J, Jezova D. Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression. Antioxidants. 2022; 11(8):1546. https://doi.org/10.3390/antiox11081546
Chicago/Turabian StyleOravcova, Henrieta, Barbora Katrencikova, Iveta Garaiova, Zdenka Durackova, Jana Trebaticka, and Daniela Jezova. 2022. "Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression" Antioxidants 11, no. 8: 1546. https://doi.org/10.3390/antiox11081546
APA StyleOravcova, H., Katrencikova, B., Garaiova, I., Durackova, Z., Trebaticka, J., & Jezova, D. (2022). Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression. Antioxidants, 11(8), 1546. https://doi.org/10.3390/antiox11081546









