Antioxidant Potential of Non-Extractable Fractions of Dried Persimmon (Diospyros kaki Thunb.) in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Plant Material
2.3. Preparation of NEP
2.4. Preparation of Persimmon-Derived Tannin
2.5. Fractionation of Hydrolyzed NEP and Persimmon-Derived Tannin
2.6. HPLC Analysis of Hydrolyzed NEP and Persimmon-Derived Tannin
2.7. Animal Studies
2.7.1. Animal and Feeding Procedures
2.7.2. ORAC Assay of Plasma
2.7.3. ORAC Assay of Liver Tissues
2.7.4. SOD Activity Assay
2.7.5. Catalase Activity Assay
2.7.6. GPx Activity Assay
2.7.7. Measurement of Total Glutathione and GSSG Concentrations
2.7.8. Measurement of Protein Concentrations in Plasma and Liver Tissues
2.7.9. Measurement of Cross-Sectional Area (CSA)
2.8. Statistical Analysis
3. Results
3.1. HPLC Analysis of Hydrolyzed NEP and Persimmon-Derived Tannin
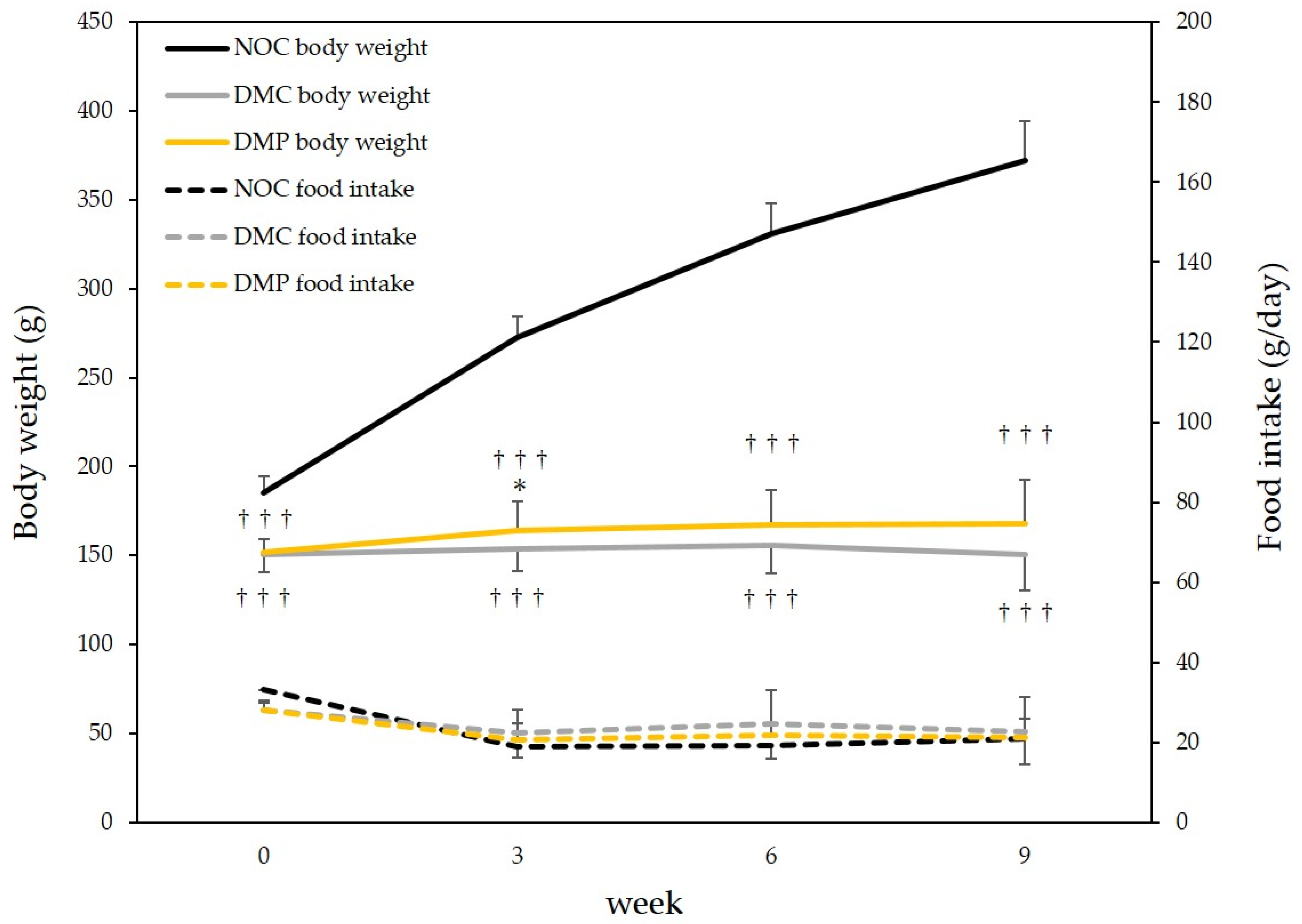
3.2. Food Intake and Body Weight Gain in Rats
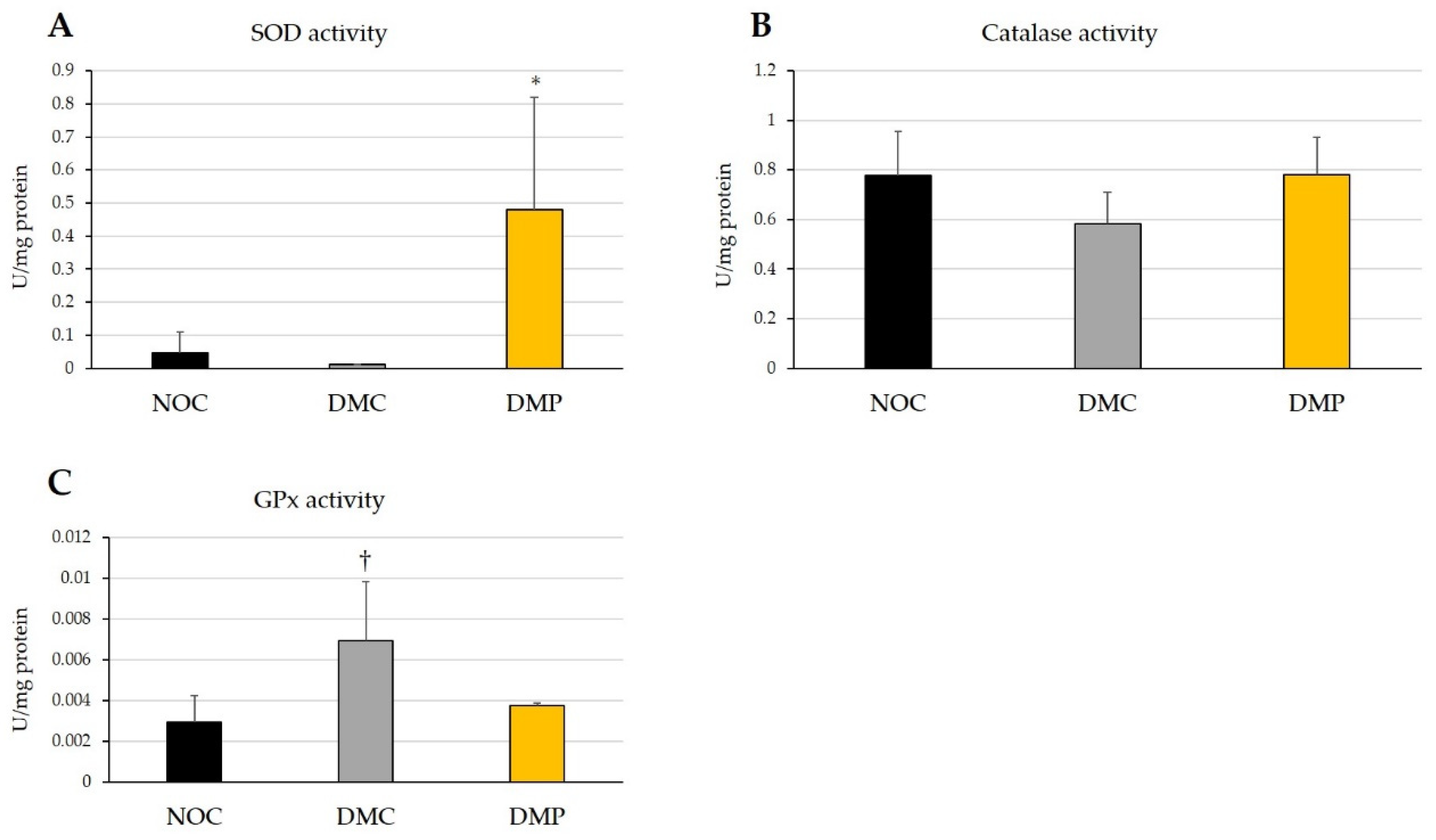
3.3. Effects of NEP on Antioxidant Activity in Plasma and Liver Tissues
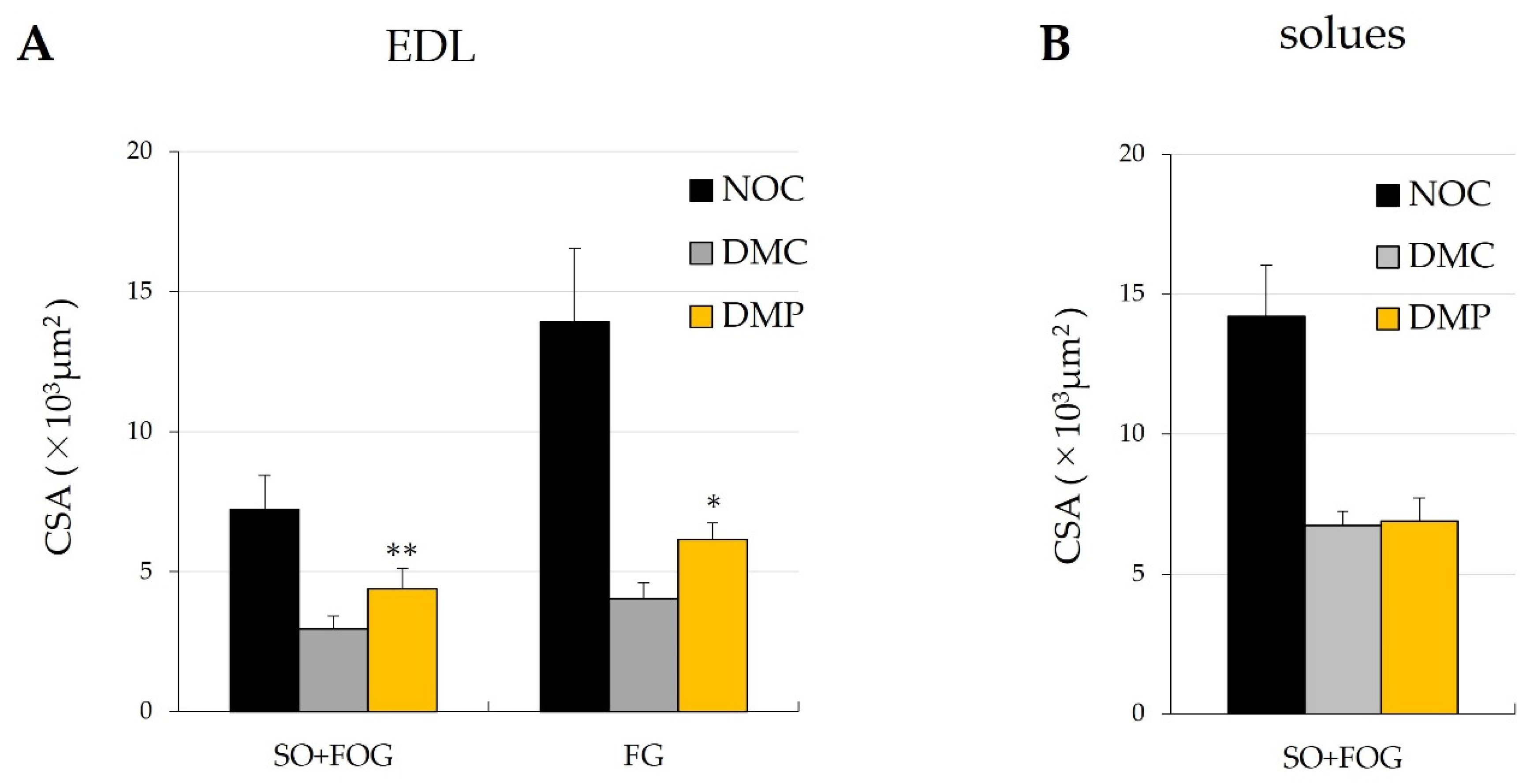
3.4. Effects of NEP on the Characterization of EDL and Soleus Muscle Fibers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michels, B.K.; Giovannucci, E.; Joshipura, J.K.; Rosner, A.B.; Stampfer, J.M.; Fuchs, S.C.; Colditz, A.G.; Speizer, E.F.; Willett, C.W. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J. Natl. Cancer Inst. 2000, 92, 1740–1752. [Google Scholar] [CrossRef] [Green Version]
- Nöthlings, U.; Schulze, B.M.; Weikert, C.; Boeing, H.; van der Schouw, T.Y.; Bamia, C.; Benetou, V.; Lagiou, P.; Krogh, V.; Beulens, J.W.J.; et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J. Nutr. 2008, 138, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, F.; Wang, L.; Zhang, D. Fruit and vegetables consumption and risk of hypertension: A meta-analysis. J. Clin. Hypertens. 