Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis
Abstract
:1. Introduction
2. Coffee as Potent Antioxidant
2.1. Coffee Roasting and Phenolic Compounds
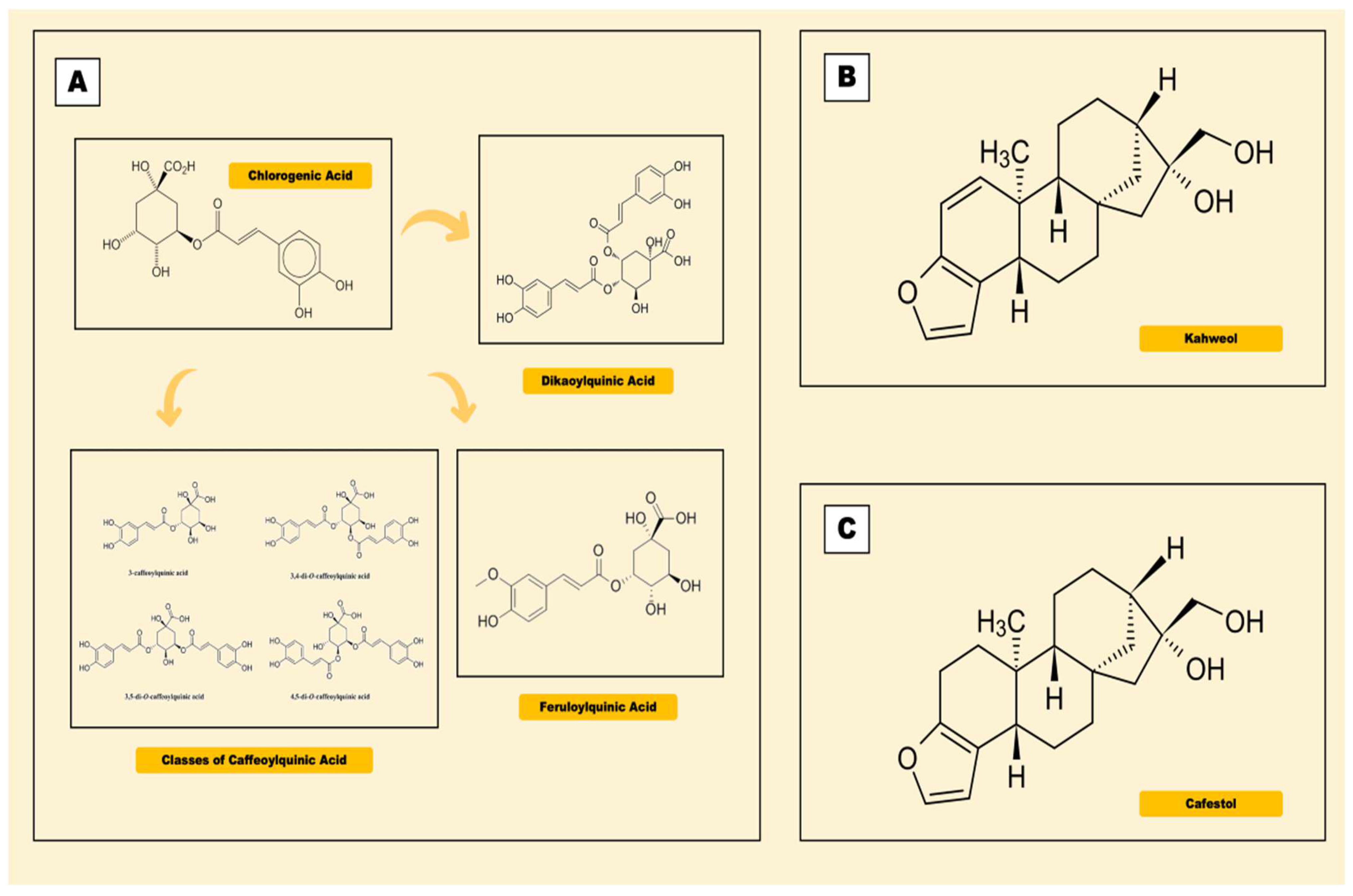
2.2. Chlorogenic Acid
2.3. Kahweol
2.4. Cafestol
3. Coffee Consumption, Gene Expressions and PD
3.1. Coffee, Adenosine A2A Receptor (A2AR) Gene Polymorphisms and PD Risk
3.1.1. Human Study
3.1.2. In Vivo Study
3.2. Caffeine, Estrogen Receptor (ESR) Genes and PD Risk
Human Study
3.3. Kahweol, Heme-Oxygenase-1 (HO-1) and Oxidative Stress in PD
In Vitro Study
3.4. Caffeine, Toxicant Responsive Genes and PD
In Vivo Study
3.5. Caffeine, Nitric Oxide Synthase (NOS) Gene Polymorphisms and PD Risk
Human Study
3.6. Coffee/Caffeine Consumption, Familial Parkinsonism Genetic Susceptibility Loci and PD Risk
Human Study
3.7. Caffeine, Cytochrome Oxidase (Cox) Expressions and PD
In Vivo Study
3.8. Caffeine Intake, Bone Marrow Stromal Cell Antigen 1 (BST1) Polymorphisms and Sporadic PD
Human Study
3.9. Coffee Consumption, Glutamate Receptor Gene Polymorphisms and PD Risk
Human Study
3.10. Coffee Consumption, Apolipoprotein E (APOE) Genetic Polymorphisms and PD Risk
Human Study
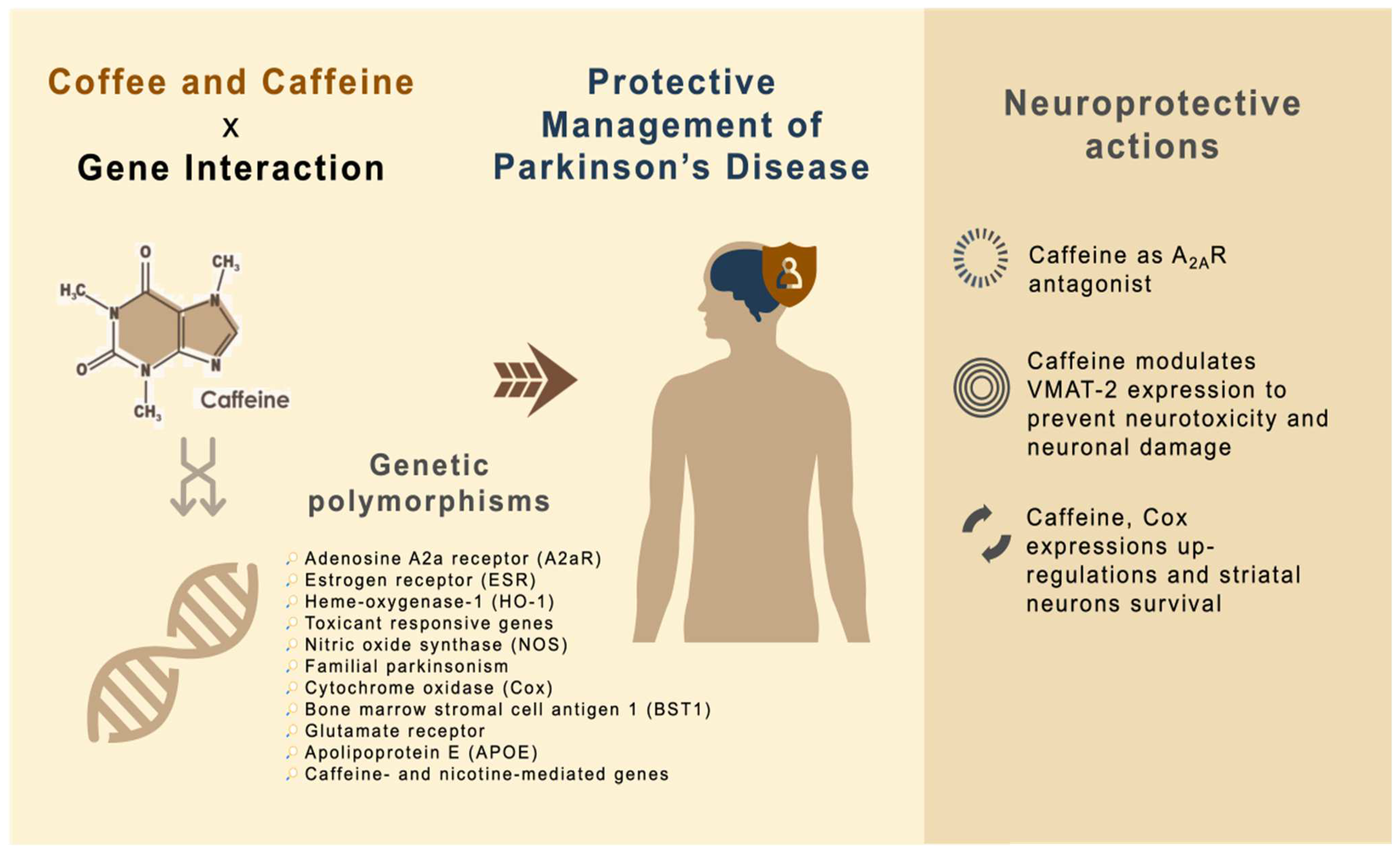
4. Neuroprotective Mechanisms of Caffeine x Gene Interaction for the Amelioration of PD
4.1. Caffeine as A2AR Antagonist
4.2. Caffeine Modulates VMAT-2 Expressions to Prevent Neurotoxicity and Neuronal Damage
4.3. Caffeine, Cox Expression Upregulations and Striatal Neuron Survival
5. The Neuroprotective Effects of Phenolic Compounds in Coffee
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2012, 51, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Rosso, A.; Mossey, J.; Lippa, C.F. Caffeine: Neuroprotective functions in cognition and Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2008, 23, 417–422. [Google Scholar] [CrossRef]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Benassi, M.T.; Bragagnolo, N. Scavenging capacity of coffee brews against oxygen and nitrogen reactive species and the correlation with bioactive compounds by multivariate analysis. Food Res. Int. 2014, 61, 228–235. [Google Scholar] [CrossRef]
- Sekeroglu, N.; Sezer Senol, F.S.; Orhan, I.E.; Rifat Gulpinar, A.; Kartal, M.; Sener, B. In vitro prospective effects of various traditional herbal coffees consumed in Anatolia linked to neurodegeneration. Food Res. Int. 2012, 45, 197–203. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo’, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef]
- Dobrev, S.M.; Angelova, S.E. Antioxidants in coffee: A DFT mechanistic study of the free radical scavenging activity. Bulg. Chem. Commun. 2020, 52, 48–53. [Google Scholar]
- Kones, R. Parkinson’s disease: Mitochondrial molecular pathology, inflammation, statins, and therapeutic neuroprotective nutrition. Nutr. Clin. Pract. 