Supplementation with Two New Standardized Tea Extracts Prevents the Development of Hypertension in Mice with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
Commercial Tea Extracts
2.2. Standards Preparation
2.3. High Performance Liquid Chromatography (HPLC)
2.4. Animals
2.5. Measurement of the Mean Arterial Pressure (MAP) in Conscious Mice by the Tail-Cuff System
2.6. Experiments of Heart Perfusion: Langendorff
2.7. Experiments of Vascular Reactivity
2.8. RNA Preparation and Quantitative Real-Time PCR
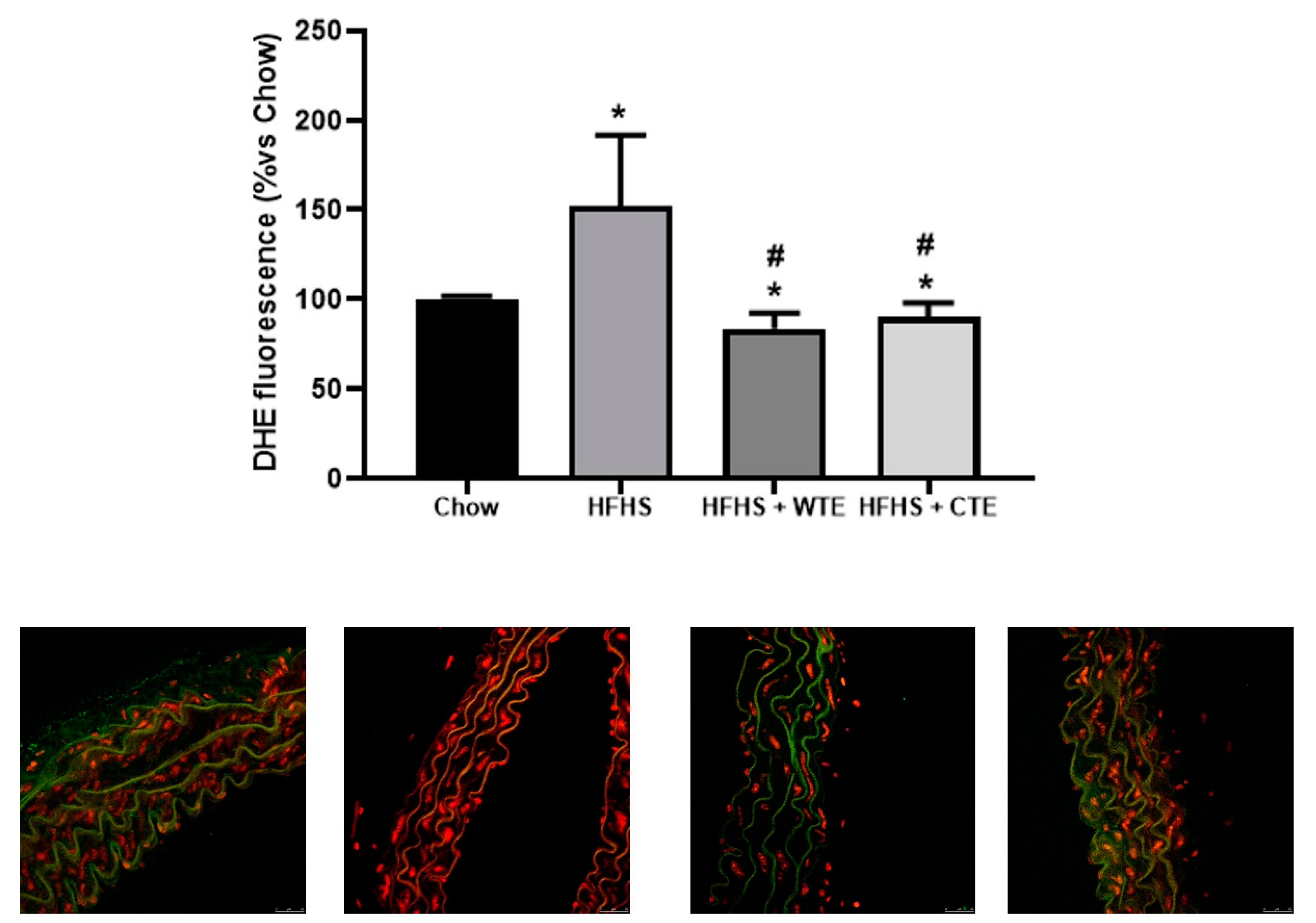
2.9. Vascular superoxide anion production
