Ramelteon Reduces Oxidative Stress by Maintenance of Lipid Homeostasis in Porcine Oocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Porcine Oocyte In Vitro Maturation
2.3. Assessment of Cumulus Cell Expansion
2.4. Detection of Nuclear Maturation
2.5. Assessment of Intracellular GSH and ROS Levels in Oocytes
2.6. Parthenogenetic Activation (PA) and In Vitro Culture (IVC)
2.7. LD, Fatty Acid (FA), and ATP Staining
2.8. Immunofluorescence Staining
2.9. Real-Time Polymerase Chain Reaction
2.10. Statistical Analyses
3. Results
3.1. Ramelteon Improved Cumulus Expansion
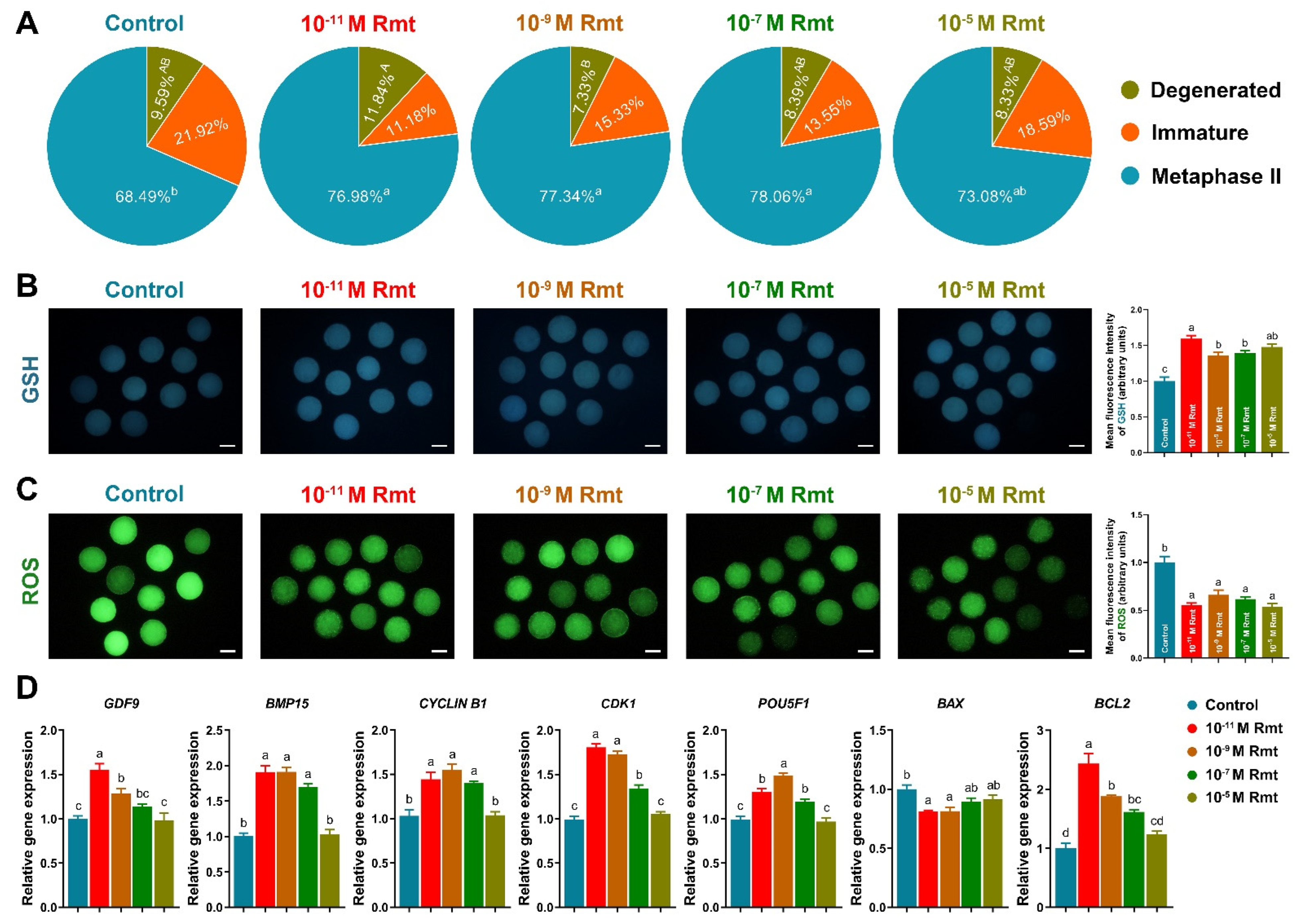
3.2. Ramelteon Improved Oocyte Maturation
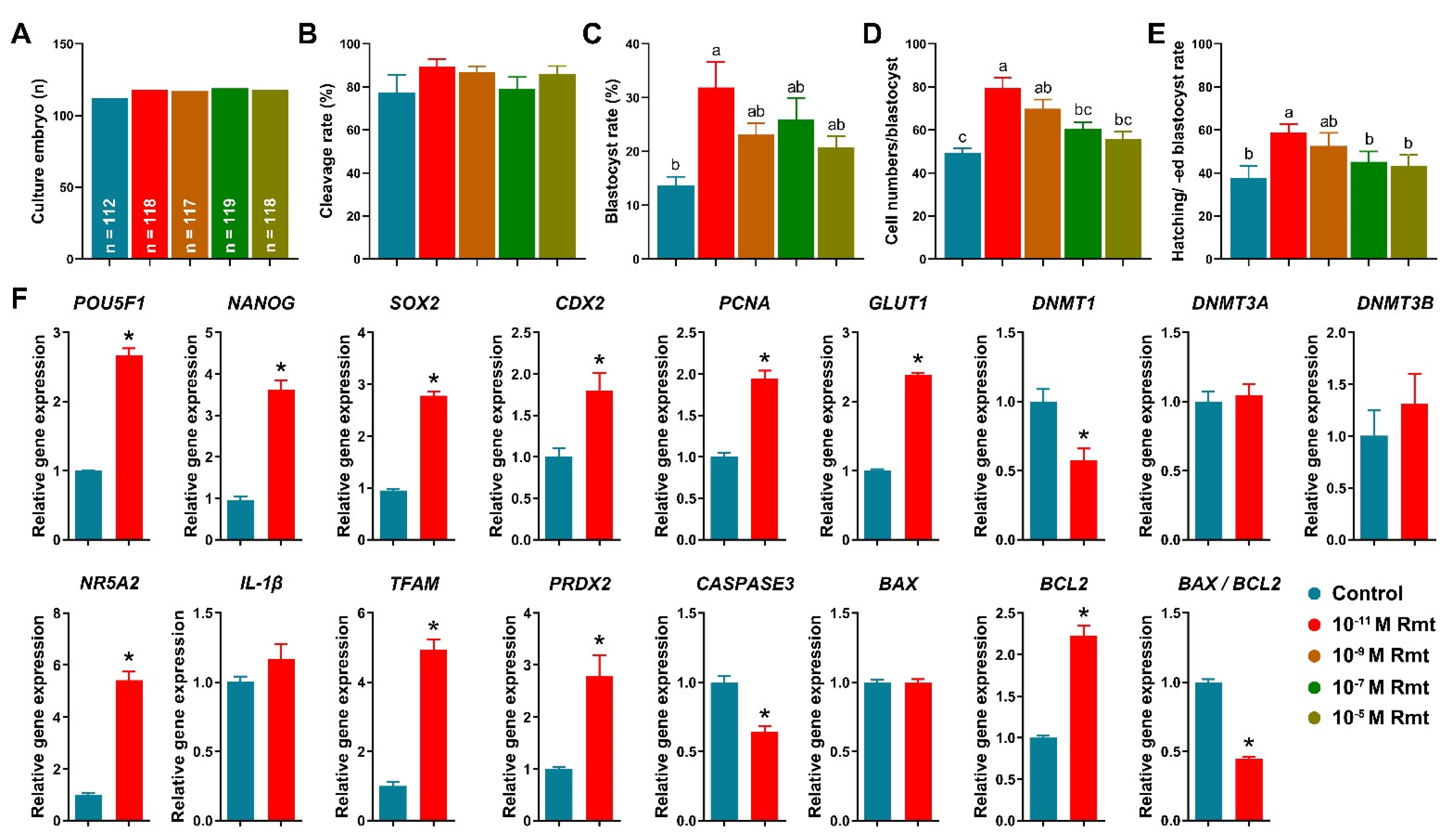
3.3. Ramelteon Improved Embryo Development after Parthenogenetic Activation
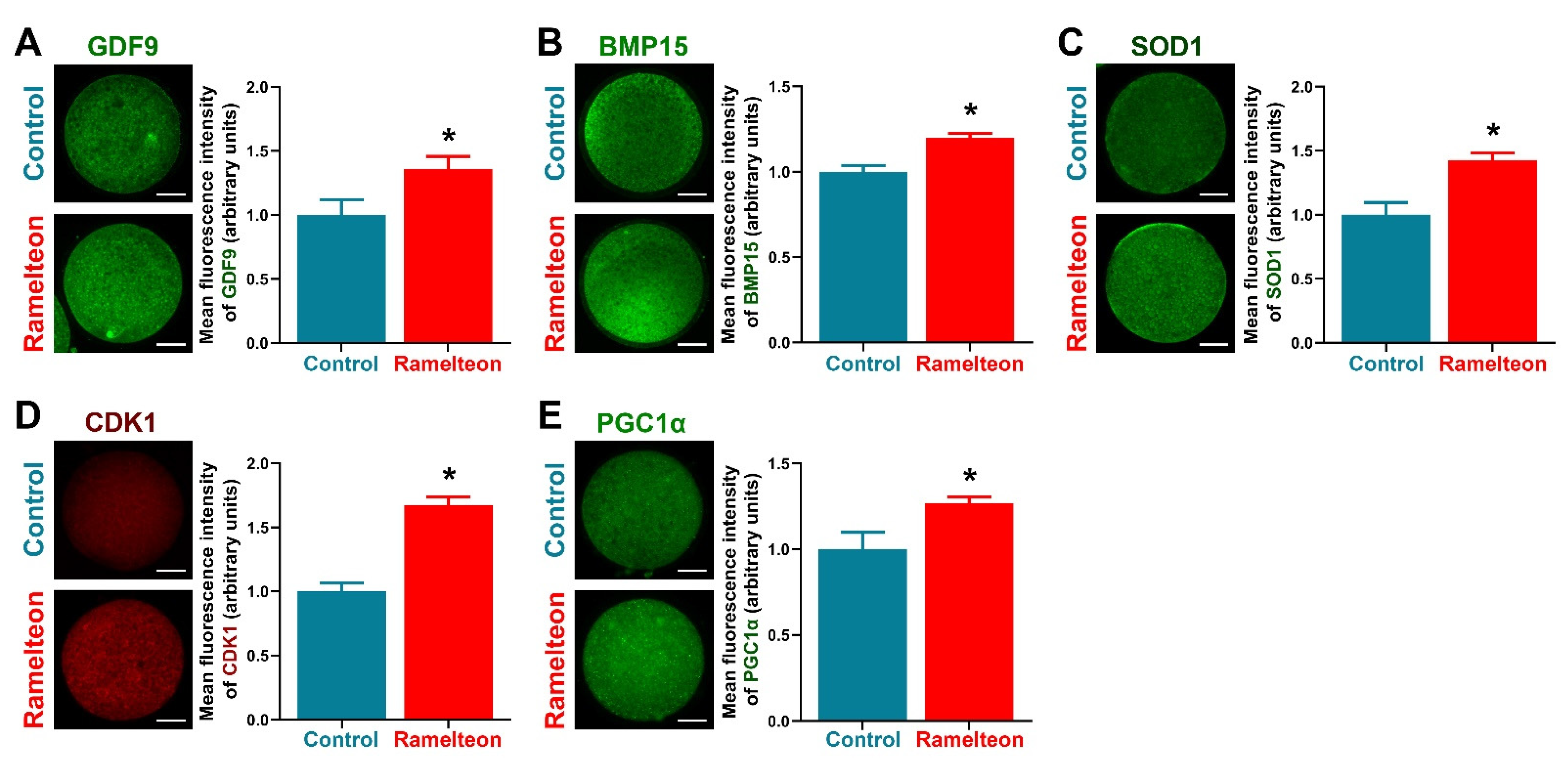
3.4. Ramelteon Participated Maintenance of Lipid Homeostasis
3.5. Ramelteon Improved Oocyte Cytoplasmic Maturation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Dokmeci Emre, S. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef] [Green Version]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. The assembly of lipid droplets and their roles in challenged cells. EMBO J. 2018, 37, e98947. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and beta-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Wang, C.; Niu, Y.; Chi, D.; Xu, M.; Si, L.; Qu, X.; Li, J. The influence of delipidation on triglyceride and LIPIN1 of porcine embryos derived from parthenogenetic activation. Reprod. Domest. Anim. 2017, 52, 842–850. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, C.; Xiong, Q.; Yang, X.; Chi, D.; Li, P.; Liu, H.; Li, J.; Huang, R. Distribution and content of lipid droplets and mitochondria in pig parthenogenetically activated embryos after delipation. Theriogenology 2015, 83, 131–138. [Google Scholar] [CrossRef]