2010, 25, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gupta, S.P.; Srivastava, G.; Srivastava, P.K.; Singh, M.P. Role of secondary mediators in caffeine-mediated neuroprotection in maneb-and paraquat-induced Parkinson’s Disease phenotype in the mouse. Neurochem. Res. 2012, 37, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Meissner, W.; Hill, M.P.; Tison, F.; Gross, C.E.; Bezard, E. Neuroprotective strategies for Parkinson’s disease: Conceptual limits of animal models and clinical trials. Trends Pharmacol. Sci. 2004, 25, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Bidel, S.; Jousilahti, P.; Antikainen, R.; Tuomilehto, J. Coffee and tea consumption and the risk of Parkinson’s disease. Mov. Disord. 2007, 22, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Sääksjärvi, K.; Knekt, P.; Rissanen, H.; Laaksonen, M.A.; Reunanen, A.; Männistö, S. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur. J. Clin. Nutr. 2008, 62, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Sipetic, S.B.; Vlajinac, H.D.; Maksimovic, J.M.; Marinkovic, J.M.; Dzoljic, E.D.; Ratkov, I.S. Cigarette smoking, coffee intake and alcohol consumption preceding Parkinson’s disease: A case-control study. Acta Neuropsychiatr. 2012, 24, 109–114. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Celeghini, R.M.S.; Debien, I.C.N.; Nogueira, G.C.; Meireles, M.A.A. Phenolic Compounds in Coffee Compared to Other Beverages. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 137–142. [Google Scholar]
- Souza, L.S.; Carrero Horta, I.P.; de Souza Rosa, L.; Barbosa Lima, L.G.; Santos da Rosa, J.; Montenegro, J.; da Silva Santos, L.; Nana de Castro, R.B.; Freitas-Silva, O.; Teodoro, A.J. Effect of the roasting levels of Coffea arabica L. extracts on their potential antioxidant capacity and antiproliferative activity in human prostate cancer cells. RSC Adv. 2020, 10, 30115–30126. [Google Scholar] [CrossRef]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, L.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health Part B 2020, 55, 495–500. [Google Scholar] [CrossRef]
- Erskine, E.; Subaşi, B.G.; Vahapoglu, B.; Capanoglu, E. Coffee phenolics and their interaction with other food phenolics: Antagonistic and synergistic effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Açıkalın, B.; Sanlier, N. Coffee and Its Effects on the Immune System. Trends Food Sci. Technol. 2021, 114, 625–632. [Google Scholar] [CrossRef]
- Lemos, M.F.; de Andrade Salustriano, N.; de Souza Costa, M.M.; Lirio, K.; da Fonseca, A.F.A.; Pacheco, H.P.; Endringer, D.C.; Fronza, M.; Scherer, R. Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J. Saudi Chem. Soc. 2022, 26, 101467. [Google Scholar] [CrossRef]
- Lonati, E.; Carrozzini, T.; Bruni, I.; Mena, P.; Botto, L.; Cazzaniga, E.; Del Rio, D.; Labra, M.; Palestini, P.; Bulbarelli, A. Coffee-derived phenolic compounds activate Nrf2 antioxidant pathway in I/R injury in vitro model: A nutritional approach preventing age related-damages. Molecules 2022, 27, 1049. [Google Scholar] [CrossRef] [PubMed]
- Alamri, E.; Rozan, M.; Bayomy, H. A study of chemical Composition, Antioxidants, and volatile compounds in roasted Arabic coffee. Saudi J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, D.H.; Jeong, H.G. Inhibitory effect of the coffee diterpene kahweol on carrageenan-induced inflammation in rats. Biofactors 2006, 26, 17–28. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Anjum, S.; Bajbouj, K.; Abu-Gharbieh, E.; Taneera, J. The coffee diterpene, kahweol, ameliorates pancreatic β-cell function in streptozotocin (STZ)-treated rat INS-1 cells through NF-kB and p-AKT/Bcl-2 pathways. Molecules 2021, 26, 5167. [Google Scholar] [CrossRef]