2.10. Statistical Analysis
3. Results
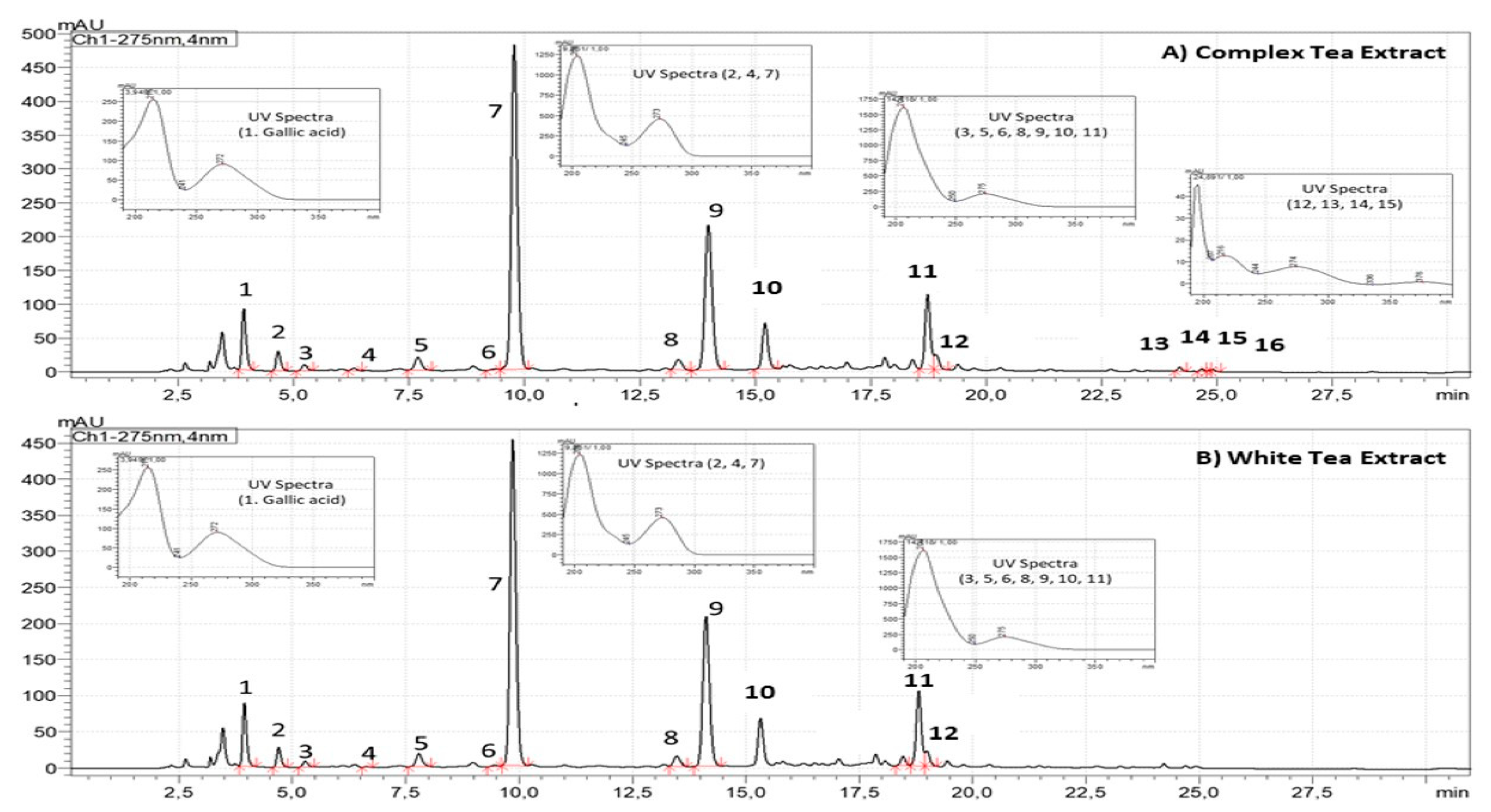
3.1. Chemical Characterization of Tea Extracts by HPLC
3.2. Results from the In Vivo Experiment
3.2.1. Body Weight, Glycaemia and Lipid Profile
3.2.2. Effects of CTE and WTE on Cardiac Function