- Prastowo, S.; Amin, A.; Rings, F.; Held, E.; Wondim, D.S.; Gad, A.; Neuhoff, C.; Tholen, E.; Looft, C.; Schellander, K.; et al. Fateful triad of reactive oxygen species, mitochondrial dysfunction and lipid accumulation is associated with expression outline of the AMP-activated protein kinase pathway in bovine blastocysts. Reprod. Fertil. Dev. 2016, 29, 890–905. [Google Scholar] [CrossRef]
- Luzzo, K.M.; Wang, Q.; Purcell, S.H.; Chi, M.; Jimenez, P.T.; Grindler, N.; Schedl, T.; Moley, K.H. High fat diet induced developmental defects in the mouse: Oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE 2012, 7, e49217. [Google Scholar] [CrossRef] [Green Version]
- Leary, C.; Leese, H.J.; Sturmey, R.G. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum. Reprod. 2015, 30, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Marei, W.F.A.; Van den Bosch, L.; Pintelon, I.; Mohey-Elsaeed, O.; Bols, P.E.J.; Leroy, J. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: A bovine in vitro model. Hum. Reprod. 2019, 34, 1984–1998. [Google Scholar] [CrossRef]
- Aizawa, R.; Ibayashi, M.; Tatsumi, T.; Yamamoto, A.; Kokubo, T.; Miyasaka, N.; Sato, K.; Ikeda, S.; Minami, N.; Tsukamoto, S. Synthesis and maintenance of lipid droplets are essential for mouse preimplantation embryonic development. Development 2019, 146, dev181925. [Google Scholar] [CrossRef]
- Jin, J.X.; Sun, J.T.; Jiang, C.Q.; Cui, H.D.; Bian, Y.; Lee, S.; Zhang, L.; Lee, B.C.; Liu, Z.H. Melatonin Regulates Lipid Metabolism in Porcine Cumulus-Oocyte Complexes via the Melatonin Receptor. Antioxidants 2022, 11, 687. [Google Scholar] [CrossRef]
- Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Neubauer, D.N. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr. Dis. Treat. 2008, 4, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Pandi-Perumal, S.R.; Spence, D.W.; Verster, J.C.; Srinivasan, V.; Brown, G.M.; Cardinali, D.P.; Hardeland, R. Pharmacotherapy of insomnia with ramelteon: Safety, efficacy and clinical applications. J. Cent. Nerv. Syst. Dis. 2011, 3, 51–65. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Sinicropi, M.S. Melatonergic drugs in development. Clin. Pharmacol. 2014, 6, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.X.; Lee, S.; Setyawan, E.M.N.; Taweechaipaisankul, A.; Kim, G.A.; Han, H.J.; Ahn, C.; Lee, B.C. A potential role of knockout serum replacement as a porcine follicular fluid substitute for in vitro maturation: Lipid metabolism approach. J. Cell Physiol. 2018, 233, 6984–6995. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, J.T.; Cui, H.D.; Jiang, C.Q.; Zhang, Y.T.; Lee, S.; Liu, Z.H.; Jin, J.X. Tannin Supplementation Improves Oocyte Cytoplasmic Maturation and Subsequent Embryo Development in Pigs. Antioxidants 2021, 10, 1594. [Google Scholar] [CrossRef]
- Wu, X.L.; Lu, S.S.; Liu, M.R.; Tang, W.D.; Chen, J.Z.; Zheng, Y.R.; Ahsan, A.; Cao, M.; Jiang, L.; Hu, W.W.; et al. Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharmacol. Sin. 2020, 41, 1016–1024. [Google Scholar] [CrossRef]
- Devi, V.; Shankar, P.K. Ramelteon: A melatonin receptor agonist for the treatment of insomnia. J. Postgrad. Med. 2008, 54, 45–48. [Google Scholar] [CrossRef]
- Markwald, R.R.; Lee-Chiong, T.L.; Burke, T.M.; Snider, J.A.; Wright, K.P., Jr. Effects of the melatonin MT-1/MT-2 agonist ramelteon on daytime body temperature and sleep. Sleep 2010, 33, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.C.; Peng, C.K.; Liao, W.I.; Pao, H.P.; Huang, K.L.; Chu, S.J. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production. Respir. Res. 2020, 21, 65. [Google Scholar] [CrossRef] [Green Version]