- Hao, W.-R.; Sung, L.-C.; Chen, C.-C.; Chen, P.-Y.; Cheng, T.-H.; Chao, H.-H.; Liu, J.-C.; Chen, J.-J. Cafestol inhibits cyclic-strain-induced interleukin-8, intercellular adhesion molecule-1, and monocyte chemoattractant protein-1 production in vascular endothelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 7861518. [Google Scholar] [CrossRef]
- Armentero, M.T.; Pinna, A.; Ferré, S.; Lanciego, J.L.; Müller, C.E.; Franco, R. Past, present and future of A2A adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol. Ther. 2011, 132, 280–299. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Kumaran, K.S. Preventive effects of caffeic acid on lipids, lipoproteins and glycoproteins in isoproterenol induced myocardial infarcted rats. Food Res. Int. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Morelli, M.; Carta, A.R.; Jenner, P. Adenosine A2A receptors and Parkinson’s disease. Handb. Exp. Pharmacol. 2009, 193, 589–615. [Google Scholar]
- Wu, Y.C.; Lai, H.L.; Chang, W.C.; Lin, J.T.; Liu, Y.J.; Chern, Y.J. A novel Gαs-binding protein, Gas-2 like 2, facilitates the signaling of the A2A adenosine receptor. Biochim. Biophys. Acta-Mol. Cell Res. 2013, 1833, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Bata-García, J.L.; Tun-Cobá, L.; Alvarez-Cervera, F.J.; Villanueva-Toledo, J.R.; Heredia-López, F.J.; Góngora-Alfaro, J.L. Improvement of postural adjustment steps in hemiparkinsonian rats chronically treated with caffeine is mediated by concurrent blockade of A1 and A2A adenosine receptors. Neuroscience 2010, 166, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Lu, Z.Y.; Fook-Chong, S.M.C.; Tan, E.; Shen, H.; Chua, E. Exploring an interaction of adenosine a2a receptor variability with coffee and tea intake in Parkinson’s Disease. Am. J. Med. Genet. Part B 2006, 141, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Facheris, M.F.; Schneider, N.K.; Lesnick, T.G.; de Andrade, M.; Cunningham, J.M.; Rocca, W.A. Coffee, caffeine-related genes, and Parkinson’s Disease: A case–control study. Mov. Disord. 2008, 23, 2033–2040. [Google Scholar] [CrossRef]
- Popat, R.A.; Van Den Eeden, S.K.; Tanner, C.M.; Kamel, F.; Umbach, D.M.; Marder, K. Coffee, ADORA2A, and CYP1A2: The caffeine connection in Parkinson’s disease. Eur. J. Neurol. 2011, 18, 756–765. [Google Scholar] [CrossRef]
- Palacios, N.; Weisskopf, M.; Simon, K.; Gao, X.; Schwarzschild, M.; Ascherio, A. Polymorphisms of caffeine metabolism and estrogen receptor genes and risk of Parkinson’s disease in men and women. Parkinsonism Relat. Disord. 2010, 16, 370–375. [Google Scholar] [CrossRef]
- Hancock, D.B.; Martin, E.R.; Vance, J.M.; Scott, W.K. Nitric oxide synthase genes and their interactions with environmental factors in Parkinson’s disease. Neurogenetics 2008, 9, 249–262. [Google Scholar] [CrossRef]
- Gao, J.J.; Nalls, M.A.; Shi, M.; Joubert, B.R.; Hernandez, D.G.; Huang, X.M. An exploratory analysis on gene-environment interactions for Parkinson disease. Neurobiol. Aging 2012, 33, e1–e6. [Google Scholar] [CrossRef]