2016, 18, 468–476. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, M.A.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.N.; Fan, J.F.; Yue, X.; Wu, X.R.; Li, L.T. Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. cv. Mopan). J. Food Sci. 2008, 73, C24–C28. [Google Scholar] [CrossRef]
- Dhawefi, N.; Jedidi, S.; Rtibi, K.; Jridi, M.; Sammeri, H.; Abidi, C.; Zouari, N.; Sebai, H. Antidiarrheal, antimicrobial, and antioxidant properties of the aqueous extract of Tunisian persimmon (Diospyros kaki Thunb.) Fruits. J. Med. Food 2021, 24, 1100–1112. [Google Scholar] [CrossRef]
- Liu, C.; Kurakane, S.; Takita, J.; Itano, R.; Soga, T.; Oikawa, A.; Igarashi, K. Antihypertensive effects of unripe persimmon (Diospyros kaki L. cv. Hiratanenashi) fruit and it component in spontaneously hypertensive rats. Food Sci. Technol. Res. 2012, 18, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Kawase, M.; Motohashi, N.; Satoh, K.; Sakagami, H.; Nakashima, H.; Tani, S.; Shirataki, Y.; Kurihara, T.; Spengler, G.; Wolfard, K.; et al. Biological activity of persimmon (Diospyros kaki) peel extracts. Phytother. Res. 2013, 17, 495–500. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yokoyama, S.; Gato, N. Bile acid-binding activity of young persimmon (Diospyros kaki) fruit and its hypolipidemic effect in mice. Phytother. Res. 2010, 24, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kadowaki, A.; Ozaki, N.; Takenaka, M.; Ono, H.; Yokoyama, S.; Gato, N. Bile acid-binding ability of kaki-tannin from young fruits of persimmon (Diospyros kaki) in vitro and in vivo. Phytother. Res. 2011, 25, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542–561. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, C.N.J.; Mbanta, C.J.; et al. IDF diabetes atlas: Grobal, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Wolff, S. Diabetes mellitus and free radicals: Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br. Med. Bull. 1993, 49, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Kiritoshi, S.; Nishikawa, T.; Sonoda, K.; Kukidome, D.; Senokuchi, T.; Matsuo, T.; Matsumura, T.; Tokunaga, H.; Brownlee, M.; Araki, E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes 2003, 52, 2570–2577. [Google Scholar] [CrossRef]
- Baynes, W.J. Chemical modification of proteins by lipids in diabetes. Clin. Chem. Lab. Med. 2003, 41, 1159–1165. [Google Scholar] [CrossRef]
- Yang, H.; Jin, X.; Lam, K.W.C.; Yan, K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31–38. [Google Scholar] [CrossRef]
- Tsubouch, H.; Ohsige, A.; Uto, H. Liver and oxidative stress. Kanzo 2015, 56, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Lakshmanan, J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct. 2013, 4, 1148–1184. [Google Scholar] [CrossRef] [PubMed]
- Bakhshaeshi, M.; Khaki, A.; Fathiazad, F.; Khaki, A.A.; Ghadamkheir, E. Anti–oxidative role of quercetin derived from Allium cepa on aldehyde oxidase (OX–LDL) and hepatocytes apoptosis in streptozotocin–induced diabetic rat. Asian Pac. J. Trop. Biomed. 2012, 2, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Oyenihi, O.; Brooks, N.; Oguntibeju, O. Effects of kolaviron on hepatic oxidative stress in streptozotocin induced diabetes. BMC Complement. Altern. Med. 2015, 15, 236. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.; Kitabatake, M.; Ouji-Sageshima, N.; Suzuki, Y.; Nakano, A.; Matsumura, Y.; Nakano, R.; Kasahara, K.; Kubo, k.; Kayano, S.; et al. Pesimmon-derived tannin has antiviral effects and reduces the severity of infection and transmission of SARS-CoV-2 in a Syrian hamster model. Nature 2021, 11, 23965. [Google Scholar]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.; Ito, T. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Nature 2021, 11, 7286. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Kitabatake, M.; Ouji-Sageshima, N.; Yasui, S.; Mochida, N.; Nakano, R.; Kasahara, K.; Tomoda, K.; Yano, H.; Kayano, S.; et al. Persimmon-derived tannin has bacteriostatic and anti-inflammatory activity in a murine model of Mycobacterium avium complex (MAC) disease. PLoS ONE 2017, 12, e0183489. [Google Scholar]
- Hamasaki, S. Quick preparation of kaki-tannin from de-astringent persimmon fruit treated by ethanol. Hortic. Res. 2010, 9, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Verdon, P.C.; Wu, H.B.A.; Wang, H.; Prior, L.R. Automated assay of oxygen radical absorbance capacity with the COBAS FARA Ⅱ. Clin. Chem. 1995, 41, 1738–1744. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, A.J.; Prior, L.R. High-Throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Leite, V.A.; Malta, G.L.; Riccio, F.M.; Eberlin, N.M.; Pastore, M.G.; Marostica Junior, R.M. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarker in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef]
- Malstrom, B.; Andreasson, L.; Reinhammer, B. The Enzymes; Boyer, P., Ed.; XIIB Academic Press: New York, NY, USA, 1975; p. 533. [Google Scholar]
- Fossati, P.; Prencipe, L.; Belti, G. Use of 3,5-dichosro-2hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, N.W. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Baker, A.M.; Cerniglia, J.G.; Zarman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Vandeputte, C.; Guizon, I.; Genestie-Denis, I.; Vannier, B.; Lorenzon, G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: Performance study of a new miniaturized protocol. Cell Biol. Toxicol. 1994, 10, 415–421. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, M. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajam, A.Y.; Rani, R.; Ganie, Y.S.; Sheikh, A.T.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, F.M.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Ayepola, R.O.; Brooks, L.N.; Oguntibeju, O.O. Kolaviron improved resistance to oxidative stress and inflammation in the blood (erythrocyte, serum and plasma) of streptozotocin-induced diabetic rats. Sci. World J. 2014, 921080. [Google Scholar] [CrossRef]
- Ighodaro, M.O.; Akinloye, A.O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, C.; Davis, J. Targeting therapeutics against glutathione depletion in diabetes and its complications. Br. J. Diabetes Vasc. Dis. 2007, 7, 258–265. [Google Scholar] [CrossRef]
- Seven, A.; Güzel, S.; Seymen, O.; Civelek, S.; Bolayırlı, M.; Uncu, M.; Burçak, G. Effects of vitamin E supplementation on oxidative stress in streptozotocin induced diabetic rats: Investigation of liver and plasma. Yonsei Med. J. 2004, 45, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.Y.; Kim, J.Y.; Cho, J.E.; Yokozawa, T. Ameliorative effects of proanthocyanidin on oxidative stress and inflammation in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2007, 55, 9395–9400. [Google Scholar] [CrossRef]
- Godin, V.D.; Wofaieb, A.S.; Garnett, E.M.; Goumeniouk, D.A. Antioxidant enzyme alterations in experimental and clinical diabetes. Mol. Cell. Biochem. 1988, 84, 223–231. [Google Scholar] [CrossRef]
- Wohaieb, A.S.; Godin, V.D. Alterations in free radical tissue-defense mechanisms in streptozotocin-induced diabetes in rat. Diabetes 1987, 36, 1014–1018. [Google Scholar] [CrossRef]
- Ishihara, A.; Itoh, K.; Itoh, M.; Hirohufuji, C. Effect of hypobaric hypoxia on rat soleus muscle fibers and their innervating motoneurons: A review. Jpn. J. Physiol. 2000, 50, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, A.; Yasuda, K.; Tsuda, K. Diabetes and skeletal muscle. J. Jpn. Diabet. Soc. 2008, 51, 459–463. [Google Scholar]