- Yokoo, M.; Sato, E. Cumulus-oocyte complex interactions during oocyte maturation. Int. Rev. Cytol. 2004, 235, 251–291. [Google Scholar]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: Role of cumulus cells. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Turathum, B.; Gao, E.M.; Chian, R.C. The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Zhou, C.J.; Wu, S.N.; Shen, J.P.; Wang, D.H.; Kong, X.W.; Lu, A.; Li, Y.J.; Zhou, H.X.; Zhao, Y.F.; Liang, C.G. The beneficial effects of cumulus cells and oocyte-cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. PeerJ 2016, 4, e1761. [Google Scholar] [CrossRef]
- Sugiura, K.; Su, Y.Q.; Eppig, J.J. Targeted suppression of Has2 mRNA in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol. Reprod. Dev. 2009, 76, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Fulop, C.; Szanto, S.; Mukhopadhyay, D.; Bardos, T.; Kamath, R.V.; Rugg, M.S.; Day, A.J.; Salustri, A.; Hascall, V.C.; Glant, T.T.; et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 2003, 130, 2253–2261. [Google Scholar] [CrossRef] [Green Version]
- Salustri, A.; Garlanda, C.; Hirsch, E.; De Acetis, M.; Maccagno, A.; Bottazzi, B.; Doni, A.; Bastone, A.; Mantovani, G.; Beck Peccoz, P.; et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 2004, 131, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- da Luz, C.M.; da Broi, M.G.; Donabela, F.C.; Paro de Paz, C.C.; Meola, J.; Navarro, P.A. PTGS2 down-regulation in cumulus cells of infertile women with endometriosis. Reprod. Biomed. Online 2017, 35, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Adriaenssens, T.; Segers, I.; Wathlet, S.; Smitz, J. The cumulus cell gene expression profile of oocytes with different nuclear maturity and potential for blastocyst formation. J. Assist. Reprod. Genet. 2011, 28, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.X.; Lee, S.; Khoirinaya, C.; Oh, A.; Kim, G.A.; Lee, B.C. Supplementation with spermine during in vitro maturation of porcine oocytes improves early embryonic development after parthenogenetic activation and somatic cell nuclear transfer. J. Anim. Sci. 2016, 94, 963–970. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.X.; Taweechaipaisankul, A.; Kim, G.A.; Ahn, C.; Lee, B.C. Melatonin influences the sonic hedgehog signaling pathway in porcine cumulus oocyte complexes. J. Pineal Res. 2017, 63, e12424. [Google Scholar] [CrossRef]