- Chung, S.J.; Armasu, S.M.; Anderson, K.J.; Biernacka, J.M.; Lesnick, T.G.; Rider, D.N.; Cunningham, J.M.; Ahlskog, J.E.; Frigerio, R.; Maraganore, D.M. Genetic susceptibility loci, environmental exposures, and Parkinson’s disease: A case-control study of gene-environment interactions. Parkinsonism Relat. Disord. 2013, 19, 595–599. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Sasaki, S.; Tsuboi, Y. Lack of association between BST1polymorphisms and sporadic Parkinson’s disease in a Japanese population. J. Neurol. Sci. 2012, 323, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Hamza, T.H.; Chen, H.L.; Hill-Burns, E.M.; Rhodes, S.L.; Montimurro, J.; Kay, D.M. Genome-wide gene-environment study identifies glutamate receptor gene grin2a as a Parkinson’s Disease modifier gene via interaction with coffee. PLoS Genet. 2011, 7, e1002237. [Google Scholar] [CrossRef]
- Yamada-Fowler, N.; Fredrikson, M.; Söderkvist, P. Caffeine Interaction with Glutamate Receptor Gene GRIN2A: Parkinson’s Disease in Swedish Population. PLoS ONE 2014, 9, e99294. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, C.C.; Kay, D.M.; Factor, S.A.; Smii, A.; Nutt, J.G.; Higgins, D.S. Exploring gene-environment interactions in Parkinson’s disease. Hum. Genet. 2008, 123, 257–265. [Google Scholar] [CrossRef]
- Singh, S.; Singh, K.; Gupta, S.P.; Patel, D.K.; Singh, V.K.; Singh, R.K. Effect of caffeine on the expression of cytochrome P450 1A2, adenosine A2Areceptor and dopamine transporter in control and 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine treated mouse striatum. Brain Res. 2009, 1283, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, S.; Singhal, N.K.; Sharma, A.; Parmar, D.; Singh, M.P. Nicotine- and caffeine-mediated changes in gene expression patterns of MPTP-lesioned mouse striatum: Implications in neuroprotection mechanism. Chem.-Biol. Interact. 2010, 185, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, K.; Patel, S.; Patel, D.K.; Singh, C.; Nath, C. Nicotine and caffeine-mediated modulation in the expression of toxicant responsive genes and vesicular monoamine transporter-2 in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease phenotype in mouse. Brain Res. 2008, 1207, 193–206. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Lerner, A. Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3624. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.S.; Jing, J.; Stonehouse, A.H.; Stevens, A.; Edelman, G.M. Caffeine stimulates cytochrome oxidase expression and activity in the striatum in a sexually dimorphic manner. Mol. Pharmacol. 2008, 74, 673–684. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; de Souza, I.C.C.; Fürstenau, C.R. Mitochondrial protection promoted by the coffee diterpene kahweol in methylglyoxal-treated human neuroblastoma SH-SY5Y Cells. Neurotox. Res. 2020, 37, 100–110. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008, 582, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, C.R.; de Souza, I.C.C.; de Oliveira, M.R. The effects of kahweol, a diterpene present in coffee, on the mitochondria of the human neuroblastoma SH-SY5Y cells exposed to hydrogen peroxide. Toxicol. In Vitro 2019, 61, 104601. [Google Scholar] [CrossRef] [PubMed]