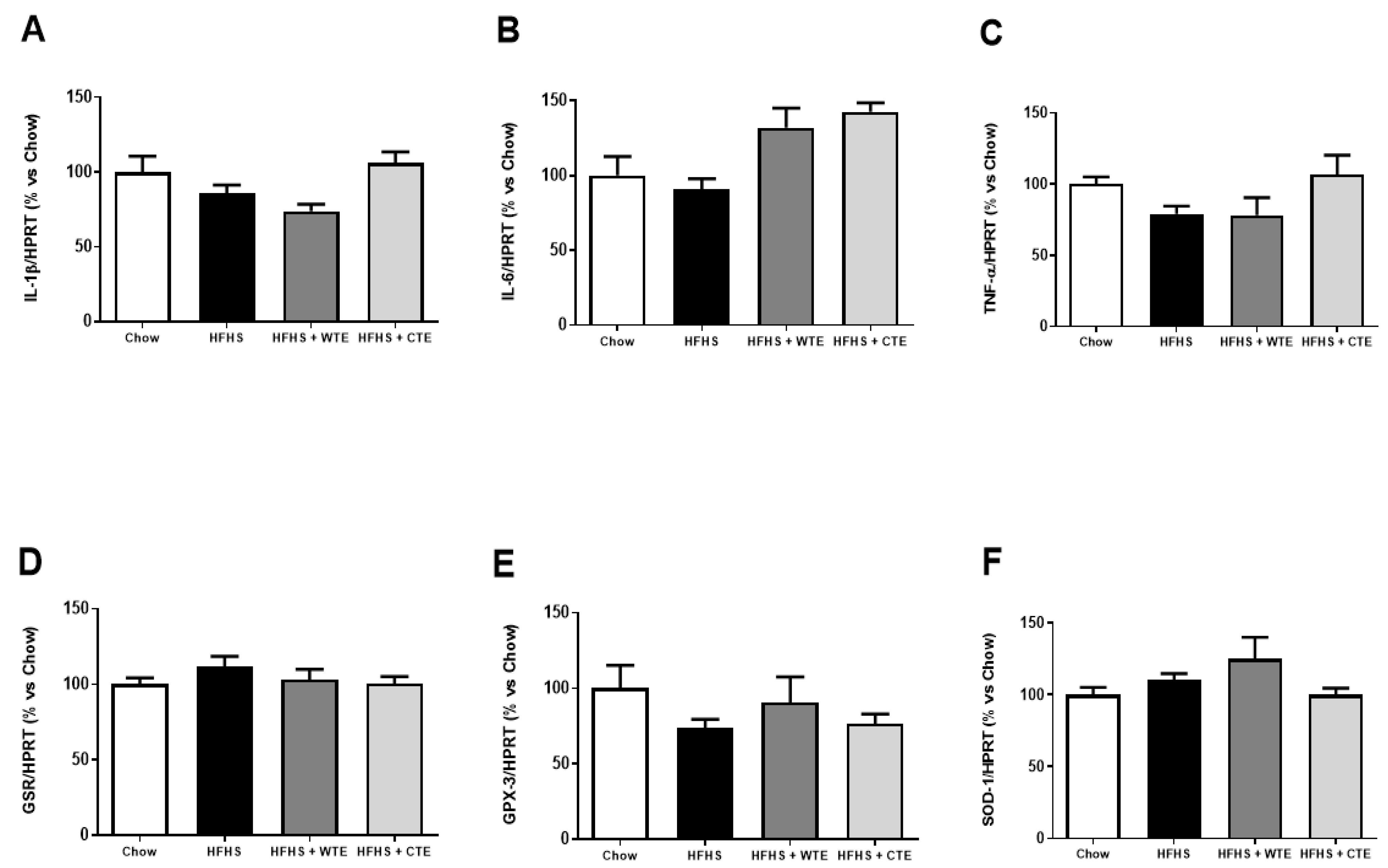
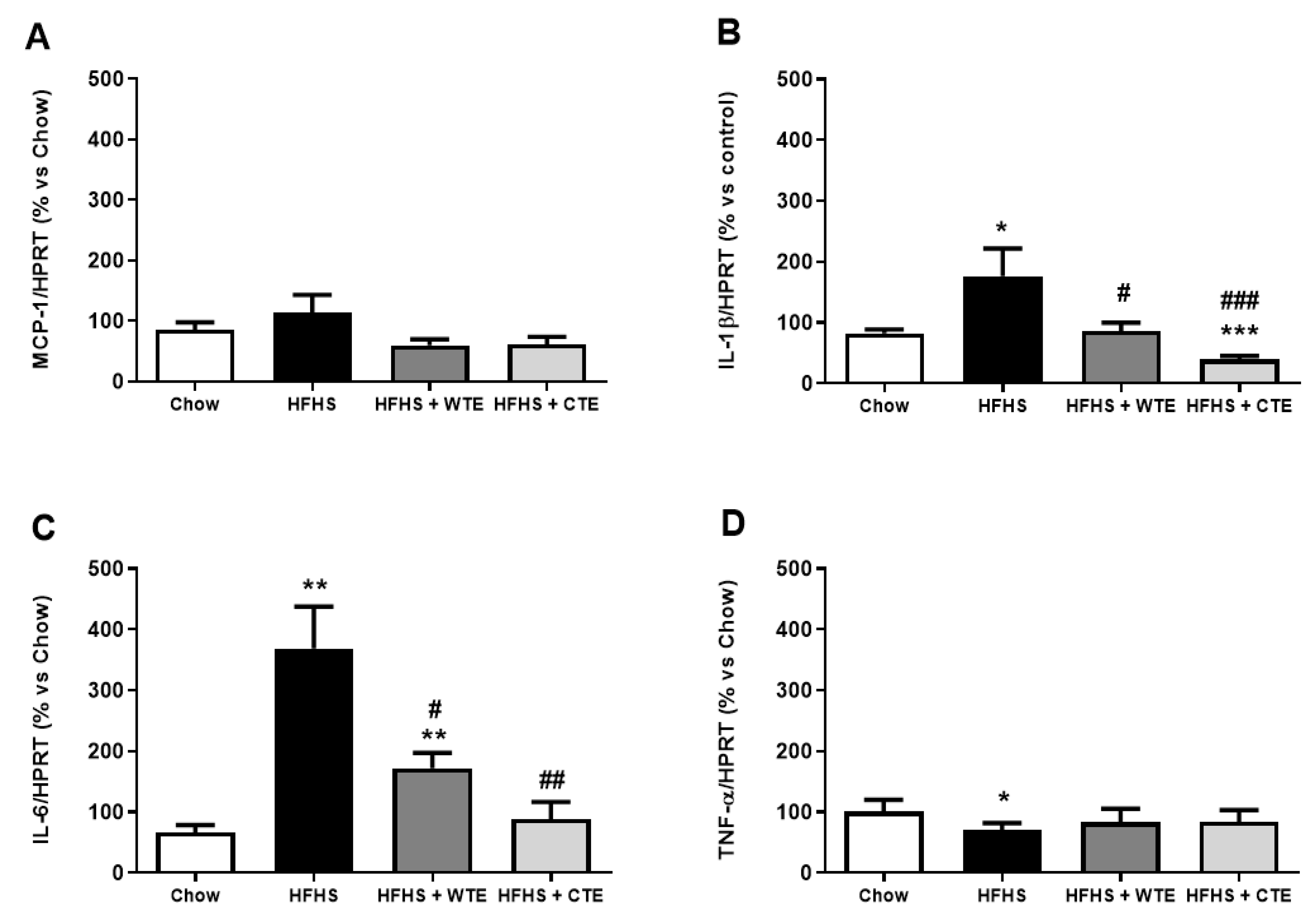
3.2.3. Effects of CTE and WTE on the Gene Expression of Inflammatory and Oxidative Stress Related Markers in Cardiac Tissue
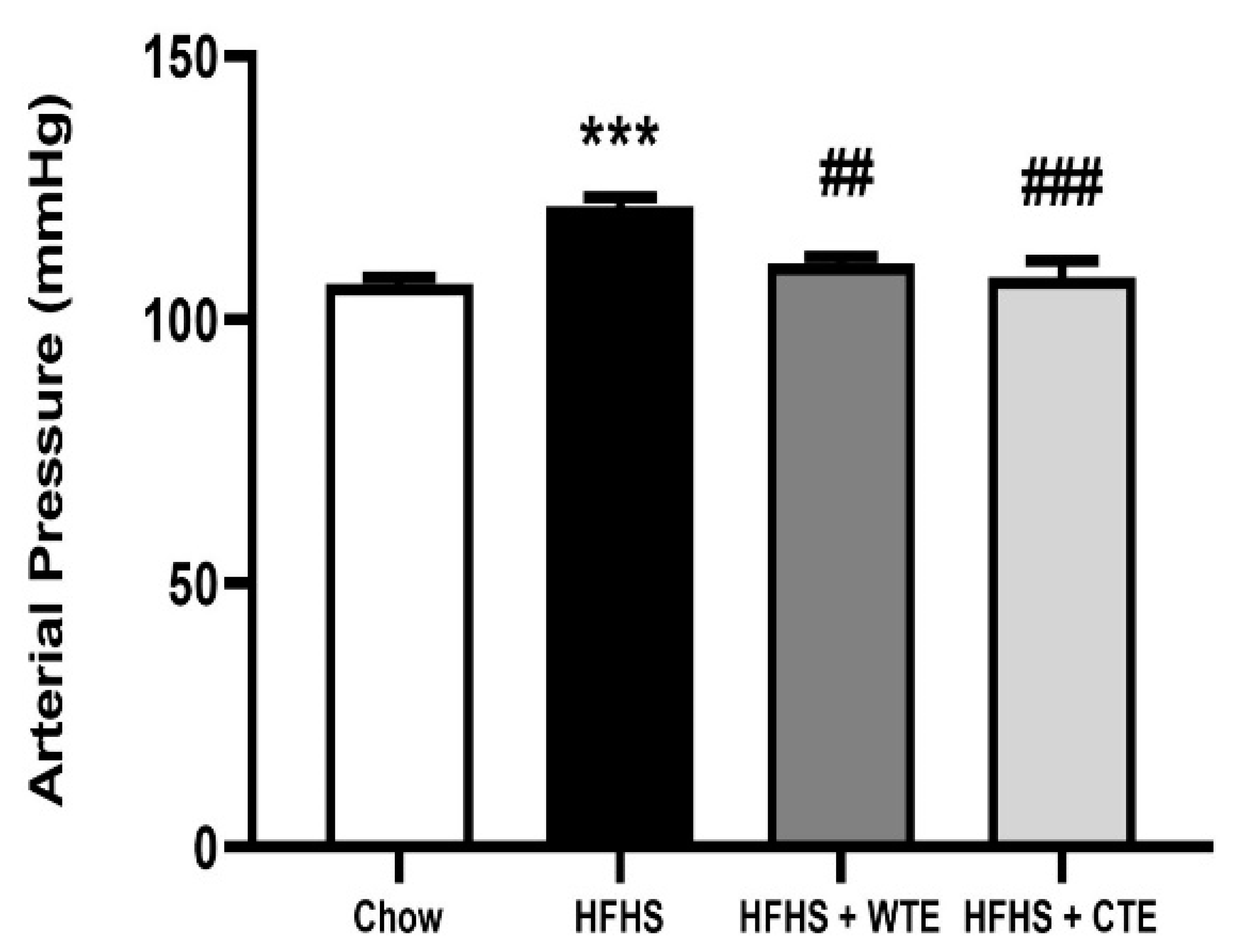
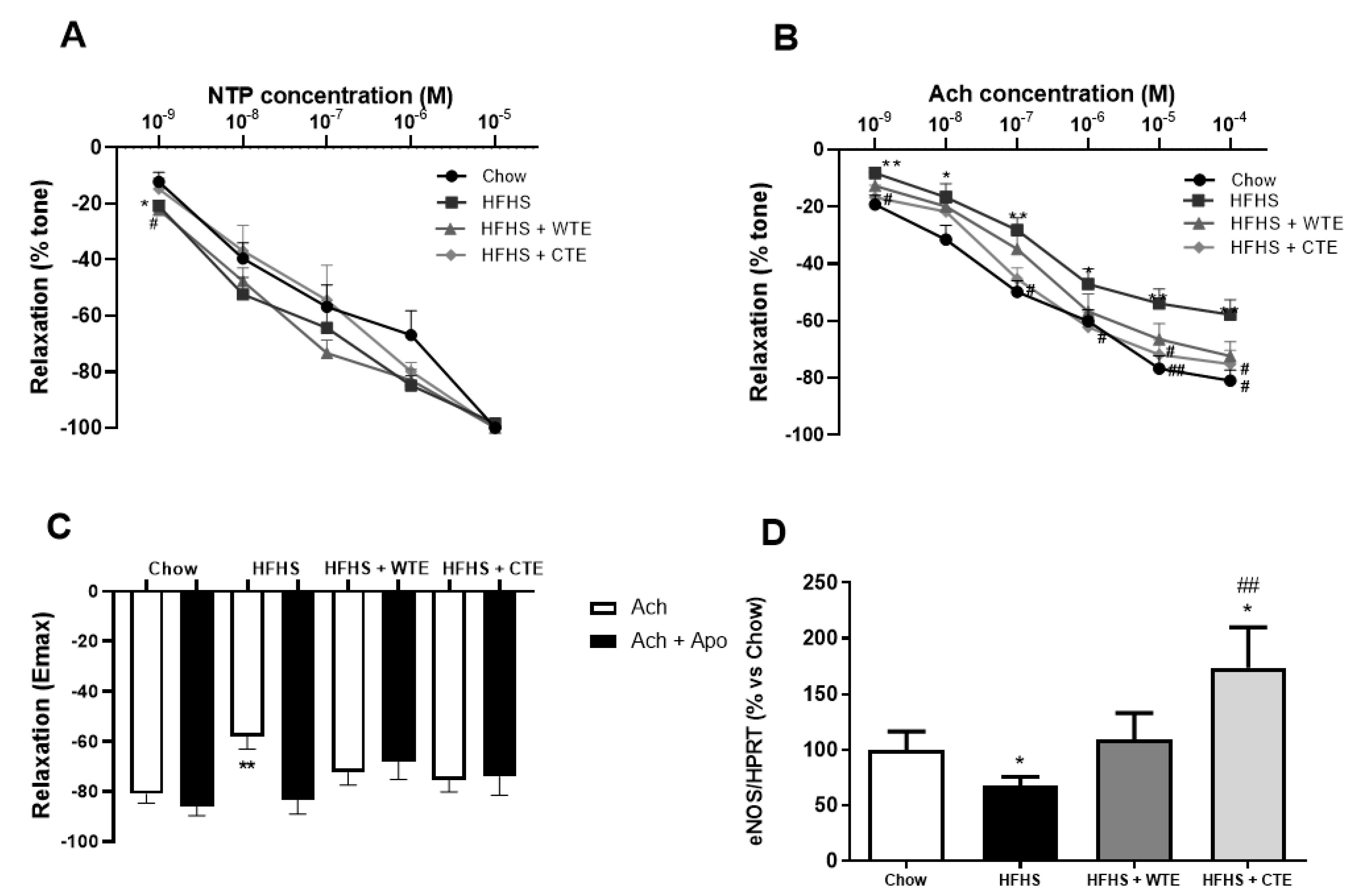
3.3. Effects of CTE and WTE on Blood Pressure and Vascular Reactivity
3.4. Effects of CTE and WTE on the Gene Expression of Inflammatory and Oxidative Stress-Related Markers in Arterial Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. 2021. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 8 August 2022).