- Rodrigues-Cunha, M.C.; Mesquita, L.G.; Bressan, F.; Collado, M.D.; Balieiro, J.C.; Schwarz, K.R.; de Castro, F.C.; Watanabe, O.Y.; Watanabe, Y.F.; de Alencar Coelho, L.; et al. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequent embryo development. Theriogenology 2016, 86, 1685–1694. [Google Scholar] [CrossRef]
- Noda, Y.; Ota, K.; Shirasawa, T.; Shimizu, T. Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol. Reprod. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Setyawan, E.M.N.; Kim, G.A.; Lee, B.C. Effects of manganese on maturation of porcine oocytes in vitro and their subsequent embryo development after parthenogenetic activation and somatic cell nuclear transfer. J. Reprod. Dev. 2019, 65, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Taweechaipaisankul, A.; Jin, J.X.; Lee, S.; Kim, G.A.; Lee, B.C. The effects of canthaxanthin on porcine oocyte maturation and embryo development in vitro after parthenogenetic activation and somatic cell nuclear transfer. Reprod. Domest. Anim. 2016, 51, 870–876. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Leem, J.; Bai, G.Y.; Kim, J.S.; Oh, J.S. Melatonin protects mouse oocytes from DNA damage by enhancing nonhomologous end-joining repair. J. Pineal Res. 2019, 67, e12603. [Google Scholar] [CrossRef]
- Shi, J.M.; Tian, X.Z.; Zhou, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009, 47, 318–323. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Shimasaki, S. Molecular Aspects and Clinical Relevance of GDF9 and BMP15 in Ovarian Function. Vitam. Horm. 2018, 107, 317–348. [Google Scholar]