- Al-Sweidi, S.; Morissette, M.; Bourque, M.; Di Paolo, T. Estrogen receptors and gonadal steroids in vulnerability and protection of dopamine neurons in a mouse model of Parkinson’s disease. Neuropharmacology 2011, 61, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, Y.; Brown-Jermyn, D.; Chen, J.F.; Ascherio, A.; Dluzen, D.E. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neurosci. 2006, 11, 535–541. [Google Scholar] [CrossRef]
- Jazwa, A.; Cuadrado, A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets 2010, 11, 1517–1531. [Google Scholar] [CrossRef]
- Lin, H.Y.; Huang, B.R.; Yeh, W.L.; Lee, C.H.; Huang, S.S.; Lai, C.H. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol. Aging 2014, 35, 191–202. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Chuang, J.-I. Fibroblast Growth Factor 9 Upregulates Heme Oxygenase-1 and γ-Glutamylcysteine Synthetase Expression to Protect Neurons from 1-Methyl-4-Phenylpyridinium Toxicity. Free. Radic. Biol. Med. 2010, 49, 1099–1108. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Joniec, I.; Ciesielska, A.; Kurkowska-Jastrzebska, I.; Przybylkowski, A.; Czlonkowska, A.; Czlonkowski, A. Age-and sex-differences in the nitric oxide synthase expression and dopamine concentration in the murine model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2009, 1261, 7–19. [Google Scholar] [CrossRef]
- Wu, D.; Tseng, I.J.; Yuan, R.Y.; Hsieh, C.Y.; Hu, C.J. Memory consolidation and inducible nitric oxide synthase expression during different sleep stages in Parkinson disease. Sleep Med. 2014, 15, 116–120. [Google Scholar] [CrossRef]
- Padovan-Neto, F.E.; Echeverry, M.B.; Tumas, V.; Del-Bel, E.A. Nitric oxide synthase inhibition attenuates L-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neuroscience 2009, 159, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Camandola, S.; Plick, N.; Mattson, M.P. Impact of Coffee and Cacao Purine Metabolites on Neuroplasticity and Neurodegenerative Disease. Neurochem. Res. 2019, 44, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Peeraully, T.; Tan, E.K. Genetic variants in Sporadic Parkinson’s Disease: East vs West. Parkinsonism Relat. Disord. 2012, 18, S63–S65. [Google Scholar] [CrossRef]
- Schulte, C.; Gasser, T. Genetic basis of Parkinson’s disease: Inheritance, penetrance, and expression. Appl. Clin. Genet. 2011, 4, 67–80. [Google Scholar] [PubMed]
- Satake, W.; Nakabayashi, Y.; Mizuta, I.; Hirota, Y.; Ito, C.; Kubo, M. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2010, 41, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Hernández, M.E.; Padilla, E.; Gonzalez-Lima, F.; Jiménez-Rivera, C.A. Cocaine reduces cytochrome oxidase activity in the prefrontal cortex and modifies its functional connectivity with brainstem nuclei. Brain Res. 2014, 1542, 56–69. [Google Scholar] [CrossRef]
- Arnold, S. The power of life-Cytochrome c oxidase takes center stage in metabolic control, cell signaling and survival. Mitochondrion 2012, 12, 46–56. [Google Scholar] [CrossRef]