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Carretero, O.A.; Oparil, S. Essential hypertension. Part I: Definition and etiology. Circulation 2000, 101, 329–335. [Google Scholar] [CrossRef]
- Hall, J.E.; Do Carmo, J.M.; Da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Turtle, J.R. The economic burden of insulin resistance. Int. J. Clin. Pract. Suppl. 2000, 23–28. [Google Scholar] [PubMed]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Kendall, D.M.; Harmel, A.P. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: Understanding the role of insulin resistance. Am. J. Manag. Care 2002, 8, S635–S653. [Google Scholar]
- Mancusi, C.; Izzo, R.; Di Gioia, G.; Losi, M.A.; Barbato, E.; Morisco, C. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High Blood Press. Cardiovasc. Prev. 2020, 27, 515–526. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Lembo, G.; Napoli, R.; Capaldo, B.; Rendina, V.; Iaccarino, G.; Volpe, M.; Trimarco, B.; Sacca, L. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. J. Clin. Investig. 1992, 90, 24–29. [Google Scholar] [CrossRef]
- Underwood, P.C.; Adler, G.K. The renin angiotensin aldosterone system and insulin resistance in humans. Curr. Hypertens. Rep. 2013, 15, 59–70. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Dange, R.B.; Agarwal, D.; Teruyama, R.; Francis, J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J. Neuroinflammation 2015, 12, 31. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y. Oxidative stress in the brain causes hypertension via sympathoexcitation. Front. Physiol. 2012, 3, 335. [Google Scholar] [CrossRef]
- De la Fuente-Fernandez, M.; Gonzalez-Hedstrom, D.; Amor, S.; Tejera-Munoz, A.; Fernandez, N.; Monge, L.; Almodovar, P.; Andres-Delgado, L.; Santamaria, L.; Prodanov, M.; et al. Supplementation with a Carob (Ceratonia siliqua L.) Fruit Extract Attenuates the Cardiometabolic Alterations Associated with Metabolic Syndrome in Mice. Antioxidants 2020, 9, 339. [Google Scholar] [CrossRef]
- Rocha, N.G.; Templeton, D.L.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Metabolic syndrome and endothelin-1 mediated vasoconstrictor tone in overweight/obese adults. Metabolism 2014, 63, 951–956. [Google Scholar] [CrossRef]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef]
- Krzeminska, J.; Wronka, M.; Mlynarska, E.; Franczyk, B.; Rysz, J. Arterial Hypertension-Oxidative Stress and Inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The Effect of Steaming and Fermentation on Nutritive Values, Antioxidant Activities, and Inhibitory Properties of Tea Leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; Di Serio, M.; Amoresano, A. Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry. Foods 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Zagula, G.; Bajcar, M.; Saletnik, B.; Czernicka, M.; Puchalski, C.; Kapusta, I.; Oszmianski, J. Comparison of the Effectiveness of Water-Based Extraction of Substances from Dry Tea Leaves with the Use of Magnetic Field Assisted Extraction Techniques. Molecules 2017, 22, 1656. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, S.; Huang, J.; Wei, C.C.; Wan, X.; Sang, S.; Ho, C.T. Importance of the Nucleophilic Property of Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 5379–5383. [Google Scholar] [CrossRef]
- Chen, Z.M.; Lin, Z. Tea and human health: Biomedical functions of tea active components and current issues. J. Zhejiang Univ. Sci. B 2015, 16, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-García, A.M.; Helbig, I.; Klette, P.; Weber, S.; Maeder, J.; Morlock, G.E. Authentication of Commercial Powdered Tea Extracts (Camellia sinensis L.) by Gas Chromatography. ACS Food Sci. Technol. 2021, 1, 596–604. [Google Scholar] [CrossRef]
- Lee, B.L.; Ong, C.N. Comparative analysis of tea catechins and theaflavins by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 881, 439–447. [Google Scholar] [CrossRef]
- Bruno, R.S.; Dugan, C.E.; Smyth, J.A.; DiNatale, D.A.; Koo, S.I. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 2008, 138, 323–331. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhao, Y.; Zhu, H.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.S.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, M.; Wang, R.; Li, M.; Li, D.; Xie, Z. Dietary supplement of Yunkang 10 green tea and treadmill exercise ameliorate high fat diet induced metabolic syndrome of C57BL/6 J mice. Nutr. Metab. 2020, 17, 14. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS): Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Lookian, F.; Hosseinzadeh, H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed. Pharmacother. 2017, 92, 726–731. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef]