- Peng, J.; Li, Q.; Wigglesworth, K.; Rangarajan, A.; Kattamuri, C.; Peterson, R.T.; Eppig, J.J.; Thompson, T.B.; Matzuk, M.M. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 2013, 110, E776–E785. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Ignotz, G.; Currie, W.B.; Yang, X. Dynamics of maturation-promoting factor and its constituent proteins during in vitro maturation of bovine oocytes. Biol. Reprod. 1997, 56, 253–259. [Google Scholar] [CrossRef] [Green Version]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Del Collado, M.; da Silveira, J.C.; Sangalli, J.R.; Andrade, G.M.; Sousa, L.; Silva, L.A.; Meirelles, F.V.; Perecin, F. Fatty Acid Binding Protein 3 and Transzonal Projections Are Involved in Lipid Accumulation during In Vitro Maturation of Bovine Oocytes. Sci. Rep. 2017, 7, 2645. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.T.; Koo, O.J.; Kwon, D.K.; Park, H.J.; Jang, G.; Kang, S.K.; Lee, B.C. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 2009, 46, 22–28. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.-T.; Yuan, J.-D.; Zhang, Q.; Luo, X.; Qi, X.-Y.; Liu, J.-H.; Jiang, X.-Q.; Lee, S.; Taweechaipaisankul, A.; Liu, Z.-H.; et al. Ramelteon Reduces Oxidative Stress by Maintenance of Lipid Homeostasis in Porcine Oocytes. Antioxidants 2022, 11, 1640. https://doi.org/10.3390/antiox11091640
Sun J-T, Yuan J-D, Zhang Q, Luo X, Qi X-Y, Liu J-H, Jiang X-Q, Lee S, Taweechaipaisankul A, Liu Z-H, et al. Ramelteon Reduces Oxidative Stress by Maintenance of Lipid Homeostasis in Porcine Oocytes. Antioxidants. 2022; 11(9):1640. https://doi.org/10.3390/antiox11091640
Chicago/Turabian StyleSun, Jing-Tao, Jin-Dong Yuan, Qi Zhang, Xin Luo, Xin-Yue Qi, Jia-Hui Liu, Xi-Qing Jiang, Sanghoon Lee, Anukul Taweechaipaisankul, Zhong-Hua Liu, and et al. 2022. "Ramelteon Reduces Oxidative Stress by Maintenance of Lipid Homeostasis in Porcine Oocytes" Antioxidants 11, no. 9: 1640. https://doi.org/10.3390/antiox11091640