- Sedlák, E.; Fabian, M.; Robinson, N.C.; Musatov, A. Ferricytochrome c protects mitochondrial cytochrome c oxidase against hydrogen peroxide-induced oxidative damage. Free. Radic. Biol. Med. 2010, 49, 1574–1581. [Google Scholar] [CrossRef]
- Quarona, V.; Zaccarello, G.; Chillemi, A.; Brunetti, E.; Singh, V.K.; Ferrero, E. CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytom. B Clin. Cytom. 2013, 84B, 207–217. [Google Scholar] [CrossRef]
- Chen, M.L.; Lin, C.H.; Lee, M.J.; Wu, R.M. BST1 rs11724635 interacts with environmental factors to increase the risk of Parkinson’s Disease in a Taiwanese population. Parkinsonism Relat. Disord. 2014, 20, 280–283. [Google Scholar] [CrossRef]
- Mellone, M.; Gardoni, F. Modulation of NMDA receptor at the synapse: Promising therapeutic interventions in disorders of the nervous system. Eur. J. Pharmacol. 2013, 719, 75–83. [Google Scholar] [CrossRef]
- Federoff, M.; Jimenez-Rolando, B.; Nalls, M.A.; Singleton, A.B. A large study reveals no association between APOE and Parkinson’s disease. Neurobiol. Dis. 2012, 46, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H. Effect of APOE genotype on gray matter density in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2013, 19, 138–140. [Google Scholar]
- Hong, C.T.; Chan, L.; Bai, C.-H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Froestl, W.; Muhs, A.; Pfeifer, A. Cognitive Enhancers (Nootropics). Part 1: Drugs Interacting with Receptors. Update 2014. J. Alzheimer’s Dis. 2014, 41, 961–1019. [Google Scholar] [CrossRef] [PubMed]
- Ho, A. Two wrong makes a right: Nicotine and caffeine as defensive agents against Parkinson’s disease. Nutr. Bytes 2002, 8, 3–7. [Google Scholar]
- Pinna, A.; Serra, M.; Morelli, M.; Simola, N. Role of adenosine A2A receptors in motor control: Relevance to Parkinson’s disease and dyskinesia. J. Neural Transm. 2018, 125, 1273–1286. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Chen, J.F.; Ascherio, A. Caffeinated clues and the promise of adenosine A (2A) antagonists in PD. Neurology 2002, 58, 1154–1160. [Google Scholar] [CrossRef]
- Ochi, M.; Koga, K.; Kurokawa, M.; Kase, H.; Nakamura, J.; Kuwana, Y. Systemic administration of adenosine A (2A) receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: A microdialysis study. Neuroscience 2000, 100, 53–62. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Wagatsuma, K.; Toyohara, J.; Ishiwata, K.; Ishii, K. Adenosine A2A Receptor Occupancy by Caffeine After Coffee Intake in Parkinson’s Disease. Mov. Disord. 2022, 37, 853–857. [Google Scholar] [CrossRef]
- Xu, Z.; Cawthon, D.; McCastlain, K.A.; Slikker, W.; Ali, S.F. Selective alterations of gene expression in mice induced by MPTP. Synapse 2005, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Munoz, D.G.; Fujioka, S. Caffeine and Parkinson disease: A possible diagnostic and pathogenic breakthrough. Neurology 2018, 90, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Machado-Filho, J.A.; Correia, A.O.; Montenegro, A.B.A.; Nobre, M.E.P.; Cerqueira, G.S.; Neves, K.R.T.; Naffah-Mazzacoratti, M.D.G.; Cavalheiro, E.A.; de Castro Brito, G.A.; de Barros Viana, G.S. Caffeine neuroprotective effects on 6-OHDA-lesioned rats are mediated by several factors, including pro-inflammatory cytokines and histone deacetylase inhibitions. Behav. Brain Res. 2014, 264, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W.; Gainetdinov, R.R.; Levey, A.I.; Caron, M.G. Dopamine transporters and neuronal injury. Trends Pharmacol. Sci. 1999, 20, 424–429. [Google Scholar] [CrossRef]