- Andersen, H.; Gjerstad, D.M.; Jakobsen, J. Atrophy of foot muscles. Diabetes Care 2004, 27, 2382–2385. [Google Scholar] [CrossRef] [Green Version]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, A.M.; Morio, B.; Vidal, H.; Rieusset. J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008, 118, 789–800. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | NOC and DMC Groups Control Diet a | DMP Group NEP Diet a |
|---|---|---|
| Corn starch | 39.75 | 39.75 |
| Casein | 20.0 | 20.0 |
| α-Corn starch | 13.2 | 13.2 |
| Sucrose | 10.0 | 10.0 |
| Soybean oil | 7.0 | 7.0 |
| Mineral mix b | 3.5 | 3.5 |
| Vitamin mix c | 1.0 | 1.0 |
| L-cysteine | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 |
| Crystalline cellulose | 5.0 | - |
| NEP | - | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochida, N.; Matsumura, Y.; Kitabatake, M.; Ito, T.; Kayano, S.-i.; Kikuzaki, H. Antioxidant Potential of Non-Extractable Fractions of Dried Persimmon (Diospyros kaki Thunb.) in Streptozotocin-Induced Diabetic Rats. Antioxidants 2022, 11, 1555. https://doi.org/10.3390/antiox11081555
Mochida N, Matsumura Y, Kitabatake M, Ito T, Kayano S-i, Kikuzaki H. Antioxidant Potential of Non-Extractable Fractions of Dried Persimmon (Diospyros kaki Thunb.) in Streptozotocin-Induced Diabetic Rats. Antioxidants. 2022; 11(8):1555. https://doi.org/10.3390/antiox11081555
Chicago/Turabian StyleMochida, Naoko, Yoko Matsumura, Masahiro Kitabatake, Toshihiro Ito, Shin-ichi Kayano, and Hiroe Kikuzaki. 2022. "Antioxidant Potential of Non-Extractable Fractions of Dried Persimmon (Diospyros kaki Thunb.) in Streptozotocin-Induced Diabetic Rats" Antioxidants 11, no. 8: 1555. https://doi.org/10.3390/antiox11081555