- Kang, J.; Cheng, H.; Ji, J.; Incardona, J.; Rampe, D. In vitro electrocardiographic and cardiac ion channel effects of (-)-epigallocatechin-3-gallate, the main catechin of green tea. J. Pharmacol. Exp. Ther. 2010, 334, 619–626. [Google Scholar] [CrossRef]
- Feng, W.; Hwang, H.S.; Kryshtal, D.O.; Yang, T.; Padilla, I.T.; Tiwary, A.K.; Puschner, B.; Pessah, I.N.; Knollmann, B.C. Coordinated regulation of murine cardiomyocyte contractility by nanomolar (-)-epigallocatechin-3-gallate, the major green tea catechin. Mol. Pharmacol. 2012, 82, 993–1000. [Google Scholar] [CrossRef]
- Dong, H.J.; Li, J.; Zhan, H.; Li, Y.; Su, R.B. Tea polyphenols promote cardiac function and energy metabolism in ex vivo rat heart with ischemic/reperfusion injury and inhibit calcium inward current in cultured rat cardiac myocytes. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 604–608. [Google Scholar]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1378–E1387. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, C.; Jiang, Z.; Wang, Y.; Zhu, F.; Li, T.; Wan, X.; Xu, Y.; Xie, Z.; Li, D.; et al. Epicatechin-3-Gallate Signaling and Protection against Cardiac Ischemia/Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019, 371, 663–674. [Google Scholar] [CrossRef]
- Yanagi, S.; Matsumura, K.; Marui, A.; Morishima, M.; Hyon, S.H.; Ikeda, T.; Sakata, R. Oral pretreatment with a green tea polyphenol for cardioprotection against ischemia-reperfusion injury in an isolated rat heart model. J. Thorac. Cardiovasc. Surg. 2011, 141, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Schuster, R.; Dahnert, I.; Seeger, J.; Dhein, S. Epigallocatechin Gallate Reduces Ischemia/Reperfusion Injury in Isolated Perfused Rabbit Hearts. Int. J. Mol. Sci. 2018, 19, 628. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Hotta, Y.; Ishikawa, N.; Wakida, Y.; Fukuzawa, Y.; Isobe, F.; Nakano, A.; Chiba, T.; Kawamura, N. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci. 2007, 80, 1020–1032. [Google Scholar] [CrossRef]
- Liou, Y.M.; Hsieh, S.R.; Wu, T.J.; Chen, J.Y. Green tea extract given before regional myocardial ischemia-reperfusion in rats improves myocardial contractility by attenuating calcium overload. Pflug. Arch. 2010, 460, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Modesto, P.N.; Polegato, B.F.; Dos Santos, P.P.; Grassi, L.D.V.; Molina, L.C.C.; Bazan, S.G.Z.; Pereira, E.J.; Fernandes, A.A.H.; Fabro, A.T.; Androcioli, V.N.; et al. Green Tea (Camellia sinensis) Extract Increased Topoisomerase IIbeta, Improved Antioxidant Defense, and Attenuated Cardiac Remodeling in an Acute Doxorubicin Toxicity Model. Oxid. Med. Cell. Longev. 2021, 2021, 8898919. [Google Scholar] [CrossRef]
- Nacerai, H.; Gregory, T.; Sihem, B.; Salah, A.; Souhila, A.B. Green Tea Beverage and Epigallocatecihin Gallate Attenuate Nicotine Cardiocytotoxicity in Rat. Acta Pol. Pharm. 2017, 74, 277–287. [Google Scholar]
- Li, W.; Nie, S.; Xie, M.; Chen, Y.; Li, C.; Zhang, H. A major green tea component, (-)-epigallocatechin-3-gallate, ameliorates doxorubicin-mediated cardiotoxicity in cardiomyocytes of neonatal rats. J. Agric. Food Chem. 2010, 58, 8977–8982. [Google Scholar] [CrossRef]
- Pagnotta, E.; Calonghi, N.; Hrelia, S.; Masotti, L.; Biagi, P.; Angeloni, C. Green tea protects cytoskeleton from oxidative injury in cardiomyocytes. J. Agric. Food Chem. 2006, 54, 10159–10163. [Google Scholar] [CrossRef]
- Shibu, M.A.; Kuo, C.H.; Chen, B.C.; Ju, D.T.; Chen, R.J.; Lai, C.H.; Huang, P.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Oolong tea prevents cardiomyocyte loss against hypoxia by attenuating p-JNK mediated hypertrophy and enhancing P-IGF1R, p-akt, and p-Bad(ser136) activity and by fortifying NRF2 antioxidation system. Environ. Toxicol. 2018, 33, 220–233. [Google Scholar] [CrossRef]
- Antonello, M.; Montemurro, D.; Bolognesi, M.; Di Pascoli, M.; Piva, A.; Grego, F.; Sticchi, D.; Giuliani, L.; Garbisa, S.; Rossi, G.P. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am. J. Hypertens. 2007, 20, 1321–1328. [Google Scholar] [CrossRef]