- Stonehouse, A.H.; Adachi, M.; Walcott, E.C.; Jones, F.S. Caffeine regulates neuronal expression of the dopamine 2 receptor gene. Mol. Pharmacol. 2003, 64, 1463–1473. [Google Scholar] [CrossRef]
- Kase, H. The adenosine A2A receptor selective antagonist KW6002: Research and development toward a novel nondopaminergic therapy for Parkinson’s disease. Neurology 2003, 61, S97–S107. [Google Scholar] [CrossRef]
- Gigante, A.F.; Asabella, A.N.; Iliceto, G.; Martino, T.; Ferrari, C.; Defazio, G.; Rubini, G. Chronic coffee consumption and striatal DAT-SPECT findings in Parkinson’s disease. Neurol. Sci. 2018, 39, 551–555. [Google Scholar] [CrossRef]
- Schiffmann, S.N.; Dassesse, D.; d’Alcantera, P.; Ledent, C.; Swillens, S.; Zoli, M. A2A receptor and striatal cellular functions. Neurology 2003, 61, S24–S29. [Google Scholar] [CrossRef]
- Mao, X.; Chai, Y.; Lin, Y.F. Dual regulation of the ATP-sensitive potassium channel by caffeine. Am. J. Physiol. Cell Physiol. 2007, 292, C2239–C2258. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]

| Coffee Type | Caffeoylquinic Acid (CQA) | Feruloylquinic Acid (FQA) | Dikaoylquinic Acid (diCQA) | Total Chlorogenic Acids |
|---|---|---|---|---|
| Green coffee | 3.26–7.66 | 0.19–1.43 | 0.45–2.31 | 4.10–11.30 |
| Roasted coffee | 0.38–3.23 | 0.06–0.34 | 0.03–0.24 | 0.47–2.66 |
| Decaffeinated coffee a | 5.19–6.14 | 0.32–0.45 | 0.61–0.77 | 6.13–7.47 |
| Instant regular coffee | 0.63–5.28 | 0.06–1.16 | 0.03–0.53 | 0.72–6.97 |
| Instant decaffeinated coffee | 3.33–4.73 | 0.60–0.84 | 0.17–0.28 | 4.10–5.85 |
| Genetic Polymorphisms | Reference | Genes/Cells of Interest | Results |
|---|---|---|---|
| Adenosine A2A Receptor (A2AR) | [35] | 1976T/T and 2592Tins/Tins genotypes | Independent coffee-PD association of the A2A 2592C > Tins (rs3032740) polymorphism |
| [36] | Cytochrome P450 1A2 (CYP1A2) | ≠Coffee-PD associations | |
| [37] | Four A2AR (rs5751876, rs71651683, rs3032740 and rs5996696) and three CYP1A2 (rs762551, rs2472304 and rs2470890) | Strong coffee-PD association among CYP1A2 variant allele rs762551 and rs2470890 | |
| Estrogen receptor (ESR) genes | [38] | Estrogen receptor alpha (ESR1), Estrogen receptor beta (ESR2) | ↑ PD risk among female with rs762551 polymorphism of CYP1A2 |
| Nitric Oxide Synthase (NOS) | [39] | NOS2A rs944725 | Significant inverse interaction between caffeine consumption and the NOS2A rs944725 |
| Familial Parkinsonism genetic susceptibility loci | [40] | 10 genome-wide association studies (GWAS) SNPs at or near the alpha-synuclein (SNCA), MAPT, LRRK2, and human leukocyte antigen (HLA) loci | ≠ significant interactions of caffeine intake with several SNPs at or near the SNCA, MAPT, and HLA loci |
| [41] | SNCA, MAPT and LRRK2 | Significant pairwise interaction has been observed between coffee drinking and MAPT H1/H2 haplotype (rs16940806) | |
| Bone marrow stromal cell antigen 1 (BST1) | [42] | BST1 SNPs rs11931532, rs12645693, and rs11724635 | ≠ significant associations between BST1 SNPs rs11931532, rs12645693, and rs11724635 and the risk of sporadic PD |
| Glutamate receptor gene (GRIN2A) | [43] | rs4998386 | Significant interactions from rs4998386 and the neighboring SNPs in GRIN2A |
| [44] | Glutamate receptor gene (GRIN2A) rs4998386 | Heavy caffeine intake & GRIN2A_rs4998386_TC genotype was associated with a ↓ 64% risk reduction Strong significant GRIN2A_rs4998386 genotype ∗ caffeine interaction | |
| Apolipoprotein E (APOE) | [45] | Genetic polymorphisms of APOE ε2/ε3/ε4, repeat polymorphism (REP1) in the promoter region of the SNCA, MAPT H1/H2 and ubiquitin carboxy-terminal esterase L1 (UCHL1) S18Y | Inverse association between coffee drinking and APOE genotype Most dramatic PD risk in APOE ε2-carriers |
| Adenosine A2A Receptor (A2AR) | [46,47] | CYP1A2 and dopamine transporter (DAT) | Coffee-PD partially associated by CYP1A2, A2AR and DAT |
| Toxicant responsive genes | [48] | 7-ethoxyresorufin O-deethylase (CYP1A1), p-Nitrophenol O-hydroxylase (CYP2E1), glutathione-S-transferase ya (GST-ya), glutathione-S-transferase yc (GST-yc), glutathione S-transferase alpha 4 (GSTA4-4), vesicular monoamine transporter-2 (VMAT-2) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | MPTP significantly attenuated CYP1A1 and VMAT-2, and augmented CYP2E1, GST-ya, GST-yc and GSTA4-4 expressions and activities |
| Nitric Oxide Synthase (NOS) | [49] | NOS2A rs944725 | ↑ microglial activation and iNOS expression by boosting p38 and ERK1/2 MAP kinase activities |
| Cytochrome oxidase (Cox) expressions | [50] | Cytochrome oxidase 1 (Cox1), cytochrome oxidase 4 (Cox4), cytochrome oxidase 7c (Cox7c) | ↑ Cox1, Cox4 and Cox7c in the striatum of male mice, but not in female mice after receiving a single dose of caffeine ↑ Cox7c mRNA expression in the striatum and in PC-12 cells |
| Heme oxygenase-1 (HO-1) | [51] | Human neuroblastoma SH-SY5Y cells (Pretreatment of SH-SY5Y cells with kahweol) | Pretreatment of SH-SY5Y cells with kahweol significantly reduced 6-OHDA-induced generation of ROS and caspase-3 activation. Protects against 6-OHDA-induced neuronal cell death. Kahweol activated the induction of Nrf2 and HO-1 expression via the phosphatidylinositol 3-kinase (PI3K) and p38 pathway |
| [52,53] | Human neuroblastoma SH-SY5Y cells | ↑ mitochondrial protection in SH-SY5Y cells exposed to H2O2 ↓ oxidative stress markers ↓ production of ROS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.K.; Mhd Rodzi, N.A.R. Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis. Antioxidants 2022, 11, 1587. https://doi.org/10.3390/antiox11081587
Lee LK, Mhd Rodzi NAR. Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis. Antioxidants. 2022; 11(8):1587. https://doi.org/10.3390/antiox11081587
Chicago/Turabian StyleLee, Lai Kuan, and Nur Anis Raihana Mhd Rodzi. 2022. "Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis" Antioxidants 11, no. 8: 1587. https://doi.org/10.3390/antiox11081587
APA StyleLee, L. K., & Mhd Rodzi, N. A. R. (2022). Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis. Antioxidants, 11(8), 1587. https://doi.org/10.3390/antiox11081587