- Ihm, S.H.; Jang, S.W.; Kim, O.R.; Chang, K.; Oak, M.H.; Lee, J.O.; Lim, D.Y.; Kim, J.H. Decaffeinated green tea extract improves hypertension and insulin resistance in a rat model of metabolic syndrome. Atherosclerosis 2012, 224, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jamshidi, A.; Nikbakht-Nasrabadi, E.; Khosravi-Boroujeni, H. Green tea catechins and blood pressure: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2014, 53, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Bukowski, J.F.; Percival, S.S. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 2009, 25, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, R.; Wang, B.; Yu, X.; Yang, X.; Liu, K.; Mi, M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014, 4, 6251. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Barzegar, K. The effect of green tea and sour tea on blood pressure of patients with type 2 diabetes: A randomized clinical trial. J. Diet Suppl. 2013, 10, 105–115. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Thabane, L.; Mbuagbaw, L.; Liu, A.; Levine, M.A.; Holbrook, A. Effect of green tea supplementation on blood pressure among overweight and obese adults: A systematic review and meta-analysis. J. Hypertens. 2015, 33, 243–254. [Google Scholar] [CrossRef]
- Tong, X.; Taylor, A.W.; Giles, L.; Wittert, G.A.; Shi, Z. Tea consumption is inversely related to 5-year blood pressure change among adults in Jiangsu, China: A cross-sectional study. Nutr. J. 2014, 13, 98. [Google Scholar] [CrossRef]
- Leung, F.P.; Yung, L.M.; Ngai, C.Y.; Cheang, W.S.; Tian, X.Y.; Lau, C.W.; Zhang, Y.; Liu, J.; Chen, Z.Y.; Bian, Z.X.; et al. Chronic black tea extract consumption improves endothelial function in ovariectomized rats. Eur. J. Nutr. 2016, 55, 1963–1972. [Google Scholar] [CrossRef]
- Auger, C.; Kim, J.H.; Chabert, P.; Chaabi, M.; Anselm, E.; Lanciaux, X.; Lobstein, A.; Schini-Kerth, V.B. The EGCg-induced redox-sensitive activation of endothelial nitric oxide synthase and relaxation are critically dependent on hydroxyl moieties. Biochem. Biophys. Res. Commun. 2010, 393, 162–167. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Dias, P.M.; Majumder, S.; Joshi, M.K.; Sinkar, V.P.; Banerjee, G.; Chatterjee, S. L-theanine promotes nitric oxide production in endothelial cells through eNOS phosphorylation. J. Nutr. Biochem. 2013, 24, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shreshtha, A.K.; Thakur, M.S.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, e00829. [Google Scholar] [CrossRef]
- Mena, P.; Dominguez-Perles, R.; Girones-Vilaplana, A.; Baenas, N.; Garcia-Viguera, C.; Villano, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, J.B. Sympathetic system hyperactivity in obesity. Arq. Bras. Endocrinol. Metabol. 2008, 52, 6–7. [Google Scholar] [CrossRef]



| Chow | HFHS | HFHS + WTE | HFHS + CTE | |
|---|---|---|---|---|
| Body weight (g) | 28.5 ± 0.5 | 48.7 ± 1 *** | 47.7 ± 1.1 | 44.8 ± 0.9 ***# |
| Glycaemia | 103 ± 4.9 | 138 ± 5.5 *** | 153 ± 7 *** | 142 ± 4.7 *** |
| Triglycerides | 47 ± 4 | 61 ± 3 *** | 92 ± 10 ***# | 67 ± 10 *** |
| Total Cholesterol | 104 ± 3 | 206 ± 7.4 *** | 247 ± 11 ***# | 238 ± 17 *** |
| LDL-c | 18.8 ± 1.6 | 52 ± 4 *** | 52.3 ± 4.6 *** | 55.4 ± 6 *** |
| HDL-c | 25.7 ± 1.1 | 52.2 ± 2.6 *** | 60 ± 2.7 *** | 53.7 ± 5.1 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente Muñoz, M.; de la Fuente Fernández, M.; Román-Carmena, M.; Iglesias de la Cruz, M.d.C.; Amor, S.; Martorell, P.; Enrique-López, M.; García-Villalón, A.L.; Inarejos-García, A.M.; Granado, M. Supplementation with Two New Standardized Tea Extracts Prevents the Development of Hypertension in Mice with Metabolic Syndrome. Antioxidants 2022, 11, 1573. https://doi.org/10.3390/antiox11081573
de la Fuente Muñoz M, de la Fuente Fernández M, Román-Carmena M, Iglesias de la Cruz MdC, Amor S, Martorell P, Enrique-López M, García-Villalón AL, Inarejos-García AM, Granado M. Supplementation with Two New Standardized Tea Extracts Prevents the Development of Hypertension in Mice with Metabolic Syndrome. Antioxidants. 2022; 11(8):1573. https://doi.org/10.3390/antiox11081573
Chicago/Turabian Stylede la Fuente Muñoz, Mario, María de la Fuente Fernández, Marta Román-Carmena, Maria del Carmen Iglesias de la Cruz, Sara Amor, Patricia Martorell, María Enrique-López, Angel Luis García-Villalón, Antonio Manuel Inarejos-García, and Miriam Granado. 2022. "Supplementation with Two New Standardized Tea Extracts Prevents the Development of Hypertension in Mice with Metabolic Syndrome" Antioxidants 11, no. 8: 1573. https://doi.org/10.3390/antiox11081573






